Abstract
The docking protein p130Cas is a major Src substrate involved in integrin signaling and mechanotransduction. Tyrosine phosphorylation of p130Cas in focal adhesions (FAs) has been linked to enhanced cell migration, invasion, proliferation, and survival. However, the mechanism of p130Cas targeting to FAs is uncertain, and dynamic aspects of its localization have not been explored. Using live cell microscopy, we show that fluorophore-tagged p130Cas is a component of FAs throughout the FA assembly and disassembly stages, although it resides transiently in FAs with a high mobile fraction. Deletion of either the N-terminal Src homology 3 (SH3) domain or the Cas-family C-terminal homology (CCH) domain significantly impaired p130Cas FA localization, and deletion of both domains resulted in full exclusion. Focal adhesion kinase was implicated in the FA targeting function of the p130Cas SH3 domain. Consistent with their roles in FA targeting, both the SH3 and CCH domains were found necessary for p130Cas to fully undergo tyrosine phosphorylation and promote cell migration. By revealing the capacity of p130Cas to function in FAs throughout their lifetime, clarifying FA targeting mechanism, and demonstrating the functional importance of the highly conserved CCH domain, our results advance the understanding of an important aspect of integrin signaling.
Keywords: Adhesion, Cell Migration, Phosphotyrosine Signaling, SH3 Domains, Src, Focal Adhesion Kinase (FAK), p130Cas, Paxillin
Introduction
p130Cas (Crk-associated substrate) is a Src substrate that functions in integrin signaling to promote cell motility, invasion, proliferation, and survival (1, 2). p130Cas was first recognized as a prominent tyrosine-phosphorylated protein in cells transformed by v-crk (3) and v-src (4). The observation that p130Cas interacts directly with the Src homology 2 (SH2)2 domain of the v-Crk protein (5, 6) contributed to the recognition of SH2 domains as phosphotyrosine-binding modules in signal transduction. The primary structure of p130Cas (7) indicated a function as a docking/scaffolding protein, lacking domains indicative of intrinsic enzymatic activity but having various domains and motifs for mediating interactions with other proteins.
p130Cas was independently identified in a screen for proteins that interact with focal adhesion kinase (FAK) (8), a tyrosine kinase named for its prominent localization to sites of integrin-mediated cell adhesion. A Src homology 3 (SH3) domain at the N terminus of p130Cas mediates the FAK interaction. Like FAK, p130Cas localizes to focal adhesions (FAs) and undergoes tyrosine phosphorylation in response to adhesion (9–11). Thus, p130Cas is a signaling component of the FA protein complex (“adhesome”) that assembles to bring about cellular responses to integrin engagement. A primary role for p130Cas in integrin signaling is consistent with the phenotype of p130Cas-deficient mice, which die during embryonic development due to defects associated with a disorganized actin cytoskeleton (12). Despite the direct interaction with FAK, tyrosine phosphorylation of p130Cas is attributed to Src-family kinases (13–15). However, FAK can act as a scaffold to recruit Src to phosphorylate p130Cas by virtue of the Src SH2 domain binding to the FAK autophosphorylation site (16). The SH3 and/or SH2 domains of Src can also directly bind to motifs near the p130Cas C-terminal region termed the Src binding domain (SBD) (14, 16, 17).
The major sites of p130Cas tyrosine phosphorylation lie in the substrate domain (SD) located in the central region of the protein. Much evidence has implicated p130Cas SD tyrosine phosphorylation in the promotion of cell motility and invasion (18–23). The SD is defined by 15 scattered YXXP motifs, most of which have been detected as in vivo sites of tyrosine phosphorylation (24, 25). Included are nine YDXP motifs that upon tyrosine phosphorylation are optimal binding sites for SH2 domains of the adaptor proteins Crk and Nck (26). The YDXP tyrosines are phosphorylated by Src in vitro (21), and recruitment of Crk (7) and/or Nck (27) to these sites may promote downstream signaling events leading to cell motility and invasion. p130Cas SD tyrosine phosphorylation occurs preferentially at FAs (28) and is enhanced by cellular stretching (29). Moreover, mechanical extension of the SD increases the accessibility of YXXP tyrosines for phosphorylation by Src (29). Thus, signaling via p130Cas SD tyrosine phosphorylation is further implicated in the process of mechanotransduction. p130Cas SD tyrosine phosphorylation is also greatly enhanced upon tyrosine phosphatase inhibition (28), indicating a signaling transience subject to negative regulation by tyrosine phosphatases.
p130Cas was also independently identified in a screen for genes conferring resistance to tamoxifen in estrogen receptor-positive breast cancer (30). Hence, the human homolog of p130Cas has been called BCAR1 (product of breast cancer antiestrogen resistance gene 1). Studies addressing the mechanism of antiestrogen resistance have suggested signaling roles for p130Cas in promoting cell proliferation and survival (31–34). Similarly, p130Cas coupling to Crk has been linked to cell survival during invasion (19).
p130Cas is the founding member of the “Cas family” of proteins that, in mammals, also includes HEF-1/Cas-L/NEDD9, Efs/Sin, and HEPL (35, 36). Although p130Cas is expressed ubiquitously, HEF-1/Cas-L/NEDD9, Efs/Sin, and HEPL all have more restricted tissue distributions. In addition to SH3 and SD domains, Cas family proteins feature a conserved C-terminal region of ∼140 residues, termed here the Cas-family C-terminal homology (CCH) domain. The CCH domain may adopt a tertiary structure similar to the four-helical bundle of the FA targeting (FAT) domain of FAK (37). However, the role of the p130Cas CCH domain in FA targeting remains uncertain.
A full understanding of p130Cas function requires knowledge of the dynamics of its localization to FAs and clarification of the mechanism by which it targets to these sites. We addressed these questions using microscopy techniques to visualize and quantify the FA localization of fluorophore-tagged p130Cas variants in live and fixed cells. In addition to elucidating these aspects of p130Cas FA targeting, our results further demonstrate the relationship between FA localization and the SD tyrosine phosphorylation response.
EXPERIMENTAL PROCEDURES
Cells and Cell Culture
Cas−/− mouse embryo fibroblasts (MEFs) (12) were kindly provided by Hisamaru Hirai (University of Tokyo). MEFs were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1% antibiotic/antimicotic (Mediatech, Manassas, VA), 5 μg/ml plasmocin (InvivoGen, San Diego, CA), and 1% nonessential amino acids (Invitrogen). TetFAK cells were maintained and induced as previously described (38). Phoenix Ecotropic viral packaging cells (a gift from Gary Nolan, Stanford University) were maintained as previously described (39).
Antibodies
Monoclonal mouse antibodies to total p130Cas and paxillin were obtained from BD Transduction Laboratories. Phosphospecific pCas antibodies against p130Cas SD tyrosines 165, 249, and 410 were from Cell Signaling Technology (Beverly, MA). Mouse monoclonal antibody against actin was from Sigma. Cy3-conjugated AffiniPure donkey anti-mouse IgG and horseradish peroxidase-conjugated goat secondary antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Plasmid Construction and Protein Expression
PCR-mediated mutagenesis strategies were used to create p130Cas deletion mutants lacking SH3 and/or CCH domains, and all final coding regions were confirmed by sequencing before use. Stable expression of p130Cas variants in Cas−/− MEFs and TetFAK cells was achieved using LZRS-IRES retroviral vectors and Phoenix E packaging cells.
