Abstract
The stability of the hemopexin-heme (Hx-heme) complex to dissociation of the heme prosthetic group has been examined in bicarbonate buffers in the presence and absence of various divalent metal ions. In NH4HCO3 buffer (pH 7.4, 20 mm, 25 °C) containing Zn2+ (100 μm), 14% of the heme dissociates from this complex (4.5 μm) within 10 min, and 50% dissociates within 2 h. In the absence of metal ions, the rate of dissociation of this complex is far lower, is decreased further in KHCO3 solution, and is minimal in NaHCO3. In NH4HCO3 buffer, dissociation of the Hx-heme complex is accelerated by addition of divalent metals with decreasing efficiency in the order Zn2+ > Cu2+ ≫ Ni2+ > Co2+≫Mn2+. Addition of Ca2+ prior to addition of Zn2+ stabilizes the Hx-heme complex to dissociation of the heme group, and addition of Ca2+ after Zn2+-induced dissociation of the Hx-heme complex results in re-formation of the Hx-heme complex. These effects are greatly accelerated at 37 °C and diminished in other buffers. Overall, the solution conditions that promote formation of the Hx-heme complex are similar to those found in blood plasma, and conditions that promote release of heme are similar to those that the Hx-heme complex should encounter in endosomes following endocytosis of the complex formed with its hepatic receptor.
Keywords: Blood, Calcium, Heme, Hemopexin, Myoglobin, Protein Metal Ion Interaction, Bicarbonate, Metal Ions
Introduction
The plasma protein hemopexin (Hx,2 MW ∼58,000) is a positive acute phase reactant glycoprotein with the primary role of scavenging heme released from methemoglobin and other heme proteins as the result of hemolysis, rhabdomyolysis, or ischemia-reperfusion injury (1–3). In doing so, Hx prevents the oxidative damage and pro-inflammatory effects of free heme (4–6) as demonstrated by the increased sensitivity of Hx-null mice (4, 7) to heme overload and development of heme-catalyzed oxidative damage to the vasculature, liver, and kidneys. Hx may also have a role in protection of neural tissue, and it exhibits other activities that include inhibition of necrosis and adhesion of polymorphonuclear leukocytes (8–10). The dominant form of circulating Hx is the apoprotein3 (6–25 μm (1)), which upon binding heme forms the Hx-heme complex that is recognized by hepatic receptors and removed from circulation by endocytosis (3, 11, 12). Subsequent to endocytosis, heme is dissociated from the Hx-heme complex and oxidized by heme oxygenase-1 to release iron that is then added to ferritin stores (13, 14). The protein component of the endocytosed Hx-heme complex that is liberated in this manner either returns to circulation (15–18) or undergoes lysosomal degradation (11, 18).
Heme binds to Hx with extremely high affinity (19, 20) even though the crystal structure for the rabbit Hx-heme complex indicates that the heme is more exposed to solvent than is the case for many heme proteins (21, 22). The Hx-heme complex is an unusual b-type hemeprotein in that the axial ligands that coordinate the heme iron are not located in a structurally constrained region of the protein, and one of the axial histidine ligands is located in the flexible linker region that connects the N- and C-terminal domains of the protein (10, 21). Physiologically, Hx must bind heme with extremely high affinity so that it can scavenge heme in circulating blood under conditions where it is released by myoglobin and hemoglobin, but Hx must also release heme in hepatic endosomes potentially liberating apoHx to return to circulation. While the high affinity of Hx for heme may be attributed to bisimidazole axial coordination, to packing of hydrophobic residues around the heme, and to the hydrogen bonding interactions of the heme propionate groups (21, 23), structural characteristics of the protein that might promote the release of heme when required are less evident.
Our recent studies of human Hx have focused on the effects of metal ions on the structural dynamics of this protein (24), the thermal stability of the Hx-heme complex (10, 25), and the influence of heme orientation on interaction of the protein with metal ions (26). The reduction potential of the rabbit Hx-heme complex has been shown to be sensitive to the ionic composition of the solution and pH (27), and the human Hx-ferroheme complex exhibits greater thermal instability (lower Tm (25, 28)) than does the Hx-ferriheme complex. Nevertheless, the physiological significance of the many metal ion binding sites of Hx (10, 29) and of heme insertion isoforms exhibited by the Hx-heme complex remain uncertain.
