Abstract
Class II diterpene cyclases mediate the acid-initiated cycloisomerization reaction that serves as the committed step in biosynthesis of the large class of labdane-related diterpenoid natural products, which includes the important gibberellin plant hormones. Intriguingly, these enzymes are differentially susceptible to inhibition by their Mg2+ cofactor, with those involved in gibberellin biosynthesis being more sensitive to such inhibition than those devoted to secondary metabolism, which presumably limits flux toward the potent gibberellin phytohormones. Such inhibition has been suggested to arise from intrasteric Mg2+ binding to the DXDD motif that cooperatively acts as the catalytic acid, whose affinity must then be modulated in some fashion. While further investigating class II diterpene cyclase catalysis, we discovered a conserved basic residue that seems to act as a counter ion to the DXDD motif, enhancing the ability of aspartic acid to carry out the requisite energetically difficult protonation of a carbon-carbon double bond and also affecting inhibitory Mg2+ binding. Notably, this residue is conserved as a histidine in enzymes involved in gibberellin biosynthesis and as an arginine in those dedicated to secondary metabolism. Interchanging the identity of these residues is sufficient to switch the sensitivity of the parent enzyme to inhibition by Mg2+. These striking findings indicate that this is a single residue switch for Mg2+ inhibition, which not only supports the importance of this biochemical regulatory mechanism in limiting gibberellin biosynthesis, but the importance of its release, presumably to enable higher flux, into secondary metabolism.
Keywords: Enzyme Catalysis, Enzyme Mechanisms, Evolution, Metabolism, Metabolic Regulation, Diterpene, Gibberellins, Natural Products, Terpene Synthases
Introduction
The labdane-related diterpenoids are a large superfamily of natural products, with almost 7,000 known (1). The founding members of this natural products superfamily seem to be the gibberellin (GA)4 phytohormones. Given the essential role of GA for normal growth and development in higher plants, there is an absolute requirement for the corresponding enzymatic genes (2). These then form a biosynthetic reservoir from which additional, more specialized/secondary metabolism can, and clearly has, evolved (3). Accordingly, it is perhaps not surprising that the majority of the known labdane-related diterpenoids are produced by plants.
Labdane-related diterpenoid biosynthesis is uniquely initiated by sequential cyclization and/or rearrangement reactions, the first of which is catalyzed by class II diterpene cyclases, whose bicyclic product is then elaborated by class I diterpene synthases (4, 5). However, the predominant metabolic fate of the universal diterpenoid precursor (E,E,E)-geranylgeranyl diphosphate (GGPP) in plastids, where such biosynthesis is initiated in plants, is incorporation into photosynthetic pigments (i.e. carotenoids and the phytyl side chain of chlorophyll) (Fig. 1). Indeed, the potent biological activity of GA presumably limits the amount of flux necessary for such phytohormone production (6, 7). Nevertheless, the more specialized metabolites that evolved from GA typically require higher flux (e.g. as antibiotic components of a defense response to microbial infection (8)).
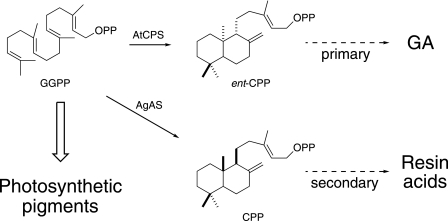
FIGURE 1.
Various metabolic fates of GGPP in plant plastids. The production of photosynthetic pigments and GA is conserved in all higher plants, whereas the production of resin acids initiated by the AgAS studied here is shown as an example of more specialized/secondary labdane-related diterpenoid metabolism. OPP, diphosphate ester group.
The enzymatic structure-function relationships underlying the acid initiated cycloisomerization reaction mediated by the class II diterpene cyclases that initiate labdane-related diterpenoid biosynthesis remain unclear. The requisite carbon-carbon double bond (C C) protonation is energetically difficult, yet appears to be catalyzed by a DXDD motif wherein the “first” and “last” aspartates act to increase the acidity of the “middle” aspartate, which then carries out such protonation. This hypothesis was originally based on the highly conserved nature of this motif and analogies to the much better understood C
C) protonation is energetically difficult, yet appears to be catalyzed by a DXDD motif wherein the “first” and “last” aspartates act to increase the acidity of the “middle” aspartate, which then carries out such protonation. This hypothesis was originally based on the highly conserved nature of this motif and analogies to the much better understood C C protonation reaction catalyzed by bacterial squalene-hopene cyclases (SHCs), which contain an analogous catalytic motif (9). Mutational analysis has since demonstrated the importance of the corresponding aspartates in class II diterpene cyclases (10, 11), and we have reported evidence in a preliminary communication strongly supporting similar cooperative catalytic acid function for this motif (12). Furthermore, it has recently been suggested that class II diterpene cyclases are descended from SHC (13). However, in SHC, it is known that the ability of the corresponding DXDD motif to carry out this energetically difficult reaction further requires the presence of a basic residue (conserved as histidine or arginine (14, 15)) to act as a counter ion (16), but this residue is not conserved in class II diterpene cyclases.