LZRS-MS-IRES-Zeo vector (a gift from Al Reynolds, Vanderbilt University) was used to express p130Cas variants (WT, ΔSH3, ΔCCH, ΔSH3/ΔCCH, and CCH only) with the fluorophore Venus-YFP (40) fused to their C termini. The SH3 deletion (lacking residues 5–65) was described previously (16). The CCH deletion, created for this study, lacked only the C-terminal 141 amino acids. The CCH only-Venus construct contains just the C-terminal 141 amino acids of p130Cas preceded by a start methionine. After three rounds of infection with viral supernatant, fluorescence activated cell sorting was used to select cells in the lower half of the fluorescence range. To achieve expression of p130Cas(ΔSH3) to levels comparable with other variants, it was necessary to further screen clonal populations by immunoblotting and pool higher expressing clones. The selected cell populations were periodically maintained in media containing 10 μg/ml Zeocin (Invitrogen) to assure continued expression from the bicistronic transcript that includes the bleomycin resistance gene product expressed from the internal ribosome entry site.
A similar approach was taken to express untagged p130Cas variants from the LZRS-MS-IRES-GFP vector (41), with fluorescence-activated cell sorting used to select for enhanced green fluorescent protein expressed separately from the internal ribosome entry site of the bicistronic transcript. LZRS-MS-IRES-GFP-p130Cas(WT) (21) and LZRS-MS-IRES-GFP-p130Cas(ΔSH3) (28) were described previously. Plasmids LZRS-MS-IRES-GFP-p130Cas(ΔCCH) and LZRS-MS-IRES-GFP-p130Cas(ΔSH3/ΔCCH) were created for this study.
Plasmid mCherry-C1-paxillin was generated to provide an FA marker for use in live cell studies. The cDNA for paxillin-α (provided by Hajime Yano, Osaka, Japan) was subcloned into plasmid mCherry-C1 (provided by Maria Nemethova, Vienna, Austria) such that paxillin is expressed with an N-terminal mCherry tag. Transient expression of mCherry-paxillin was achieved by transfection with Lipofectamine LTX (Invitrogen).
Immunoblotting
For immunoblot analysis, whole cell lysates in modified radioimmune precipitation assay buffer (39) were separated by 7% SDS-PAGE using 20 μg protein/lane. To enhance detection of p130Cas-SD phosphotyrosine, cell cultures were treated for 6 h with 500 μm sodium orthovanadate and washed in the presence of 1 μm sodium orthovanadate before lysis. Immunoblots were visualized by enhanced chemiluminescence. Tyrosine phosphorylation of p130Cas SD was quantified from scanned blots using ImageJ software to measure pixel intensities, with normalization to total p130Cas.
Analysis of p130Cas Localization to FAs in Live Cells
Cas−/− MEFs expressing p130CasVenus variants were transiently transfected with plasmid mCherry-C1-paxillin and then grown to confluence on coverslips coated with 1 μg/ml fibronectin (from human plasma, Sigma). Two hours before analysis, scratch wounds were prepared by creating denuded areas using a pipette tip. Cells migrating into the wound and expressing both fluorophores to appropriate levels were chosen for analysis of dynamic FAs using total internal reflection fluorescence (TIRF) microscopy, which was performed on a Nikon ECLIPSE TE2000-E inverted microscope equipped with a Perfect Focus System and a TIRF 100×/1.49 NA oil-immersion lens. Fluorophores were excited with an 18-milliwatt argon laser (Melles Griot, Albuquerque, NM) and 10-milliwatt DPSS laser 85YCA010 (Melles Griot). A custom-made double-dichroic TIRF mirror and emission filters (Chroma) in a Ludl filter wheel were used to make two-color movies at 25-s intervals between frames for a total of 180 frames. Images were captured by a back-illuminated EM-CCD camera Cascade 512B (Photometrics, Tucson, AZ) driven by IPLab (Scanalytics, Fairfax, VA) software using constant exposure and white/black point settings in each channel. Maintaining these values allowed us to choose cells with similar overall fluorescence intensities. Images were imported into ImageJ and converted to 8-bit grayscale, and background (non-cellular) fluorescence was then set to zero by adjusting the lower limit of the display range using the brightness/contrast function.
Quantitative assessment of the dynamic localization of p130CasVenus(WT) relative to mCherry-paxillin was made from plot profiles (obtained using ImageJ) of pixel intensities for each fluorophore within a 3-pixel-wide line through the central longitudinal axes of 12 FAs from 4 different cells. Apparent rate constants for FA assembly and disassembly were then calculated from the slopes of semilogarithmic plots of the mean pixel intensities as a function of time (42). Significance was determined using an unpaired, two-tailed, Student's t test.
For fluorescence recovery after photobleaching (FRAP) analysis, 20 FAs (each from a different cell) expressing p130CasVenus(WT) and mCherry-paxillin were photobleached for 8 s with both 10-milliwatt DPSS 85YCA010 and 30-milliwatt argon lasers (Melles Griot) by focusing the beam in the TIRF focal plane with a custom-made lens (Nikon) placed in the position of the filter cube. Two-color movies were recorded every 2 s for 4 min using TIRF microscopy as described above. FRAP curves and times of 50% recovery were calculated and adjusted for chromophore-activated laser inactivation effect according to specifications previously described (43) using single control measurements taken from each cell.
Quantitative Analysis of p130Cas Localization to FAs in Fixed Cells
Cells expressing p130CasVenus variants were fixed, immunostained for paxillin as an FA marker, and visualized by confocal spinning disk microscopy. Cells growing at low density on coverslips coated with 10 μg/ml fibronectin were fixed for 30 min in 2% paraformaldehyde (in a buffer containing 20 mm PIPES (pH 7.1), 127 mm NaCl, 5 mm KCl, 1.1 mm NaH2PO4, 0.4 mm KH2PO4, 2 mm MgCl2, 5.5 mm glucose, 1 mm EGTA) and then permeabilized for 10 min in 0.4% Triton X in phosphate-buffered saline. After blocking for 1 h in 1% bovine serum albumin in phosphate-buffered saline, the coverslips were immunostained for paxillin with detection by Cy3-conjugated secondary antibody. The coverslips were mounted in Prolong Gold Mounting Media (Invitrogen). Confocal images were captured at a single 0.2-μm slice depth (at the most appropriate ventral plane for visualizing FAs) with a Yokogawa QLC-100/CSU-10 spinning disk head attached to a Nikon TE2000E microscope using a 100×/1.4 oil Plan Apo objective lens (Nikon) and a back-illuminated EM-CCD camera Cascade 512B driven by IPLab software. Two-color excitation was achieved by a krypton-argon laser (75-milliwatt 488/568 from Melles Griot) under acousto optical tunable filter control. Custom-made double dichroic mirror and filters (Chroma Technology, Rockingham, VT) were used in a Ludl filter wheel (Hawthorne, NY) in the emission light path. Images were processed to remove noncellular background as described above. For each experimental population, 50 FAs (10 each from 5 separate cells) with robust paxillin staining were chosen for analysis. Masks of the selected FA sites were created based on the paxillin-delimited boundaries. The masks were then superimposed onto the corresponding images in the green emission channel to obtain measurements of the mean Venus-YFP fluorescence within the FAs. Non-FA-associated Venus-YFP fluorescence was subtracted from these values based on measurements of adjacent cellular areas using the same FA mask. Significance was determined using an unpaired, two-tailed, Student's t test.