Recently, we have reported the ability of metal ions to induce heme release from a variety of b-type heme proteins (30) at alkaline pH in bicarbonate buffer. We have now extended these studies to consideration of the Hx-heme complex and find that the stability of this complex also responds to electrolytes and metal ions at physiological pH but in a manner that should promote preferential binding of heme to apoHx in plasma. The possible implications of these results for the physiological function of Hx are discussed.
EXPERIMENTAL PROCEDURES
Human Hx was isolated from plasma cryosupernate (Canadian Blood Services) without heme bound (apoHx), and the Hx-ferriprotoporphyrin IX complex (Hx-heme) was prepared by addition of ferriheme (Frontier Scientific) as described previously (24, 25, 29). The electronic absorption and CD spectra of the protein used in the current study were consistent with Form β of Hx-heme (26). The Hx-heme complex was exchanged (≥4 × 105-fold) into water by ultrafiltration (AmiconUltra 10,000 NMWL Millipore) prior to dilution (∼50-fold) into buffers used for data collection. Hx-heme complex concentrations were determined on the basis of a molar absorptivity of 136,000 m−1 cm−1 at 280 nm (29, 31). Horse heart myoglobin (Mb; Sigma cat. no. M1882) was converted to metmyoglobin (metMb) with K3Fe(CN)6 and passed over a column of Dowex 1X-8 (32). The concentration of metMb was determined from the molar absorptivity (188,000 m−1 cm−1) at 408 nm (pH 6.4) (33).
Spectroscopy and kinetics experiments were conducted with Cary Models 6000 and 3 spectrophotometers. All samples were thermostatted in Teflon-stoppered cuvettes with 1 cm pathlength (Hellma). Dissociation of heme from the Hx-heme complex was monitored at the Soret maximum (413.5 nm). Complete dissociation of heme from its normal binding site resulted in a 69.2% decrease in absorbance intensity at this wavelength (pH 7.4, 25 °C). Titrations of the Hx-heme complex with Ca2+ were monitored by following the rate of heme release as a function of the amount of CaCl2 added and were fit by non-linear regression analysis (Scientist, vers. 2, MicroMath) as described previously (34). Near UV and visible CD spectra were recorded with a Jasco Model J-810 spectropolarimeter.
Prior to monitoring the exchange of heme from metMb to apoHx, each protein was exchanged (>5 × 104-fold) into buffer by centrifugal ultrafiltration. The transfer of heme from metMb to apoHx was monitored at the wavelength at which the greatest change in absorbance is observed for this reaction (404.5 nm).
Glass-distilled water passed through a Barnstead Nanopure Diamond Life Sciences purification system was used throughout. All buffers (Fisher and Sigma) and salt solutions were air-saturated at 22 °C prior to adjusting pH. Tetramethylammonium hydroxide pentahydrate was purchased from Sigma. Metal ion solutions were prepared either by dilution from Titrosol (E. Merck) standards (CoCl2, CuCl2, MnCl2, and ZnCl2) or gravimetrically from metal chloride or sulfate salts (Fisher and Sigma).
SDS-PAGE was performed under reducing conditions (29) to check for protein hydrolysis using PageRuler (Fermentas Life Sciences) molecular weight standards. Gels were analyzed with a ChemiGenius2 Bio Imaging System and associated software (GeneSnap 6.05 for image acquisition and GeneTools 3.06 for data analysis, Syngene).
RESULTS
The Effect of Zn2+ on Stability of the Hx-Heme Complex in Sodium and Ammonium Bicarbonate Buffer
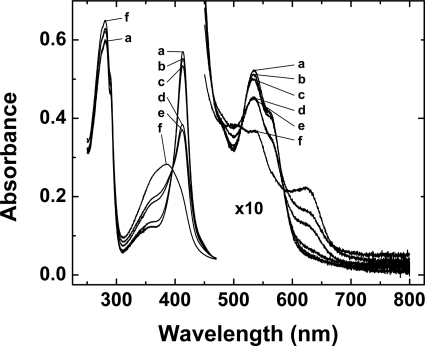
The spectrum of the Hx-heme complex is initially the same in either sodium or ammonium bicarbonate buffer (Fig. 1, spectrum a). With addition of Zn2+ (100 μm), half of the heme dissociates from this complex (4.3 μm) after 20 h in the presence of sodium bicarbonate buffer (20 mm, pH 7.4, 22 °C) (Fig. 1, spectrum e) whereas just 2.5 h is required for half of the heme to dissociate from the complex in ammonium bicarbonate buffer (20 mm, pH 7.4, 22 °C)(Fig. 1, spectrum d) and >97% of the heme was released after 22 h under these conditions. In ammonium bicarbonate buffer without added metal ions, the spectrum changes slowly in a manner that is consistent with dissociation of some (13% in 24 h) of the heme. This change in spectrum was unaffected by the presence of EDTA. In the absence of added metal ions, the spectrum of the Hx-heme complex changed <1% in sodium bicarbonate buffer in 24 h.