C protonation reaction catalyzed by bacterial squalene-hopene cyclases (SHCs), which contain an analogous catalytic motif (9). Mutational analysis has since demonstrated the importance of the corresponding aspartates in class II diterpene cyclases (10, 11), and we have reported evidence in a preliminary communication strongly supporting similar cooperative catalytic acid function for this motif (12). Furthermore, it has recently been suggested that class II diterpene cyclases are descended from SHC (13). However, in SHC, it is known that the ability of the corresponding DXDD motif to carry out this energetically difficult reaction further requires the presence of a basic residue (conserved as histidine or arginine (14, 15)) to act as a counter ion (16), but this residue is not conserved in class II diterpene cyclases.
In the course of our initial characterization of the ent-copalyl diphosphate (ent-CPP) synthase (CPS) from Arabidopsis thaliana (AtCPS), we noted that this GA-associated class II diterpene cyclase suffered from striking inhibition exerted by its divalent magnesium ion (Mg2+) cofactor, which also is synergistic with previously reported GGPP substrate inhibition, and which we hypothesized occurred via “inhibitory” Mg2+ binding to the DXDD motif (17). Intriguingly, we further found that the class II diterpene cyclase activity of the resin acid (i.e. more specialized or secondary metabolism) dedicated abietadiene synthase from Abies grandis (AgAS) was much less susceptible to such Mg2+ mediated inhibition, although it also contains the DXDD motif. We then hypothesized that the observed Mg2+ inhibitory effect represented a biochemical feed-forward inhibition mechanism acting to limit flux toward production of the potent GA phytohormones in plant plastids, with an as-yet-unidentified residue acting to determine susceptibility to such intrasteric inhibitory Mg2+ binding to the DXDD motif (17). Here, we report results that not only provide insight into the enzymatic mechanism, but also Mg2+-dependent regulation of class II diterpene cyclases, including how this is modified in response to the selective advantages provided by differential regulation of biosynthetically related/derived, but physiologically distinct (i.e. primary/GA versus more specialized/secondary) metabolic fluxes.
EXPERIMENTAL PROCEDURES
General
The preparation of reference samples for products and substrate (+/−)-14,15-oxidogeranylgeranyl diphosphate (oxido-GGPP) have been described previously (12, 17). GGPP was purchased from Sigma-Aldrich (St. Louis, MO) or Isoprenoids, LC (Tampa, FL). Unless otherwise noted, all other chemicals were purchased from Fisher Scientific (Loughborough, Leicestershire, UK), and molecular biology reagents from Invitrogen. Sequence alignments were carried out using the AlignX program from the Vector NTI software package (Invitrogen), using the default parameters.
Enzymatic Analyses
Recombinant pseudomature AtCPS and AgAS were expressed, purified, and assayed as described previously (17). Site-directed mutagenesis was carried out via PCR amplification of pENTR (Gateway, Invitrogen) clones using overlapping mutagenic primers. The resulting mutant genes were verified by complete sequencing prior to transfer via directional recombination to pDEST14 and pDEST17 expression vectors. His-tag purification was enabled by pDEST17 constructs, which encode an N-terminal His6 tag, allowing purification with nickel-nitrilotriacetic acid superflow resin (EMD Chemicals, Gibbstown, NJ), used according to the manufacturer's instructions, with the resulting enzymes being >95% pure as judged by SDS-PAGE analysis. Kinetic parameters were calculated from fitting the observed data to the substrate inhibition equation (KaleidaGraph 4.0, Synergy Software; Reading, PA). For data that could not be fit to the substrate inhibition equation (Mg2+ kinetics with AtCPS,D377A; AtCPS,D380A; AtCPS,H331A; and AgAS,D621A/R356H; as indicated by R2 < 0.9), kinetic parameters were calculated by partial double reciprocal plots (i.e. using the increasing rate data at lower Mg2+ concentrations to calculate Km and the subsequently decreasing rate data at higher Mg2+ concentrations to calculate Ki, with the resulting kinetic constants marked with an asterisk in Table 1 and supplemental Table S3), with the exception of AtCPS,H331R, for which there was no detectable substrate inhibition and was fit to the Michaelis-Menten equation (R2 = 0.97). For clarity and consistency, all kinetic plots are shown with a smooth fit line simply connecting the observed data points.