Wound Healing Migration Assay
Scratch wounds were created as described above in confluent cell monolayers on coverslips coated with 1 μg/ml fibronectin. The wounded monolayers were then placed in complete Dulbecco's modified Eagle's medium further supplemented with 10% fetal bovine serum and 10 mm HEPES, mounted into a 37 °C heating chamber on an inverted Nikon ECLIPSE TE2000-E2 microscope, and visualized through a 20× Plan Apo differential interference contrast objective lens. Frames were captured every 5 min for 8 h on a CoolSnapHQ camera (Photometrics) using IPLab software. Sixty representative cells at the wound margins (12 each from 5 independent wounds per experimental population) were followed using the ImageJ manual tracking function. The mean total distances traveled per hour were calculated, and statistical significance was determined using an unpaired, two-tailed, Student's t test.
RESULTS
Expression of p130Cas Variants Tagged with Fluorophore Venus-YFP
To study p130Cas localization to FAs, mouse p130Cas variants tagged at their C-terminal ends with the fluorophore Venus-YFP were stably expressed in Cas−/− MEFs. The p130Cas variants included WT, ΔSH3, ΔCCH, ΔSH3/ΔCCH, and CCH only (Fig. 1A). Based on sequence conservation among the members of the Cas family including a Drosophila homolog, we define the p130Cas CCH domain as 141 amino acid residues at the C terminus, beginning with an invariant proline (Fig. 1B). Within the p130Cas CCH domain are three 25–30 amino acid stretches strongly predicted to adopt α-helical secondary structure (Fig. 1B). These predicted helical regions correspond well to three of the four helices of the FAK FAT domain, but as previously noted (36), β-strand content is predicted for a highly conserved stretch in the p130Cas CCH domain that would correspond to the other FAK FAT helix (Fig. 1B).
FIGURE 1.
p130Cas deletion mutants and the CCH domain. A, p130Cas variants used in this study are shown. Indicated are positions of the N-terminal SH3 domain, the SD, SBD, and the CCH domain. To study subcellular localization, the variants were expressed with Venus-YFP fused to their C termini. B, Cas-family C-terminal homology domains. Shown in the amino acid sequence alignment are mouse p130Cas residues 734–874 (UniProt ID Q61140), mHEF1 (mouse human enhancer of filamentation 1) residues 693–833 (UniProt ID O35177), mouse EFS (embryonal Fyn-associated substrate) residues 421–560 (UniProt ID Q64355), DmCas (Drosophila melanogaster p130Cas) homolog residues 683–823 (UniProt ID A4IJ58), and mouse HEPL (HEF1-EFS-p130Cas-like) residues 672–804 (UniProt ID Q08EC4). C termini are indicated by asterisks. Positions of amino acid identity have a black background, whereas other well conserved positions have a gray background. A secondary structure prediction for the p130Cas CCH domain, made using Jpred 3 (58), is shown at the top of the alignment, with α-helices indicated by HHH and β strands by EEE. The numbers ranging from 0 to 9 indicate prediction reliability, with larger numbers indicating greater reliability.
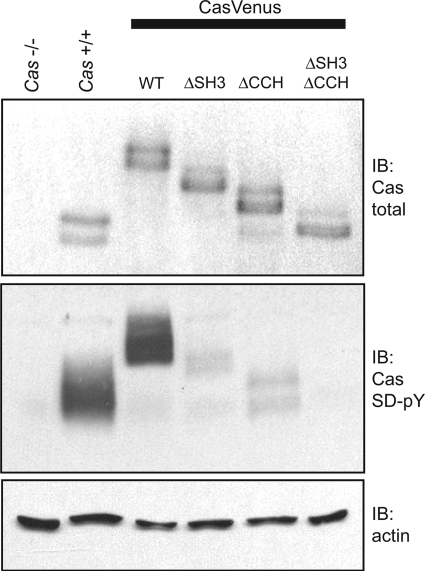
Immunoblotting of total cell lysates confirmed that the p130CasVenus variants are expressed to roughly equivalent levels and have the expected SDS-PAGE mobilities (Fig. 2, top panel). The signaling capacities of the variants were assessed by immunoblotting with a mixture of three pCas phosphospecific antibodies that recognize phosphorylated YXXP tyrosines in the p130Cas SD (28). To better enable detection of the SD phosphotyrosines, cells were pretreated with sodium vanadate to inhibit tyrosine phosphatases. p130CasVenus(WT) achieved a level of SD tyrosine phosphorylation close to that of endogenous p130Cas (Fig. 2, middle panel). However, deletion of either the SH3 or CCH domain significantly impaired SD tyrosine phosphorylation such that only faint pCas immunoreactivity could be detected (Fig. 2, middle panel). Even upon prolonged exposure of the blot, SD tyrosine phosphorylation could not be detected when both the SH3 and CCH domains were deleted. Thus, it is clear that both the SH3 and CCH domains have important roles in allowing proper p130Cas signaling.
FIGURE 2.
Venus-YFP-tagged p130Cas variants lacking either the SH3 or CCH domain are deficient in SD phosphorylation. Immunoblot (IB) analysis was carried out on whole cell lysates from Cas−/− MEFs reconstituted with either p130CasVenus(WT), p130CasVenus(ΔSH3), p130CasVenus(ΔCCH), or p130CasVenus(ΔSH3/ΔCCH). Parental Cas−/− MEFs and normal Cas+/+ MEFs were included as controls. Blotting with an antibody for total p130Cas indicated that the variants were expressed to equivalent levels (top). SD tyrosine phosphorylation (SD-pY) was assessed from vanadate-treated cells using a mixture of pCas phosphospecific antibodies that recognize SD YXXP sites (middle). Actin was detected as a loading control (bottom).