FIGURE 1.
The spectrum of the Hx-heme complex. The spectrum of the Hx-heme complex (4.35 μm) in the presence and absence of ZnSO4 (100 μm) in sodium or ammonium bicarbonate buffer (20 mm, adjusted to pH 7.4 with dilute HCl) at 22 °C: a, spectrum recorded immediately after dilution into sodium bicarbonate buffer in the absence of Zn2+; b, in ammonium bicarbonate buffer 5 min after addition of Zn2+; c, in sodium bicarbonate buffer 143 min after addition of Zn2+; d, in ammonium bicarbonate buffer 153 min after addition of Zn2+; e, in sodium bicarbonate buffer 1,236 min after addition of Zn2+; and f, in ammonium bicarbonate buffer 1320 min after addition of Zn2+.
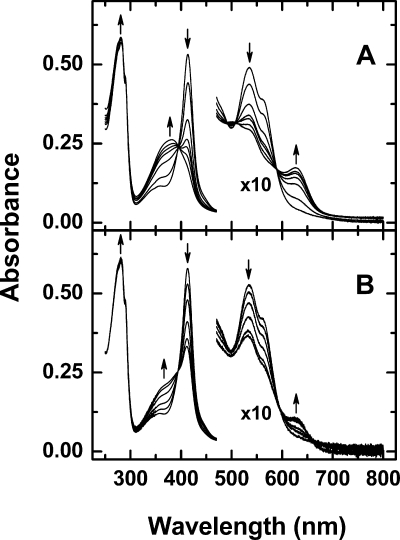
This behavior of the Hx-heme complex is in marked contrast to our previous observations with other b-type heme proteins (30) for which the dissociation of heme from the protein-heme complex was observed only after the addition of metal ions and the stability of heme binding was much greater in ammonium bicarbonate buffer than in sodium bicarbonate. While heme dissociates from the Hx-heme complex in the absence of metal ions in ammonium bicarbonate buffer (20 mm, pH 7.8), complete dissociation of the complex was not achieved even after more than 2 weeks at 15 °C (Fig. 2B). Electronic absorption spectra exhibited the same isosbestic points in the Soret and visible regions (395, 491, 507, and 590 nm) for the reaction with or without metal ions present (Figs. 1 and 2).
FIGURE 2.
Dissociation of heme from the Hx-heme complex in ammonium bicarbonate buffer (20 mm, pH 7. 8, 15 °C) in the presence and absence of Ni2+. A, Hx-heme complex (4.14 μm) with 100 μm NiCl2. The arrows indicate the change in absorption maxima with time at 0, 1240, 2900, 4300, 5200, 6800, and 11,000 min. B, Hx-heme complex (4.42 μm) with no metal ions added. The arrows indicate the change in absorption maxima with time at 0 min, 1660, 3000, 5600, 9900, and 15,700 min.