TABLE 1.
GGPP kinetics of AtCPS and mutants
| AtCPSa | kcat | Km | Ki |
|---|---|---|---|
| s−1 | μm | μm | |
| Wild-type | 3 | 3 | 5 |
| H331A | 2 × 10−4 | 2 | 3 |
| D377A | 2 × 10−4 | 2 | 6b |
| D379A | 3 × 10−7 | 16b | |
| D380A | 7 × 10−4 | 5 |
a Assays contained the 0.1 mm MgCl2 required for optimal activity with the wild type enzyme.
b Kinetic constants derived from partial double reciprocal plot fits, as described under “Experimental Procedures.”
RESULTS
To further investigate the mechanism by which the DXDD motif initiates class II diterpene cyclization, we carried out additional mutational analysis using alanine, glutamate, and asparagine substitutions for each of these aspartates in AtCPS. Appreciable mutant activity was only found with substitution of glutamate for the first, or asparagine for the last, aspartates (supplemental Table S1). These results are consistent with previous mutational analysis of this motif in AgAS (10), and previous reports of CPS that contain glutamate in place of the first aspartate (18, 19). Together, this suggests the possibility of a polar interaction wherein the first aspartate carries a negative charge that is presumably stabilized by interaction with the last aspartate.
To identify a catalytic basic residue that might act as a counter ion to the DXDD motif in class II diterpene cyclases, the domains that contain the class II active site (20) were examined for similarly conserved (His/Arg) positions. Only a single such position was found (supplemental Fig. S1), and an alanine substitution for the corresponding His in AtCPS (H331A) led to a significant reduction in enzymatic activity.
Expanding upon our initial study of the catalytic aspartate residues Asp379 and Asp380 (12), we examined the kinetic parameters of the D377A and H331A mutants (Table 1). Each mutant exhibited a significant reduction in activity (>1,000-fold decrease in kcat), but neither appeared to drastically affect substrate binding (<5-fold increase in Km). In combination with our previous study, these results are consistent with the hypothesis that the DXDD motif is not involved in productive GGPP binding. However, the previously noted GGPP substrate inhibition effect was slightly more pronounced in the H331A mutant, although no such substrate inhibition was seen with the D379A or D380A mutants, indicating a drastic increase in Ki for GGPP.
Additional kinetic studies were carried out with the GGPP analog (+/−)-14,15-oxidogeranylgeranyl diphosphate (oxido-GGPP). This substrate is more easily protonated, due to the epoxide ring substitution for the C14–C15 C C of GGPP, and its cyclization yields 3-hydroxycopalyl diphosphate epimers (Fig. 2). We have characterized previously AtCPS,D379A and AtCPS,D380A with this substrate analog (12), and here, we report analogous investigations with AtCPS,D377A and AtCPS,H331A. As previously reported, AtCPS,D379A is essentially unable to cyclize either GGPP or oxido-GGPP, whereas AtCPS,D380A is significantly more active with oxido-GGPP than GGPP, as were the AtCPS,D377A and AtCPS,H331A reported here (Table 2). Together, these results support a model of class II diterpene cyclase activity with a catalytic tetrad composed of the DXDD motif and a basic (His/Arg) residue counter ion, much as found in SHC.
C of GGPP, and its cyclization yields 3-hydroxycopalyl diphosphate epimers (Fig. 2). We have characterized previously AtCPS,D379A and AtCPS,D380A with this substrate analog (12), and here, we report analogous investigations with AtCPS,D377A and AtCPS,H331A. As previously reported, AtCPS,D379A is essentially unable to cyclize either GGPP or oxido-GGPP, whereas AtCPS,D380A is significantly more active with oxido-GGPP than GGPP, as were the AtCPS,D377A and AtCPS,H331A reported here (Table 2). Together, these results support a model of class II diterpene cyclase activity with a catalytic tetrad composed of the DXDD motif and a basic (His/Arg) residue counter ion, much as found in SHC.