p130Cas Localizes to FAs throughout Their Lifetime
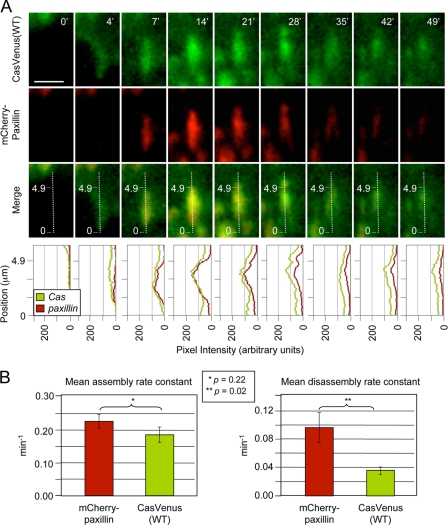
To study the dynamic aspects of p130Cas targeting to FAs, p130CasVenus(WT) was visualized at the ventral interface of living cells using TIRF microscopy. As a control FA marker, cells were transiently transfected to express paxillin tagged with the mCherry fluorophore. Paxillin is an established marker of FAs throughout their lifetime (42). Dynamic adhesions in motile cells at the margins of monolayer scratch wounds were analyzed. Fig. 3A shows images of a representative FA recorded over a 50-min time period. Similar to mCherry-paxillin, p130CasVenus(WT) localizes to FAs at the outset of their assembly and persists throughout the process of disassembly. As a further measure of the FA targeting dynamics of p130CasVenus(WT), assembly and disassembly rates were determined by quantifying fluorescence pixel intensities over time through the centers of the long axes of FAs. The bottom panels of Fig. 3A show the resulting fluorescence intensity plots obtained for the representative FA shown. Such measurements obtained from 12 different FAs indicated that the rate of p130CasVenus(WT) assembly into FAs was not significantly different from that of mCherry-paxillin (Fig. 3B). However, p130CasVenus(WT) appeared to disassemble from FAs at a slower rate than mCherry-paxillin (Fig. 3B). Clearly, p130Cas localizes to FAs throughout their lifetime.
FIGURE 3.
p130Cas localizes to FAs throughout their lifetime. p130CasVenus(WT) cells transiently expressing mCherry-paxillin were imaged by live TIRF microscopy at the leading edge of wound margins to assess the dynamics of p130Cas localization to FAs relative to paxillin. A, images of a representative FA taken over a 49-min time period are shown. The graphs show fluorescence intensity profiles obtained through the center of the long axis of the FA (dotted line) at each time point. The scale bar is 5 μm. B, mean assembly and disassembly rates for p130Cas and paxillin, calculated from the fluorescence intensity profiles of 12 FAs from four different cells. Bars indicate S.E. Significance values were determined by Student's t test.
p130Cas Exists in FAs with a High Mobile Fraction
To investigate the dynamic mobility of p130CasVenus(WT) in existing FAs, FRAP experiments were performed using TIRF microscopy. Again, mCherry-paxillin was used as the point of reference. FAs were photobleached for 8 s, and the subsequent recovery of unbleached fluorophores was observed at 2-s intervals. The mean time of 50% fluorescence recovery for p130CasVenus(WT) was ∼6 s, whereas 50% recovery of mCherry-paxillin was significantly slower at ∼43 s (Fig. 4). The recovery curve for p130CasVenus(WT) plateaued at a higher level compared with that of mCherry-paxillin. Thus, p130Cas exists dynamically in FAs with a high mobile fraction.
FIGURE 4.
p130Cas exists in FAs with a high mobile fraction. TIRF-FRAP was used to study the exchange of p130CasVenus(WT) and mCherry-paxillin in individual FAs at the leading edge of a wounded cell monolayer. After photobleaching, fluorescence recovery was recorded at 2-s intervals. A, a representative FA is shown before photobleaching (left panels), immediately after photobleaching (0”), and during fluorescence recovery (10”–100”). The scale bar is 5 μm. B, shown is a plot comparing the fluorescence recoveries after photobleaching of p130CasVenus(WT) and mCherry-paxillin. Data points represent the mean values of 20 individual FAs, with error bars indicating S.E. The 50% mean fluorescence recovery time points are marked by arrows. Student's t test showed the difference in 50% recovery points for p130Cas and paxillin was significant at p < 0.001.
Both the SH3 and CCH Domains Are Important for Targeting p130Cas to FAs
Using TIRF microscopy, p130CasVenus(WT) is observed to localize quite distinctly to FAs, with little Venus-YFP signal seen in adjacent ventral areas (Figs. 3A and 5A and see supplemental Movie 1). However, when either p130CasVenus(ΔCCH) or p130CasVenus(ΔSH3) were similarly examined, it was apparent that neither was able to achieve the same discrete FA localization. Rather, both of these deletion mutants became more broadly distributed in the ventral region, with only modest enrichment in and around the vicinity of FAs (Fig. 5, B and C, and see supplemental Movie 1). The CCH domain alone also exhibited this limited capacity for FA targeting, similar to the ΔCCH and ΔSH3 variants (Fig. 5D).
FIGURE 5.
p130Cas mutants lack discrete localization to FAs. Live TIRF microscopy was used to assess the localization of p130CasVenus variants (WT, ΔCCH, ΔSH3, or CCH only) within the ventral plane of cells migrating from the edge of a wounded cell monolayer. To mark adhesion sites, cells were transfected to transiently express mCherry-paxillin. The larger panels show merged images of representative single cells at the wound margin, with arrows indicating direction of migration. Boxed areas enclosing well defined FAs are shown slightly enlarged in adjacent panels that separately show the Venus, mCherry, and merged fluorescence. Note that both ΔSH3 (B) and ΔCCH (C) variants lack the discrete FA localization that is apparent for WT (A). Also note that the CCH only variant (D) localizes poorly to FAs. The scale bar is 5 μm. Time sequences for the FAs shown are presented in supplemental Movie 1.
To further assess the mechanism of p130Cas targeting to FAs, the Venus-tagged variants, including the ΔSH3/ΔCCH double-deletion, were visualized at the ventral region of fixed cells using confocal microscopy. Paxillin immunostaining served to mark the FAs. By this method, the prominent FA localization of p130CasVenus(WT) was again evident, although cytoplasmic localization is more apparent than with TIRF microscopy (Fig. 6A, top panels). The much weaker FA targeting of p130CasVenus(ΔSH3) and p130CasVenus(ΔCCH) was also apparent (Fig. 6A, middle panels). For p130CasVenus(ΔSH3/ΔCCH), however, there was no FA localization (Fig. 6A, bottom panels). To quantify the FA targeting efficiencies of the variants, mean Venus fluorescence values were determined from measurements of 50 FAs, within the boundaries set by paxillin staining. Relative to p130CasVenus(WT), the FA targeting efficiency was significantly reduced by ∼3-fold for p130CasVenus(ΔCCH) and ∼6-fold for p130CasVenus(ΔSH3) (Fig. 6B). The difference in FA targeting between p130CasVenus(ΔCCH) and p130Cas(ΔSH3) is also significant. The FA localization of p130CasVenus(ΔSH3/ΔCCH) was below the background level of fluorescence in adjacent areas (Fig. 6B). Thus, it is clear that both the SH3 and CCH domains make important contributions to the proper FA targeting of p130Cas and that no FA targeting is achieved in the absence of both domains.
FIGURE 6.
Both the CCH and SH3 domains play important roles in targeting p130Cas to FAs. The localization of p130CasVenus variants to FAs was assessed by fluorescence confocal microscopy at a single ventral slice. Immunostaining for endogenous paxillin served to mark the FAs. A, shown are representative images of cells expressing the p130CasVenus variants: WT, ΔCCH, ΔSH3, or ΔSH3/ΔCCH. p130CasVenus fluorescence is shown as green (left panels), paxillin immunofluorescence as red (center panels), and co-localization as yellow in the merged images (right panels). Insets represent 2-fold enlargements of selected areas containing FAs. The scale bar is 10 μm. B, shown is a quantitative analysis of mean p130CasVenus fluorescence at FAs. Paxillin immunofluorescence was used to delineate FA borders, and mean Venus-YFP fluorescence values were measured from within the FA boundary. For each p130Cas variant, mean Venus-YFP fluorescence was determined from 50 FAs (10 each from 5 separate cells). Bars indicate S.E., and statistical significance was determined by Student's t test.