The Effect of the Counter Ion on the Stability of the Hx-Heme Complex in Bicarbonate Buffer
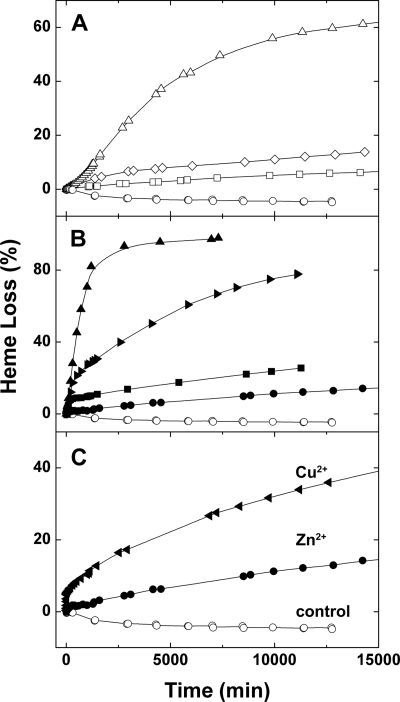
The influence of monovalent cations on the stability of the Hx-heme complex was examined in bicarbonate buffer (20 mm, pH 8.0 ± 0.2) by monitoring the decrease in absorbance at the Soret band (Fig. 3). These data were collected at 15 °C to ensure the stability of any apoHx formed during these extended reaction times. While the stability of the Hx-heme complex was only slightly diminished in potassium relative to sodium bicarbonate, ammonium bicarbonate had a pronounced destabilizing effect. This effect could result from an increase in the rate of release of heme from the Hx-heme complex (off-rate) or a decrease in the rate at which heme rebinds to apoHx (on-rate) or some combination of both as the counter ion is changed from sodium to potassium to ammonium.
FIGURE 3.
The effect of cations on the dissociation of heme from the Hx-heme complex. The change in the absorbance at 413.5 nm was used to calculate the extent of heme dissociation from the Hx-heme complex (4.2 μm) in (□) sodium, (◇) potassium, or (○) ammonium bicarbonate buffer (20 mm, 15 °C). The pH values of the bicarbonate solutions (pH 8.0 ± 0.2) were not adjusted.
The Effect of Divalent Metal Ions on the Stability of the Hx-Heme Complex in Bicarbonate Buffer
The effects of divalent metal cations (100 μm) on the rate of heme dissociation from the Hx-heme complex (4.3 μm) in ammonium bicarbonate buffer (20 mm, pH 7.4, 25 °C) are shown in Fig. 4. Metal ion concentrations of 100 μm were selected to saturate the principal metal ion binding sites of the protein as determined from potentiometric titrations (29). The enhancement of heme dissociation from the Hx-heme complex in ammonium bicarbonate buffer by various metal ions decreased in the order Zn2+ > Cu2+ ≫ Ni2+ > Co2+. The kinetics of all of these reactions are multiphasic (Figs. 3 and 4). In the presence of divalent metal ions, the rate constant for association of heme with Hx is likely to be diminished because the metal ions presumably compete with the heme iron for coordination to the histidyl residues that normally provide axial ligands (10). Subtle changes in protein structure lower the Tm for the Hx-heme complex in the presence of these four metal ions (24, 25). These metal ion-induced structural changes are also expected to influence the rate constants for both association and dissociation of the Hx-heme complex.
FIGURE 4.
The effect of divalent metal ions (100 μm) on the dissociation of heme from the Hx-heme complex (4.3 μm) in ammonium bicarbonate buffer (20 mm, pH 7.4 (adjusted with dilute HCl), 25 °C). (□) no additions, (♦) MnCl2, (▾) CoCl2, (▴)NiCl2, (●) CuCl2, or (■) ZnSO4.
Notably, the addition of Mn2+ had little or no effect on the release of heme from the Hx-heme complex (Fig. 4). Previous potentiometric studies of Mn2+ binding to apoHx (29) demonstrated the presence of a high affinity binding site for Mn2+ on apoHx that is not exhibited by the Hx-heme complex. Changing the counter ion of the added metal ions from Cl− (200 μm) to SO42− (100 μm) produced no significant differences in the reaction rates. However, we note that the concentration of counter ion introduced with the metal salts is small relative to the concentration of Cl− (∼1.7 mm) required to lower the pH of the bicarbonate solutions to 7.4.