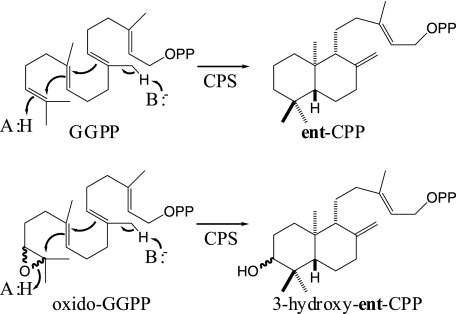
FIGURE 2.
Class II cycloisomerization reaction. CPS typically cyclize GGPP to ent-CPP and will readily cyclize (+/−)-oxido-GGPP into 3α- and 3β-hydroxy-ent-CPP as well. OPP, diphosphate ester group.
TABLE 2.
Observed rates of AtCPS and mutants with GGPP relative to (+/−)-oxido-GGPP
| AtCPSa | VobsGGPP | Vobsoxido | Vobsoxido/VobsGGPP |
|---|---|---|---|
| s−1 | s−1 | ||
| Wild-type | 1.2 ± 0.1 | 0.7 ± 0.1 | 0.6 |
| H331A | (5 ± 1) × 10−5 | (7 ± 1) × 10−4 | 14 |
| D377A | (6 ± 1) × 10−5 | (2 ± 1) × 10−3 | 33 |
| D379A | <10−6 | <10−6 | <10 |
| D380A | (4 ± 2) × 10−4 | (2 ± 1) × 10−2 | 50 |
a Error represents standard error from two independent measurements.
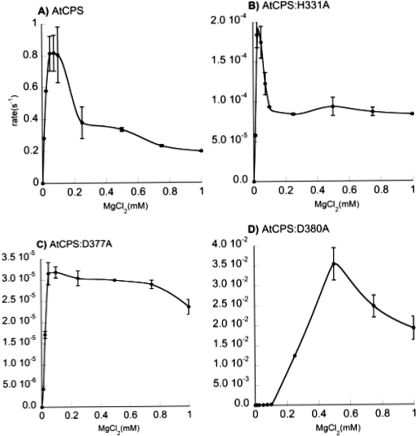
We have previously proposed that the DXDD motif acts as an inhibitory Mg2+ binding site (17) and here investigated the effects of alanine substitution for the catalytic tetrad on the response of AtCPS activity to varying concentrations of Mg2+ (Fig. 3). Both the D377A and D380A mutants exhibited reduced Mg2+ inhibition relative to wild-type AtCPS, with considerably less drastic decreases in activity above the optimal concentration of Mg2+, indicating that at least the first and last aspartates of the DXDD motif are involved in inhibitory Mg2+ binding. (Unfortunately, AtCPS,D379A did not retain sufficient activity for such analysis.) In addition, AtCPS,D379A exhibited positive cooperativity, indicating complex interactions between multiple activating Mg2+. By contrast, the H331A mutant exhibited accentuated Mg2+ inhibition. While of the opposite effect, this result also indicates proximity to the DXDD motif and further supports the role of this residue as a catalytic counter ion to the DXDD motif. Furthermore, this indicates that His331 acts antagonistically to reduce the affinity of the DXDD motif for inhibitory Mg2+ binding, presumably due to electrostatic repulsion between this divalent metal cation and the proposed catalytically relevant positively charged form of the histidine.
FIGURE 3.
Effect of magnesium on AtCPS activity. A, wild-type (WT). B, AtCPS,H331A. C, AtCPS,D377A. D, AtCPS,D380A. The measured data are simply connected by smooth fit lines, as not all plots can be fitted to the standard substrate inhibition equation. Error bars represent the S.D. from two independent measurements in all cases.
Intriguingly, we noted that the catalytic basic residue is conserved as a His in class II diterpene cyclases with a demonstrated role in GA biosynthesis, whereas those involved in secondary/specialized metabolism exclusively contained an Arg at this position (Fig. 4). On the basis of this striking conservation pattern, we hypothesized that this residue might control the differential susceptibility of class II diterpene cyclases to inhibition by Mg2+. This was investigated by making reciprocal mutations in AtCPS, which is involved in GA (i.e. primary) metabolism and exhibits strong synergistic GGPP and Mg2+ inhibition, and AgAS, which is a bifunctional diterpene synthase dedicated to resin acid (i.e. secondary) metabolism and is much less susceptible to this substrate/cofactor inhibition effect (17). Specifically, we constructed a set of “switch” mutants at the conserved His/Arg site, AtCPS,H331R and AgAS,D621A/R356H. (AgAS, D621A has been characterized previously as lacking class I activity without any effect on class II activity (11), allowing examination of the effect of mutations on class II activity in isolation.)