FAK Has a Role in the FA Targeting of p130Cas
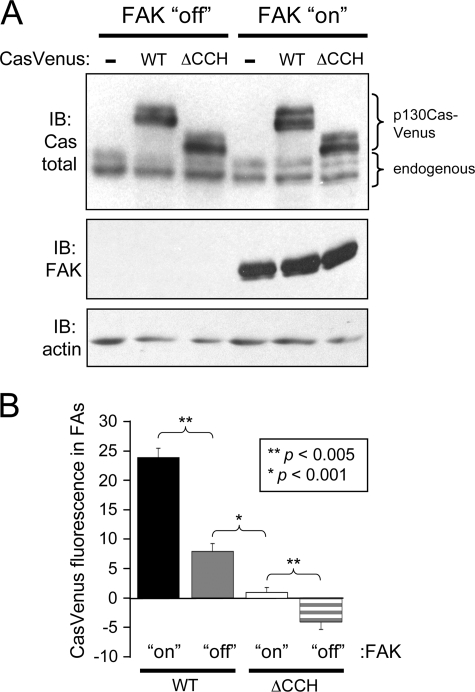
The finding that the SH3 domain is required for the proper targeting p130Cas to FAs suggests that the interaction of this domain with FAK may be involved in the process. To evaluate the role of FAK, p130CasVenus(WT) and p130Cas(ΔCCH) were stably expressed in TetFAK cells, which are FAK−/− MEFs engineered for inducible FAK expression upon tetracycline withdrawal (38). The equivalent expression of the p130CasVenus variants in the TetFAK cells was confirmed by immunoblot analysis under both non-induced and FAK-induced conditions (Fig. 7A). The FA targeting efficiencies of the two p130CasVenus variants both in the presence and absence of FAK were quantified as described above. FAK expression significantly increased FA targeting efficiency of both p130CasVenus(WT) and p130Cas(ΔCCH) (Fig. 7B and supplemental Fig. 1). For p130CasVenus(WT), the increase was ∼3-fold. Even in the presence of FAK, p130CasVenus(ΔCCH) localized very poorly to FAs and was ∼10-fold reduced in comparison to p130CasVenus(WT). In the absence of FAK, the FA targeting of p130CasVenus(ΔCCH) was no longer apparent. Thus, FAK has an important role in the FA targeting of p130Cas, and without FAK the FA targeting function achieved by the p130Cas SH3 domain is lost.
FIGURE 7.
FAK plays a role in localizing p130Cas to FAs. A, shown is an immunoblot (IB) analysis of whole cell lysates from TetFAK cells expressing either p130CasVenus(WT) or p130CasVenus(ΔCCH) that were either induced to express FAK or kept in the non-induced condition. Detection with a total p130Cas antibody (top) shows the expression of the two p130CasVenus variants in comparison to endogenous p130Cas. A total FAK antibody was used to confirm the induced FAK expression (middle), whereas actin detection was used as a loading control (bottom). B, quantitative assessment is shown of the localization of WT and ΔCCH p130CasVenus variants to FAs in the presence or absence of FAK expression. Paxillin immunofluorescence was used to delineate FA borders, and mean Venus-YFP fluorescence values were measured from within the FA boundary. For each p130Cas variant, mean Venus-YFP fluorescence was determined from 50 FAs (10 each from 5 separate cells). Bars indicate S.E., and statistical significance was determined by Student's t test.
Both the SH3 and CCH Domains Are Required for Efficient Promotion of Cell Motility by p130Cas
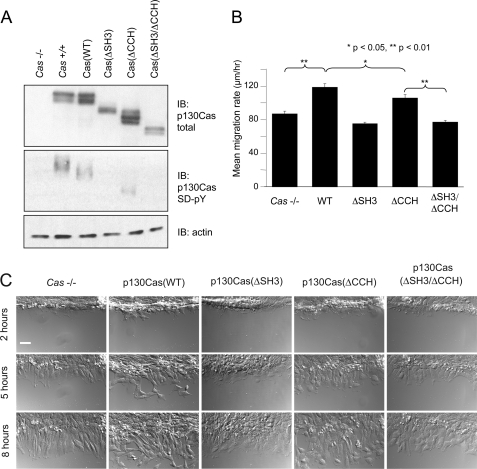
Results presented above document the importance of the CCH domain in p130Cas FA targeting and signaling. To extend these observations, we investigated the requirement of the CCH domain in the ability of p130Cas to promote cell motility. For this investigation, p130Cas variants without the Venus tag were stably expressed in Cas−/− MEFs. By using a retroviral vector that co-expresses GFP from a bicistronic transcript, cell populations were obtained that express the p130Cas variants to levels comparable with the endogenous protein (Fig. 8A, top). Vanadate treatment followed by immunoblotting with pCas antibodies showed the extent of SD tyrosine phosphorylation to be reduced in all of the deletion mutants in comparison to WT (Fig. 8A, middle panel). SD tyrosine phosphorylation was barely detectible in the ΔSH3 and ΔSH3/ΔCCH mutants, whereas quantitative analysis indicated that SD tyrosine phosphorylation of the ΔCCH mutant was reduced by 50% in comparison to WT p130Cas. The impairment of SD tyrosine phosphorylation observed for the ΔCCH mutant is not as striking as the defect observed for p130CasVenus(ΔCCH), indicating that the Venus tag has a limited negative effect on this signaling function. Nevertheless, it remains evident that the CCH domain has an important role in p130Cas SD signaling.
FIGURE 8.
Both the SH3 and CCH domains are required for p130Cas to efficiently promote cell migration. A, shown is an immunoblot (IB) analysis of whole cell lysates from Cas−/− MEFs reconstituted with untagged p130Cas(WT), p130Cas(ΔSH3), p130Cas(ΔCCH), or p130Cas(ΔSH3/ΔCCH). Parental Cas−/− MEFs and Cas+/+ MEFs were included as controls. Expression of the p130Cas variants was demonstrated using an antibody that recognizes both phosphorylated and unphosphorylated protein (top), whereas SD tyrosine phosphorylation was assessed using a mixture of pCas antibodies (middle). Before lysis, cells were treated with vanadate to enhance detection of SD phosphorylation. Actin was detected as a loading control (bottom). B and C, shown is an analysis of cell migration. Scratch wounds made in confluent cell monolayers were monitored by live differential interference contrast microscopy. For quantitative analysis (B), 60 individual cells (12 cells per 5 separate wounds) were tracked from each cell population, and mean migration rates were determined from the total distances migrated over a 6-h period. Bars indicate S.E., and significance was determined by the Student's t test. C, representative images of wound margins were taken at 2, 5, and 8 h post-wounding. The scale bar is 50 μm.