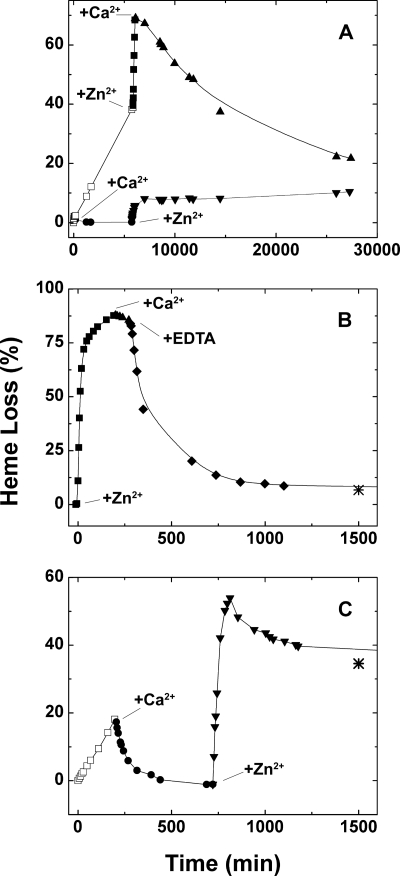
In contrast to the other metal ions evaluated in this work, Ca2+ did not induce the release of heme from the Hx-heme complex in bicarbonate buffers but promoted the rebinding of any heme that had been released from the Hx-heme complex in either bicarbonate buffer alone or in bicarbonate with other metal ions such as Zn2+ added (Fig. 5). This observation suggests that binding of Ca2+ to the Hx-heme complex stabilizes the complex and decreases the rate of complex dissociation. This stabilization of heme binding to Hx by Ca2+ is observed in both ammonium (Fig. 5A) and potassium bicarbonate (Fig. 5C) and at 37 °C (Fig. 5, B and C) as well as at 15 °C (Fig. 5A). The rebinding of heme at 15 °C is slow and is likely influenced by the aggregation state of the released heme (30, 35). Stabilization of heme binding to Hx by Ca2+ is consistent with the previous observation that Ca2+ increases the Tm for loss of tertiary structure and for release of heme from the Hx-heme complex in sodium phosphate buffer (pH 7.4, 10 mm) (10).
FIGURE 5.
Kinetics of heme dissociation from the Hx-heme complex following addition of CaCl2 (100 μm), ZnSO4 (100 μm) and/or EDTA in various sequences. A, upper line: (□) Hx-heme complex (4.5 μm) in NH4HCO3 buffer (20 mm, 15 °C) and no additions, (■) with Zn2+ added, (▴) with Ca2+ added. Lower line: (●) Hx-heme complex in NH4HCO3 buffer (20 mm, 15 °C) with Ca2+ added, (▾) with Zn2+ added. B, loss and recovery of heme binding (■) upon addition of Zn2+ (▴) followed by Ca2+ and (♦) then EDTA (final concentration 100 μm) in KHCO3 buffer (20 mm, 37 °C, [Hx-heme] = 3.9 μm). C, effect of addition of Ca2+ followed by Zn2+, (□) Hx-heme (3.9 μm) in KHCO3 buffer (20 mm, 37 °C) and no additions, (●) with Ca2+ added, (▾) with Zn2+ added. Asterisks in frames B and C indicate the percentage of heme released at 3500 min. The pH values of the bicarbonate buffers (pH 8.0 ± 0.2) were not adjusted.
The CD spectrum of the Hx-heme complex formed as the result of Ca2+-induced rebinding of heme (data not shown) indicates regeneration of Form β (26), which was the original form of the Hx-heme complex used in this study. The effects of metal ions described here are not expected to depend on the orientation of heme binding within the Hx-heme complex because Forms α and β exhibit the same metal ion-induced decreases in Tm for thermally-induced dissociation of the Hx-heme complex (25, 26).
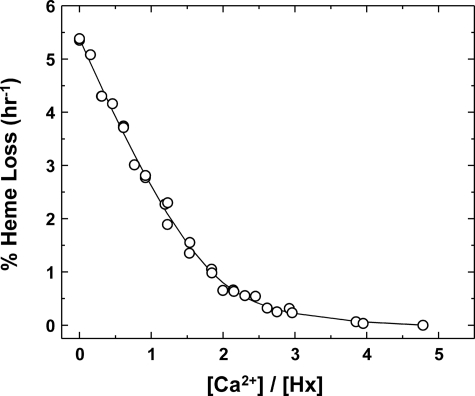
The stoichiometry of Ca2+ binding was examined in potassium bicarbonate buffer (20 mm, pH 8.0, 37 °C) by monitoring the rate of heme release as a function of added Ca2+. The data were fitted (Fig. 6) to a two-site binding model (34). Although this fit suggests the occurrence of two binding sites with affinities that differ by ∼3-fold, the inherent uncertainty of the data that could be collected for this process was sufficiently great that a more quantitative numerical analysis was not possible.
FIGURE 6.