FIGURE 4.
Multiple sequence alignment of class II diterpene synthases with known metabolic function (the catalytic DXDD motif is underlined, whereas the catalytic basic residue position is indicated with an asterisk, and the enzymes are separated according to physiological function by a line, as indicated). Those enzymes with known roles in GA metabolism from mutant plant analysis (i.e. severe dwarf phenotype): AtCPS (21), PsCPS from Pisum sativum (27), ZmCPS1 (also known as An1) from Zea mays (28), and OsCPS1 from Oryza sativa (29). Those devoted to more specialized/secondary metabolism (2°), either from mutant analysis, such as OsCPS2 from Oryza sativa (18, 30) or because they do not produce the ent-CPP required for GA biosynthesis, such as the syn-CPP synthase OsCPS4 from Oryza sativa (18, 30), and two representative bifunctional diterpene cyclases from gymnosperms, which produce CPP of normal stereochemistry as an intermediate: AgAS (31), and GbLS from Gingko biloba (32). A more extensive alignment is available in supplemental Fig. S1.
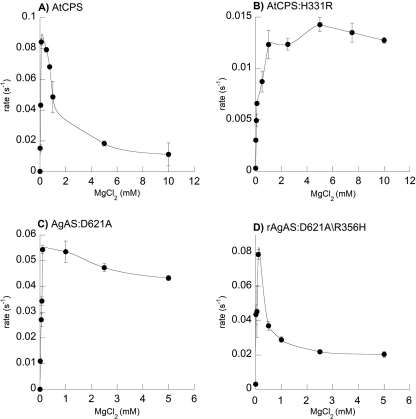
Both mutants were active but were not well expressed. Addition of a His6 tag improved the yields and allowed for rapid purification. Kinetic parameters were obtained for these constructs and compared with equivalently tagged parental constructs (i.e. wild-type AtCPS and AgAS,D621A). The tagged parental constructs exhibited <10-fold loss of catalytic activity (i.e. kcat) and retained Km and Ki for GGPP equivalent to that of the untagged enzymes (supplemental Table S2). The effects of exchanging the His/Arg residues on sensitivity to inhibitory Mg2+ binding were obtained by assaying each enzyme across a range of Mg2+ concentrations. Critically, these exchange mutants retain significant catalytic activity, with a <10-fold effect on kcat. Nevertheless, both exhibited striking alteration of their susceptibility to inhibition by Mg2+ relative to the parental wild-type enzymes (Fig. 5). In particular, whereas the AtCPS wild-type enzyme has a Ki for Mg2+ of 0.7 mm, AtCPS:H331R is no longer appreciably inhibited by Mg2+ in the range of concentrations tested here. Further, whereas AgAS:D621A has a Ki for Mg2+ of 12 mm, AgAS:D621A/R356H has a dramatically increased Ki of 0.25 mm (supplemental Table S3).
FIGURE 5.
Effect of varying Mg2+ concentration on His6 tagged wild-type and His/Arg switch mutants of class II diterpene cyclases involved in primary or more specialized/secondary metabolism. A, AtCPS. B, AtCPS,H331R. C, AgAS,D621A. D, AgAS,D621A/R356H. The measured data are simply connected by smooth fit lines, as not all plots can be fit to the standard substrate inhibition equation. Error bars represent the S.D. from two independent measurements in all cases.
DISCUSSION
Class II diterpene cyclases catalyze the committed step in biosynthesis of the large class of labdane-related diterpenoid natural products, which range from the gibberellin (GA) phytohormones to more specialized/secondary metabolites typically utilized in defense (3). In plants, these enzymes are localized in plastids (21) and divert GGPP away from the production of phytosynthetic pigments (i.e. carotenoids and the phytyl side chain of chlorophyll) (22). Thus, understanding the mechanism and regulation of these important, gate-keeping enzymes can provide key insights into understanding how plants mediate and control metabolic flux within their plastids.