To study cell motility, scratch wounds were made in confluent cell monolayers, and live cell microscopy was used to monitor the movement of cells into the wounds for determination of mean cell migration rates. Consistent with results from other studies (20, 21), p130Cas(WT) expression significantly increased the cell migration rate as compared with parental Cas−/− cells (Fig. 8B). Notably, the abilities of the deletion mutants to promote migration correlated with their extent of tyrosine phosphorylation. Thus, neither the ΔSH3 nor the ΔSH3/ΔCCH mutants promoted migration above the level observed for Cas−/− cells, whereas the ΔCCH mutant only partially rescued the defect (Fig. 8B). As evident in the representative wounds shown in Fig. 8C, many p130Cas(WT) cells are well separated from the margin at the 5-h time point, whereas Cas−/− cells and cells expressing the signaling-deficient p130Cas mutants still maintain a uniform wound edge. Thus, in addition to promoting p130Cas FA targeting, both the SH3 and CCH domains are important for achieving proper SD tyrosine phosphorylation to promote cell motility.
DISCUSSION
Although it is recognized that p130Cas localizes to FAs where it engages in integrin-mediated signaling via tyrosine phosphorylation (9–11), the dynamics and mechanism of p130Cas targeting to these sites remain poorly understood. To investigate these issues, we used TIRF microscopy to visualize fluorophore-tagged proteins in migrating fibroblasts. This approach showed that p130Cas arrives early during the assembly of FAs at the leading edge of motile cells, reaches peak levels as FAs reach full maturity, and departs late during FA disassembly. Thus, p130Cas has the capacity to function in FAs throughout their lifetime. The rate of p130Cas assembly in FAs is similar to that of paxillin, which is one of the first FA proteins detected in nascent FAs (44). The early localization of p130Cas to assembling FAs supports the notion that p130Cas engages in signaling at the leading edge of migrating cells to promote plasma membrane protrusion. During FA disassembly, p130Cas actually appears to persist longer than paxillin. This persistence is supportive of a proposed role for p130Cas in promoting FA disassembly (42).
Using FRAP, we found that p130Cas exchanges rapidly with its binding partners in FAs. Thus, the interactions made by p130Cas in FAs are quite transient, which could indicate a low affinity. We note that p130Cas exists in cells with a large cytoplasmic component, which may reflect the high mobility of the fraction of the protein that becomes localized to FAs. As p130Cas SD phosphorylation is linked to its localization in FAs (28), the high mobility of p130Cas in FAs can also account for the rapid turnover of SD tyrosine phosphorylation, as is indicated by the sodium vanadate treatment.
In addition to revealing the dynamic aspects of p130Cas localization to FAs, a major objective of our study was to clarify the FA targeting mechanism. Two past studies addressed this question using immunostaining to detect epitope-tagged p130Cas variants (45, 46). Both studies found that deletion of the SD had no effect on FA targeting, indicating that SD tyrosine phosphorylation was not involved. However, the two studies reached fundamentally different conclusions as to the domain requirements for FA targeting of p130Cas. Nakamoto et al. (45) found that the SH3 domain was essential for p130Cas targeting to FAs. In contrast, Harte et al. (46) reported no inhibitory effect on FA localization when only the SH3 domain was functionally impaired by mutation. Both studies supported the existence of an FA targeting sequence lying at or near the p130Cas C terminus but disagreed as to which domain is involved. Nakamoto et al. (45) concluded that the SBD is required for efficient FA targeting based on studies of a deletion mutant lacking this domain. However, Harte et al. (46) saw no effect on the FA targeting of a p130Cas variant with SBD mutations and instead reported that the C-terminal 182 amino acid residues contained a region essential for FA targeting. Neither of these previous studies included quantitative analyses to evaluate the p130Cas FA targeting mechanism, which may have contributed to the different conclusions reached.
Our studies using Venus-tagged p130Cas variants have clarified the domain requirements for FA targeting of p130Cas. In contrast to the conclusions reached by either Nakamoto et al. (45) or Harte et al. (46), our results clearly show that both the N-terminal SH3 and C-terminal CCH domains are necessary for p130Cas to properly localize to FAs. Quantitative analysis of fixed cells showed that deletion of either the SH3 or CCH domain led to a significant impairment in p130Cas-Venus FA localization, whereas complete loss of FA targeting occurred only when both domains were deleted. Live cell TIRF imaging of the SH3 or CCH domain deletion mutants provided further evidence of their targeting defects. Even when these mutants were detected at FAs, it was apparent that this was an abnormally indiscrete association. Our identification of a C-terminal FA targeting sequence is consistent with the findings of Harte et al. (46) while further defining this region to include just the CCH domain. Although the main conclusion of Harte et al. (46) was that the C-terminal region is sufficient to target p130Cas to FAs, they did recognize that the SH3 domain has the capacity for FA targeting and must be functionally impaired in the context of their C-terminal deletion mutant before FA localization was fully lost. Our findings, however, emphasize that the CCH domain is unable to properly localize p130Cas to FAs in the absence of the SH3 domain.
Importantly, we note that the SBD deletion mutant used in the Nakamoto et al. (45) study was derived by excision of a large HindIII fragment (17) that encodes not only the SBD but also a significant portion of the CCH domain. Thus, the observed deficiency in the FA targeting of this mutant is likely due to the partial loss of the CCH domain rather than loss of SBD function.
FAK is a prominent FA protein that interacts with the p130Cas SH3 domain (8, 47, 48). This interaction contributes to SD tyrosine phosphorylation by virtue of Src bound to the FAK autophosphorylation site (16), and deletion of the p130Cas SH3 domain impairs p130Cas SD tyrosine phosphorylation (28). Our results using an inducible FAK expression system provide the first solid evidence that the FAK interaction is the main mechanism by which the p130Cas SH3 domain targets to FAs. Thus, FAK serves the dual functions of recruiting p130Cas to FAs and recruiting Src to phosphorylate the p130Cas SD to promote downstream signaling.
Early studies of the p130Cas relative HEF-1 focused on a region consisting primarily of the CCH domain that promoted pseudohyphal growth when ectopically expressed in yeast (49). Within this region is a divergent helix-loop-helix motif (50) that corresponds roughly to the N-terminal half of the CCH domain. The entire CCH domain may adopt a fold similar to the FAK FAT domain, a four-helix bundle stabilized by a hydrophobic core (37, 51, 52). CCH domains are compatible with the FAK FAT structure by homology modeling, and the CCH domain of HEF-1 has been shown to share some biophysical properties with the FAT domain (37, 53). Our study demonstrates that the p130Cas CCH domain does indeed have the ability to function as an FA targeting domain. Consistent with a role for the CCH domain in FA targeting, we further showed that the CCH domain is required for maximal SD tyrosine phosphorylation and promotion of cell motility by p130Cas. However, our results emphasize that the CCH domain by itself is a rather inefficient FA targeting domain. We further note that, unlike the FAK FAT domain, only three helices are strongly predicted to lie within the CCH domain. Until the CCH domain tertiary structure is determined, it will remain a matter of speculation as to whether or not the CCH domain actually adopts a four-helical bundle structure.