Effect of Ca2+ on the rate of heme release from the Hx-heme complex in KHCO3 buffer (20 mm, 37 °C). The line is a fit to an equation describing a two-site model (34). The data are the sum of three separate titrations with [Hx-heme] = 4.1 μm.
The influence of Ca2+ on the kinetics of heme transfer from metMb to apoHx was investigated in an initial effort to evaluate the effect of this metal ion on heme exchange between proteins (Fig. 7). This reaction was monitored at the wavelength expected to exhibit the greatest change in absorbance (404.5 nm) as determined from the Hx-heme complex minus metMb difference spectrum (Fig. 7, inset) under conditions similar to those used to evaluate the effect of Ca2+ on the stability of the Hx-heme complex (Figs. 5 and 6). The concentration of Ca2+ used (10 μm) was based on the stoichiometry of Ca2+ binding (Fig. 6). No precipitation of either apoMb or apoHx was observed under these conditions. Notably, both the rate and the extent of heme transfer from met Mb to apoHx increase in the presence of Ca2+ (Fig. 7).
FIGURE 7.
Effect of Ca2+ on the kinetics of heme transfer from metMb to apoHx in KHCO3 buffer (20 mm, pH 8, 25 °C). The kinetics of heme transfer from metMb (1.97 μm) to apoHx (2.46 μm) in the (●) absence or (■) presence of CaCl2 (10 μm). Inset, Hx-heme complex (2 μm) minus metMb (2 μm) difference spectrum.
The Stability of the Hx-Heme Complex in Ammonium Salt Buffers
The relative stability of the Hx-heme complex (15 °C, pH 7.9) in the presence of ammonium salts (∼20 mm) of several common anions is shown in Fig. 8. As can be seen, these anions stabilize heme binding to Hx in the order Cl− ≥ HPO42− > HCO3−. The NH4Cl (20 mm) and NH4H2PO4 (10 mm) solutions were adjusted to pH 7.9 ± 0.1 with NH4OH such that the final ammonium concentrations were 21.6 and 22.1 mm, respectively. The extent of stabilization by chloride was difficult to establish quantitatively because maintaining the pH of NH4Cl solutions under aerobic conditions is difficult, and additionally, the chief contaminant in NH4Cl is NaCl (36). As noted above, Na+ has a pronounced stabilizing effect on the Hx-heme complex relative to NH4+. Further clarification of the effect of chloride was sought by examining the stability of the Hx-heme complex in tetramethylammonium chloride (tetramethyl ammonium hydroxide (20 mm) adjusted to pH 8 with HCl ([Cl−]final = ∼18.6 mm)). Under these conditions, the stability of heme binding to Hx was intermediate between that observed in the presence of ammonium chloride and ammonium phosphate during initial incubation at 15 °C (Fig. 8). However, after prolonged exposure (∼5 days) to tetramethylammonium chloride, the Hx-heme complex exhibited sufficient turbidity that further analysis was precluded.
FIGURE 8.
The effect of anions on dissociation of heme from the Hx-heme complex (4.1 μm) in ammonium salt buffers (pH 7.9 ± 0.1, 15 °C). (□) ammonium bicarbonate buffer (20 mm), (◇) ammonium bicarbonate (10 mm) plus ammonium chloride (10 mm); (▿), ammonium phosphate (10 mm NH4H2PO4, pH adjusted with ammonium hydroxide); (○), ammonium chloride (20 mm, pH adjusted with ammonium hydroxide); and (◁) tetramethylammonium chloride (20 mm (CH3)4N(OH), pH adjusted with HCl).
The stabilizing effects of various electrolytes described above appear to be additive such that sodium phosphate buffer stabilizes the Hx-heme complex more than any of the other buffer combinations examined (Fig. 9). Interestingly, the electronic absorption spectrum of Hx-heme samples diluted into sodium phosphate buffers exhibited an increase in intensity of up to ∼4% at the Soret maximum (413.5 nm) over 24–48 h, and no further change was observed following prolonged incubation in this buffer (Fig. 9A). We attribute this increase in absorbance to rebinding of the small amount of heme that can be released from the Hx-heme during extended buffer exchange and storage in water. Addition of metal ions to the Hx-heme complex also promotes dissociation of heme in phosphate buffers although the effect is much smaller than observed in bicarbonate buffers as shown for addition of Zn2+ in Fig. 9B. The relationship between the ability of metal ions to destabilize the Hx-heme complex and the buffer composition is complex. For example, in sodium phosphate buffer (Fig. 9C) the relative effectiveness of Zn and Cu in promoting heme release is the opposite of that observed in ammonium bicarbonate buffer (Fig. 3).