Of particular relevance to the studies discussed here, GA is only required in very small amounts in planta, whereas secondary metabolites can be produced in very large quantities. Because A. thaliana only produces GA and no other labdane-related diterpenoid natural products, AtCPS is dedicated to GA biosynthesis. Furthermore, AtCPS is found throughout fast growing tissues, as well as in the vasculature (23). Such widespread enzymatic distribution indicates that relatively limited activity would be sufficient to mediate the small amounts of metabolic flux necessary for GA biosynthesis. By contrast, conifer resin is found in anatomically specialized structures, such as the resin blisters in Abies grandis saplings. It has been shown that the relevant diterpene synthases, such as AgAS, are localized in the surrounding epithelial cells (24), which are then presumably responsible for production of all the observed resin, necessitating an enormous flux into such biosynthesis (i.e. these cells produce several times their own volume of resin). Thus, these disparate (i.e. primary/GA versus secondary) metabolic processes presumably require distinct regulatory mechanisms.
Although no structural information has yet been reported for class II diterpene cyclases, it has been suggested that these are descended from the triterpene SHCs, which catalyze mechanistically similar reactions (13). Based on analogies to the better understood SHC, it has been suggested that the class II diterpene cyclases utilize the DXDD motif they share in common with SHC, to act as the catalytic acid in C C protonation-initiated cyclization (10, 11). In particular, that the first and last aspartates carry a negative charge that activates the middle aspartate to carry out the requisite energetically difficult C
C protonation-initiated cyclization (10, 11). In particular, that the first and last aspartates carry a negative charge that activates the middle aspartate to carry out the requisite energetically difficult C C protonation (12), a hypothesis consistent with the data reported here.
C protonation (12), a hypothesis consistent with the data reported here.
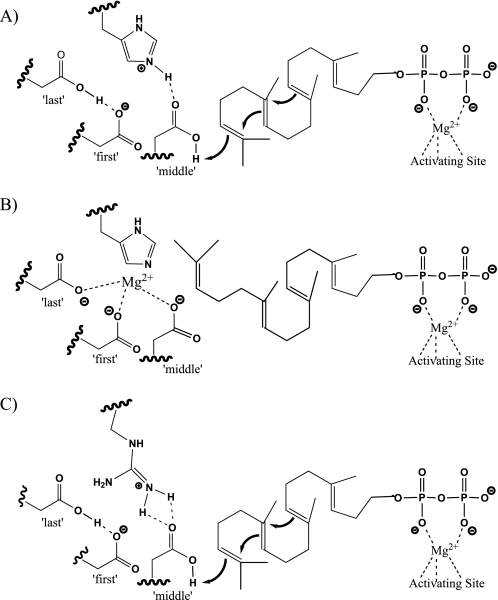
In addition to the DXDD motif, SHC further require a catalytic basic residue, conserved as a His or Arg (14, 15) that seems to act as a counter ion to the negative charge carried by the first and last aspartates and which directly interacts with the middle aspartate (16). Although this residue is not conserved in class II diterpene cyclases, we also have identified here a catalytic basic residue, similarly conserved as His or Arg, in plant class II diterpene cyclases that seems to have an analogous function, serving to increase the ability of the middle aspartate to carry out C C protonation. While clearly not phylogenetically conserved (i.e. in plant class II diterpene cyclases this catalytic counter ion precedes, while that in SHC follows, the DXDD motif) these mechanistically similar enzymes then both utilize a DXDD+His/Arg tetrad that acts cooperatively as the catalytic acid, providing significant insight into the enzymatic mechanism utilized by class II diterpene cyclases.
C protonation. While clearly not phylogenetically conserved (i.e. in plant class II diterpene cyclases this catalytic counter ion precedes, while that in SHC follows, the DXDD motif) these mechanistically similar enzymes then both utilize a DXDD+His/Arg tetrad that acts cooperatively as the catalytic acid, providing significant insight into the enzymatic mechanism utilized by class II diterpene cyclases.
Class II diterpene cyclases require the presence of Mg2+ for full catalytic activity (10, 17), presumably for binding of the diphosphate moiety of their GGPP substrate, whereas divalent metal ions are not required for binding of the squalene olefin substrate of SHC (25). Intriguingly, Mg2+ exhibits a dramatic biphasic effect on the activity of the AtCPS involved in GA biosynthesis, with a very rapid decrease in activity above the optimal concentration, and this effect is much less pronounced with class II enzymes involved in more specialized/secondary metabolism (i.e. AgAS) (17). Furthermore, Mg2+ levels vary in plant plastids where class II diterpene cyclases are found, specifically in response to light (26), over a similar sub- to low millimolar range as that observed to affect class II enzymatic activity. Thus, we have previously hypothesized that the observed selective Mg2+-dependent suppression may act as a physiologically relevant feed-forward inhibition mechanism limiting flux toward the potent GA hormones, with inhibitory Mg2+ binding to the catalytic DXDD motif, whereas an unidentified residue acts as a regulatory switch that modulates this interaction (17).