The nature of the target protein(s) for the p130Cas CCH domain in promoting FA localization is also uncertain. One possibility is paxillin, which plays a major role in targeting FAK to FAs through interaction with the FAK FAT domain (54), with paxillin LD motifs binding to opposite faces of the 4-helix bundle (55). However, in preliminary studies using mCherry-zyxin as an FA marker, we did not observe an obvious deficiency in the FA targeting of p130CasVenus(WT) in paxillin−/− MEFs. The comparatively slower disassembly rate of p130Cas in FAs further argues against a role for paxillin in the FA targeting of p130Cas. Two proteins known to interact with the C-terminal region of p130Cas are AND-34/BCAR3 (33) and Ajuba (56). However, neither protein is a strong candidate as the CCH domain target in localizing p130Cas to FAs. AND-34/BCAR3 is not a prominent FA protein and was actually implicated in recruiting p130Cas away from FAs to the general cell periphery (57). From studies of Ajuba−/− cells, it is also evident that Ajuba is not required for p130Cas localization to FAs, and Ajuba has no effect on p130Cas tyrosine phosphorylation (56). Thus, additional studies are needed to identify the CCH domain-interacting protein(s) responsible for the recruitment of p130Cas to FAs.
Finally, we note the relevance of our findings to the proposed roles for p130Cas signaling in cell motility and mechanotransduction. Tyrosine phosphorylation of the p130Cas SD has been strongly linked to its ability to promote cell motility, with subsequent recruitment of Crk and/or Nck adaptors acting to promote actin dynamics and plasma membrane protrusion (18–23). Our findings that p130Cas mutants that do not properly target to FAs are also deficient in their abilities to undergo tyrosine phosphorylation and promote cell migration are consistent with these past studies and others showing that p130Cas SD tyrosine phosphorylation occurs primarily in association with FAs (28). The morphology of FAs (as marked by paxillin) appears normal in cells expressing p130Cas mutants deficient in FA targeting, which is not surprising as this is also true for the Cas−/− MEFs (12). However, p130Cas has been implicated in promoting FA disassembly (42), and SD tyrosine phosphorylation in FAs could be involved in this process. Recently, a mechanosensing role for p130Cas in FAs has been proposed whereby the physical extension of the SD makes it more accessible for phosphorylation by Src (29). The extension requires anchor points on both sides of the SD, and it was proposed that this is achieved by distinct interactions made in FAs by the p130Cas SH3 domain and SBD. The proposed role for the SBD in this mechanosensing model is based on the above-mentioned studies of Nakamoto et al. (45) that used a deletion mutant lacking the SBD but also part of the CCH domain. Our studies now establish the CCH domain as the C-terminal FA targeting region of p130Cas and thereby implicate the CCH domain rather than the SBD as the C-terminal anchor point in the mechanosensing mechanism.
Supplementary Material
Acknowledgments
We thank Paul M. Miller for help with FRAP analysis, Catherine Alford for assistance with cell sorting, Hisamaru Hirai for providing Cas−/− MEFs, Gary Nolan for Phoenix E cells, Hajime Yano for paxillin cDNA, Al Reynolds for the LZRS-MS-IRES-GFP vector, Maria Nemethova for the mCherry-C1 vector, and Aaron Wiles for assistance with data analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-GM049882, R01-GM78373, T32-CA09592, T32-GM07347, and P30-DK058404. This work was also supported by American Heart Association Predoctoral Fellowship 0815136E (to L. M. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1 and Movie 1.
- SH2
- Src homology 2
- FAK
- focal adhesion (FA) kinase
- SH3
- Src homology 3
- CCH
- Cas-family C-terminal homology
- FRAP
- fluorescence recovery after photobleaching
- MEF
- mouse embryonic fibroblast
- SBD
- Src binding domain
- SD
- substrate domain
- TIRF
- total internal reflection fluorescence
- YFP
- yellow fluorescent protein
- WT
- wild type
- PIPES
- 1,4-piperazinediethanesulfonic acid
- GFP
- green fluorescent protein
- FAT
- focal adhesion targeting.
REFERENCES
- 1.Bouton A. H., Riggins R. B., Bruce-Staskal P. J. (2001) Oncogene 20, 6448–6458 [DOI] [PubMed] [Google Scholar]
- 2.Defilippi P., Di Stefano P., Cabodi S. (2006) Trends Cell Biol. 16, 257–263 [DOI] [PubMed] [Google Scholar]
- 3.Mayer B. J., Hamaguchi M., Hanafusa H. (1988) Nature 332, 272–275 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds A. B., Kanner S. B., Wang H. C., Parsons J. T. (1989) Mol. Cell. Biol. 9, 3951–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuda M., Mayer B. J., Fukui Y., Hanafusa H. (1990) Science 248, 1537–1539 [DOI] [PubMed] [Google Scholar]
- 6.Matsuda M., Mayer B. J., Hanafusa H. (1991) Mol. Cell. Biol. 11, 1607–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakai R., Iwamatsu A., Hirano N., Ogawa S., Tanaka T., Mano H., Yazaki Y., Hirai H. (1994) EMBO J. 13, 3748–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polte T. R., Hanks S. K. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 10678–10682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nojima Y., Tachibana K., Sato T., Schlossman S. F., Morimoto C. (1995) Cell. Immunol. 161, 8–13 [DOI] [PubMed] [Google Scholar]
- 10.Petch L. A., Bockholt S. M., Bouton A., Parsons J. T., Burridge K. (1995) J. Cell Sci. 108, 1371–1379 [DOI] [PubMed] [Google Scholar]
- 11.Vuori K., Ruoslahti E. (1995) J. Biol. Chem. 270, 22259–22262 [DOI] [PubMed] [Google Scholar]
- 12.Honda H., Oda H., Nakamoto T., Honda Z., Sakai R., Suzuki T., Saito T., Nakamura K., Nakao K., Ishikawa T., Katsuki M., Yazaki Y., Hirai H. (1998) Nat. Genet. 19, 361–365 [DOI] [PubMed] [Google Scholar]
- 13.Hamasaki K., Mimura T., Morino N., Furuya H., Nakamoto T., Aizawa S., Morimoto C., Yazaki Y., Hirai H., Nojima Y. (1996) Biochem. Biophys. Res. Commun. 222, 338–343 [DOI] [PubMed] [Google Scholar]
- 14.Vuori K., Hirai H., Aizawa S., Ruoslahti E. (1996) Mol. Cell. Biol. 16, 2606–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai R., Nakamoto T., Ozawa K., Aizawa S., Hirai H. (1997) Oncogene 14, 1419–1426 [DOI] [PubMed] [Google Scholar]