FIGURE 9.
Kinetics of heme dissociation from the Hx-heme complex in phosphate and bicarbonate buffers. A, (○) Hx-heme (4.2 μm, pH 8.0 ± 0.2, 15 °C) in 10 mm sodium phosphate ([Na+]final = 19.3 mm), (□) 20 mm sodium bicarbonate, (◇) 10 mm ammonium phosphate ([NH4]final = 22.1 mm), and (▵) 20 mm ammonium bicarbonate. B, effect of ZnSO4 (100 μm) (denoted by filled symbols) on Hx-heme (conditions and symbols as in panel A except (▶) in 20 mm potassium bicarbonate)). C, effect of metal ions on Hx-heme in sodium phosphate buffer (conditions as in panel A). (○) no metal ion, (●) with ZnSO4 (100 μm), and (◀) with CuCl2 (100 μm).
Susceptibility of Hx to Metal Ion-catalyzed Peptide Bond Hydrolysis
The possibility of metal ion promoted hydrolysis of Hx during the kinetics experiments was evaluated by analysis of these samples by SDS-PAGE in the presence of dithiothreitol (data not shown). No cleavage of Hx (apparent MW ∼74,000 (29)) was observed even after exposure to metal ions for 2 weeks at 15 °C except for samples containing Ni2+ (100 μm with 4–6 μm Hx). Analysis of samples of the Hx-heme complex following incubation with Ni2+ at 15 °C detected no hydrolysis of the Hx-heme complex but did detect cleavage of apoHx that formed during incubation (∼30% of the apoHx present hydrolyzed each day). The products of the Ni2+-catalyzed reaction exhibit apparent MWs of 28 kDa (one band) and 46 kDa (two bands that differ by ∼2 kDa). This banding pattern does not change upon further incubation at 15 °C, indicating no further hydrolysis occurred. At temperatures >22 °C, cleavage of apoHx by Ni2+ was significantly diminished or undetectable, consistent with our previous observations (24).
Ni2+ has been reported recently to hydrolyze the peptide bond preceding the Thr residue in model peptides possessing several sequences, including the sequence Thr-His-His (37, 38), which occurs in human Hx (residues 225–227). While the hydrolysis we observe occurred at 15 °C, suggesting a limited physiological importance, we interpret the products formed by exposure of Hx to Ni2+ to correspond to the C-terminal residues 225–439 (MWcalc = 23,772 plus 1 carbohydrate chain) and the N-terminal residues 1–224 (MWcalc = 25,542 plus 5 carbohydrate chains). We assign the 46 kDa band from the SDS-PAGE to the N-domain based on this larger than anticipated apparent MW as well as the electrophoretic heterogeneity of native Hx reported previously (26, 29, 39). Our fragment sizes also agree well with apparent molecular weights reported for the N- and C-domains generated by proteolytic hydrolysis (40, 41). The Thr-His-His sequence occurs in the flexible linker region (residues 209–231 (10, 21)) that connects the N- and C-domains of Hx. One of the axial His ligands to the heme iron also occurs in this region, so coordination of this residue to the heme iron presumably constrains the linker region such that formation of the square-planar Ni2+ complex with the Thr-His-His sequence that is required for peptide bond hydrolysis (37) cannot occur with the Hx-heme complex.
DISCUSSION
The influence of solution conditions on the stability of the Hx-heme complex reported here stands in marked contrast to the results we reported recently concerning the response of several other b-type heme proteins to the addition of divalent metal ions in bicarbonate buffers (30). In sodium bicarbonate buffer, the effects of metal ions on the dissociation of the Hx-heme complex are modest relative to their effects in ammonium bicarbonate buffer while the opposite is true for the b-type heme proteins studied previously (30). Moreover, the Hx-heme complex dissociates at a significant rate in ammonium bicarbonate buffer even in the absence of exogenous metal ions while the other b-type heme proteins we have studied exhibit no significant dissociation in the absence of metal ions. These observations merit consideration with regard to the physiological function of hemopexin.