This hypothesis was further bolstered by the investigation of enzymatic activity presented here, which also indicated an interaction between such inhibitory Mg2+ binding and the catalytic counter ion basic residue. In addition to being consistent with the postulated direct interaction of this residue with the DXDD motif, the enhanced inhibitory Mg2+ binding observed upon removal of the basic catalytic counter ion suggested the possibility that this residue might be the postulated regulatory switch.
Close examination of the alignment of class II enzymes focusing on those with known physiological roles revealed a striking conservation pattern wherein this position is conserved as a His in those CPS with a demonstrated role in GA biosynthesis and as an Arg in those class II enzymes functioning in more specialized/secondary metabolism (Fig. 4). Hypothesizing that this might represent the previously postulated regulatory switch, we reciprocally exchanged the corresponding catalytic basic residues in AtCPS and AgAS and found that this led to dramatically different sensitivity to inhibition by Mg2+ in both cases (Fig. 5). These results strongly indicate that the identity of the residue at this catalytic basic counter ion position represents the previously hypothesized regulatory switch for plant class II diterpene cyclases, consistent with the conservation pattern noted above. We hypothesize that the positively charged form of this catalytic basic residue repels Mg2+, with the lower pKa of His relative to Arg providing a higher probability that this side chain will be in the unprotonated/neutral form, allowing inhibitory Mg2+ binding to the DXDD motif (Fig. 6), although steric effects also are possible.
FIGURE 6.
Proposed model for the DXDD+His/Arg catalytic tetrad, with speculative hydrogen bonding interactions among the catalytic residues and the natural GGPP substrate. A, productive binding in the active site of CPS involved in GA biosynthesis. B, inhibitory binding of Mg2+ preventing GGPP cyclization in the active site of CPS involved in GA biosynthesis. C, productive binding of GGPP in the class II active site of diterpene synthases involved in more specialized/secondary metabolism. The catalytic aspartates are labeled by their relative position within the DXDD motif.
Regardless of the exact mechanism, the striking conservation pattern and biochemical effect revealed here strongly support physiological relevance for the previously hypothesized synergistic feed-forward inhibition exerted by GGPP and Mg2+ on the CPS involved in GA biosynthesis to limit flux toward this potent phytohormone. In addition, these results further suggest the importance of removing such limitation to enable higher flux in the context of more specialized/secondary metabolism, where rapid production of relatively large amounts of (e.g. antibiotic) natural products would provide obvious advantages for such a defensive response. Accordingly, our results provide insight into the enzymatic mechanism of plant class II diterpene cyclases and also support the relevance of an intertwined biochemical regulatory mechanism, which appears to operate as a single residue switch that is flipped in response to the selective advantages provided by differential regulation of biosynthetically related/derived but physiologically distinct (i.e. primary/GA versus more specialized/secondary) metabolic fluxes.
Supplementary Material
This work was supported by grants from the National Science Foundation (MCB0919735) and National Institutes of Health (GM076324) (to R. J. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3, Fig. S1, and additional references.
- GA
- gibberellin phytohormones
- SHC
- squalene-hopene cyclase
- AgAS
- abietadiene synthase from Abies grandis
- CPP
- copalyl diphosphate
- ent-CPP
- ent-copalyl diphosphate
- CPS
- CPP synthase
- AtCPS
- CPS from Arabidopsis thaliana
- GGPP
- [E,E,E]-geranylgeranyl diphosphate
- oxido-GGPP
- (+/−)-14,15-oxidogeranylgeranyl diphosphate.