- 16.Ruest P. J., Shin N. Y., Polte T. R., Zhang X., Hanks S. K. (2001) Mol. Cell. Biol. 21, 7641–7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamoto T., Sakai R., Ozawa K., Yazaki Y., Hirai H. (1996) J. Biol. Chem. 271, 8959–8965 [DOI] [PubMed] [Google Scholar]
- 18.Klemke R. L., Leng J., Molander R., Brooks P. C., Vuori K., Cheresh D. A. (1998) J. Cell Biol. 140, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho S. Y., Klemke R. L. (2000) J. Cell Biol. 149, 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J., Hamasaki H., Nakamoto T., Honda H., Hirai H., Saito M., Takato T., Sakai R. (2002) J. Biol. Chem. 277, 27265–27272 [DOI] [PubMed] [Google Scholar]
- 21.Shin N. Y., Dise R. S., Schneider-Mergener J., Ritchie M. D., Kilkenny D. M., Hanks S. K. (2004) J. Biol. Chem. 279, 38331–38337 [DOI] [PubMed] [Google Scholar]
- 22.Brábek J., Constancio S. S., Siesser P. F., Shin N. Y., Pozzi A., Hanks S. K. (2005) Mol. Cancer Res. 3, 307–315 [DOI] [PubMed] [Google Scholar]
- 23.Rivera G. M., Antoku S., Gelkop S., Shin N. Y., Hanks S. K., Pawson T., Mayer B. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9536–9541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. (2005) Nat. Biotechnol. 23, 94–101 [DOI] [PubMed] [Google Scholar]
- 25.Luo W., Slebos R. J., Hill S., Li M., Brábek J., Amanchy R., Chaerkady R., Pandey A., Ham A. J., Hanks S. K. (2008) J. Proteome Res. 7, 3447–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantley L. C., Songyang Z. (1994) J. Cell Sci. Suppl. 18, 121–126 [DOI] [PubMed] [Google Scholar]
- 27.Schlaepfer D. D., Broome M. A., Hunter T. (1997) Mol. Cell. Biol. 17, 1702–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca P. M., Shin N. Y., Brábek J., Ryzhova L., Wu J., Hanks S. K. (2004) Cell. Signal. 16, 621–629 [DOI] [PubMed] [Google Scholar]
- 29.Sawada Y., Tamada M., Dubin-Thaler B. J., Cherniavskaya O., Sakai R., Tanaka S., Sheetz M. P. (2006) Cell 127, 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkman A., van der Flier S., Kok E. M., Dorssers L. C. (2000) J. Natl. Cancer Inst. 92, 112–120 [DOI] [PubMed] [Google Scholar]
- 31.Cabodi S., Moro L., Baj G., Smeriglio M., Di Stefano P., Gippone S., Surico N., Silengo L., Turco E., Tarone G., Defilippi P. (2004) J. Cell Sci. 117, 1603–1611 [DOI] [PubMed] [Google Scholar]
- 32.Cabodi S., Tinnirello A., Di Stefano P., Bisarò B., Ambrosino E., Castellano I., Sapino A., Arisio R., Cavallo F., Forni G., Glukhova M., Silengo L., Altruda F., Turco E., Tarone G., Defilippi P. (2006) Cancer Res. 66, 4672–4680 [DOI] [PubMed] [Google Scholar]
- 33.Riggins R. B., Thomas K. S., Ta H. Q., Wen J., Davis R. J., Schuh N. R., Donelan S. S., Owen K. A., Gibson M. A., Shupnik M. A., Silva C. M., Parsons S. J., Clarke R., Bouton A. H. (2006) Cancer Res. 66, 7007–7015 [DOI] [PubMed] [Google Scholar]
- 34.Soni S., Lin B. T., August A., Nicholson R. I., Kirsch K. H. (2009) J. Cell. Biochem. 107, 364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill G. M., Fashena S. J., Golemis E. A. (2000) Trends Cell Biol. 10, 111–119 [DOI] [PubMed] [Google Scholar]
- 36.Singh M. K., Dadke D., Nicolas E., Serebriiskii I. G., Apostolou S., Canutescu A., Egleston B. L., Golemis E. A. (2008) Mol. Biol. Cell 19, 1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arold S. T., Hoellerer M. K., Noble M. E. (2002) Structure 10, 319–327 [DOI] [PubMed] [Google Scholar]
- 38.Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. (1999) Mol. Cell. Biol. 19, 4806–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brábek J., Constancio S. S., Shin N. Y., Pozzi A., Weaver A. M., Hanks S. K. (2004) Oncogene 23, 7406–7415 [DOI] [PubMed] [Google Scholar]
- 40.Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 41.Ireton R. C., Davis M. A., van Hengel J., Mariner D. J., Barnes K., Thoreson M. A., Anastasiadis P. Z., Matrisian L., Bundy L. M., Sealy L., Gilbert B., van Roy F., Reynolds A. B. (2002) J. Cell Biol. 159, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. (2004) Nat. Cell Biol. 6, 154–161 [DOI] [PubMed] [Google Scholar]
- 43.Puthenveedu M. A., Bachert C., Puri S., Lanni F., Linstedt A. D. (2006) Nat. Cell Biol. 8, 238–248 [DOI] [PubMed] [Google Scholar]
- 44.Zaidel-Bar R., Ballestrem C., Kam Z., Geiger B. (2003) J. Cell Sci. 116, 4605–4613 [DOI] [PubMed] [Google Scholar]
- 45.Nakamoto T., Sakai R., Honda H., Ogawa S., Ueno H., Suzuki T., Aizawa S., Yazaki Y., Hirai H. (1997) Mol. Cell. Biol. 17, 3884–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harte M. T., Macklem M., Weidow C. L., Parsons J. T., Bouton A. H. (2000) Biochim. Biophys. Acta 1499, 34–48 [DOI] [PubMed] [Google Scholar]
- 47.Harte M. T., Hildebrand J. D., Burnham M. R., Bouton A. H., Parsons J. T. (1996) J. Biol. Chem. 271, 13649–13655 [DOI] [PubMed] [Google Scholar]
- 48.Polte T. R., Hanks S. K. (1997) J. Biol. Chem. 272, 5501–5509 [DOI] [PubMed] [Google Scholar]
- 49.Law S. F., Estojak J., Wang B., Mysliwiec T., Kruh G., Golemis E. A. (1996) Mol. Cell. Biol. 16, 3327–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law S. F., Zhang Y. Z., Fashena S. J., Toby G., Estojak J., Golemis E. A. (1999) Exp. Cell Res. 252, 224–235 [DOI] [PubMed] [Google Scholar]
- 51.Hayashi I., Vuori K., Liddington R. C. (2002) Nat. Struct. Biol. 9, 101–106 [DOI] [PubMed] [Google Scholar]
- 52.Liu G., Guibao C. D., Zheng J. (2002) Mol. Cell. Biol. 22, 2751–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garron M. L., Arsenieva D., Zhong J., Bloom A. B., Lerner A., O'Neill G. M., Arold S. T. (2009) J. Mol. Biol. 386, 190–203 [DOI] [PubMed] [Google Scholar]
- 54.Tachibana K., Sato T., D'Avirro N., Morimoto C. (1995) J. Exp. Med. 182, 1089–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertolucci C. M., Guibao C. D., Zheng J. (2005) Protein Sci. 14, 644–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pratt S. J., Epple H., Ward M., Feng Y., Braga V. M., Longmore G. D. (2005) J. Cell Biol. 168, 813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riggins R. B., Quilliam L. A., Bouton A. H. (2003) J. Biol. Chem. 278, 28264–28273 [DOI] [PubMed] [Google Scholar]
- 58.Cole C., Barber J. D., Barton G. J. (2008) Nucleic Acids Res. 36, W197–W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.