REFERENCES
- 1.Buckingham J. (2002) Dictionary of Natural Products, www.chemnetbase.com
- 2.Fleet C. M., Sun T. P. (2005) Curr. Opin. Plant Biol. 8, 77–85 [DOI] [PubMed] [Google Scholar]
- 3.Peters R. J. (2006) Phytochemistry 67, 2307–2317 [DOI] [PubMed] [Google Scholar]
- 4.Christianson D. W. (2006) Chem. Rev. 106, 3412–3442 [DOI] [PubMed] [Google Scholar]
- 5.Wendt K. U., Schulz G. E. (1998) Structure 6, 127–133 [DOI] [PubMed] [Google Scholar]
- 6.Alabadí D., Gil J., Blázquez M. A., García-Martínez J. L. (2004) Plant Physiol. 134, 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Martinez J. L., Gil J. (2001) J. Plant Growth Regul. 20, 354–368 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd J. C., Zakhleniuk O. V. (2004) J. Exp. Bot. 55, 1221–1230 [DOI] [PubMed] [Google Scholar]
- 9.Bohlmann J., Meyer-Gauen G., Croteau R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters R. J., Croteau R. B. (2002) Biochemistry 41, 1836–1842 [DOI] [PubMed] [Google Scholar]
- 11.Peters R. J., Ravn M. M., Coates R. M., Croteau R. B. (2001) J. Am. Chem. Soc. 123, 8974–8978 [DOI] [PubMed] [Google Scholar]
- 12.Prisic S., Xu J., Coates R. M., Peters R. J. (2007) ChemBioChem 8, 869–874 [DOI] [PubMed] [Google Scholar]
- 13.Cao R., Zhang Y., Mann F. M., Huang C., Mukkamala D., Hudock M. P., Mead M., Prisic S., Wang K., Lin F. Y., Chang T. K., Peters R. J., Oldfield E. (2010) Proteins, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T., Hoshino T. (1999) Biosci. Biotechnol. Biochem. 63, 2189–2198 [DOI] [PubMed] [Google Scholar]
- 15.Merkofer T., Pale-Grosdemange C., Wendt K. U., Rohmer M., Poralla K. (1999) Tetrahedron Lett. 40, 2121–2124 [Google Scholar]
- 16.Wendt K. U. (2005) Angew. Chem. Int. Ed. Engl. 44, 3966–3971 [DOI] [PubMed] [Google Scholar]
- 17.Prisic S., Peters R. J. (2007) Plant Physiol. 144, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prisic S., Xu M., Wilderman P. R., Peters R. J. (2004) Plant Physiol. 136, 4228–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris L. J., Saparno A., Johnston A., Prisic S., Xu M., Allard S., Kathiresan A., Ouellet T., Peters R. J. (2005) Plant Mol. Biol. 59, 881–894 [DOI] [PubMed] [Google Scholar]
- 20.Peters R. J., Carter O. A., Zhang Y., Matthews B. W., Croteau R. B. (2003) Biochemistry 42, 2700–2707 [DOI] [PubMed] [Google Scholar]
- 21.Sun T. P., Kamiya Y. (1994) Plant Cell 6, 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGarvey D. J., Croteau R. (1995) Plant Cell 7, 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverstone A. L., Chang C., Krol E., Sun T. P. (1997) Plant J. 12, 9–19 [DOI] [PubMed] [Google Scholar]
- 24.Keeling C. I., Bohlmann J. (2006) Phytochemistry 67, 2415–2423 [DOI] [PubMed] [Google Scholar]
- 25.Wendt K. U., Schulz G. E., Corey E. J., Liu D. R. (2000) Angew. Chem. Int. Ed. Engl. 39, 2812–2833 [PubMed] [Google Scholar]
- 26.Ishijima S., Uchibori A., Takagi H., Maki R., Ohnishi M. (2003) Arch. Biochem. Biophys. 412, 126–132 [DOI] [PubMed] [Google Scholar]
- 27.Ait-Ali T., Swain S. M., Reid J. B., Sun T., Kamiya Y. (1997) Plant J. 11, 443–454 [DOI] [PubMed] [Google Scholar]
- 28.Bensen R. J., Johal G. S., Crane V. C., Tossberg J. T., Schnable P. S., Meeley R. B., Briggs S. P. (1995) Plant Cell 7, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto T., Miura K., Itoh H., Tatsumi T., Ueguchi-Tanaka M., Ishiyama K., Kobayashi M., Agrawal G. K., Takeda S., Abe K., Miyao A., Hirochika H., Kitano H., Ashikari M., Matsuoka M. (2004) Plant Physiol. 134, 1642–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otomo K., Kenmoku H., Oikawa H., König W. A., Toshima H., Mitsuhashi W., Yamane H., Sassa T., Toyomasu T. (2004) Plant J. 39, 886–893 [DOI] [PubMed] [Google Scholar]
- 31.Vogel B. S., Wildung M. R., Vogel G., Croteau R. (1996) J. Biol. Chem. 271, 23262–23268 [DOI] [PubMed] [Google Scholar]
- 32.Schepmann H. G., Pang J., Matsuda S. P. (2001) Arch. Biochem. Biophys. 392, 263–269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.