The physiological role of hemopexin is to scavenge heme released from hemoglobin, myoglobin, and other heme proteins in plasma following hemolysis or rhabdomyolysis to prevent oxidative damage catalyzed by heme and to release heme following uptake of the Hx-heme complex by the liver to permit recovery of heme and its iron for re-use (3). Heme binding and subsequent release occur in distinctly different environments that involve distinctly different ionic compositions. As discussed below, the results of the current study in combination with our previous findings (30) demonstrate that the stability of heme binding to Hx and other b-type heme proteins is modulated by the solution conditions in a manner that promotes transfer and binding of heme to Hx in blood plasma and release of heme from the Hx-heme complex in the endosome.
Factors Favoring Transfer of Heme to Hemopexin in Blood Plasma
The stabilization of the Hx-heme complex by sodium (136–145 mm (42)), chloride (98–107 mm (42)), and calcium ([Ca2+]free ∼1.2 mm (43, 44)) in bicarbonate buffer (21–29 mm (42, 45)) are noteworthy because their concentrations (as indicated in parentheses) are relatively high in plasma. Such conditions should promote the binding to Hx of heme that may be released from other b-type heme proteins present in plasma as the result of trauma or disease. The stabilization of the Hx-heme complex by Ca2+ is expected to promote transfer of heme (including heme bound to heme proteins) to hemopexin because the stability of heme binding to ferrimyoglobin is unaffected by Ca2+ in the presence or absence of other metal ions under these conditions (data not shown). Thus, although several divalent metal ions studied here promote dissociation of heme from both ferrimyoglobin (30) and the Hx-heme complex, Ca2+ greatly diminished this effect for the Hx-heme complex but not for myoglobin. In health, essentially all of the plasma Cu2+ (11–22 μm (46, 47)) and Zn2+ (15–20 μm (48, 49)) are bound to ceruloplasmin (Cu2+ (46)) or albumin (Cu2+ (47) and Zn2+ (50)), so they are unlikely to influence exchange of heme between proteins. However, the concentrations and distribution of these metal ions among plasma proteins in disease states that result in release of heme to blood plasma remain unknown.
Factors Favoring Dissociation of the Hx-Heme Complex in the Endosome
Upon binding to its hepatic receptor (11, 16) and internalization within an early endosome, the Hx-heme complex encounters conditions that are destabilizing relative to the conditions in plasma. The newly formed endosome has a low concentration of Cl− as the result of the interior-negative Donnan potential (51, 52), the concentration of Na+ in early endosomes decreases rapidly by Na+/H+ exchange (53), and Ca2+ taken up during endocytosis is decreased to <5 μm within 10 min after endosome formation (54). Thus, the conditions in early endosomes should promote dissociation of heme from the Hx-heme complex and rapid recycling of Hx and the cell surface receptor.
With time, endosomal pH decreases by ATP-dependent processes (51, 55, 56) to a value (pH ∼5) at which the thermal stability of the Hx-heme complex is greatly diminished (25). Although the metal ion content of endosomes is currently unknown, a recent report suggests that endocytosis of the Hx-heme complex increases cytosolic copper levels in Hepa hepatoma cells (12). Certainly the availability of Zn2+ or Cu2+ in early endosomes could accelerate the release of heme from the Hx-heme complex prior to a significant decrease in pH. Also unknown is the oxidation state of the heme iron in the Hx-heme-receptor complex and in the early endosome. Reduction of the heme iron does, however, destabilize the Hx-heme complex (25, 28), an effect that is significantly enhanced at low pH (25). Nevertheless, the results reported here indicate that reduction of the heme iron may not be required for the release of heme from the Hx-heme complex in the endosome.
The major route by which the Hx-heme complex is removed from circulation involves specific receptor-mediated endocytosis at the hepatic parenchymal cell surface although Hx-heme receptors are also expressed on certain circulating cells as well as various barrier tissues. The protective effects of the Hx-heme complex and the cellular metabolism of the heme component have been well reviewed recently (1, 3). However, a similarly detailed understanding of functioning of the specific Hx-heme receptor(s) or the fate of apoHx in vivo remains elusive. The conditions under which heme is released from Hx-heme in vivo following receptor mediated endocytosis is intricately coupled to the mechanism by which Hx and its receptor are processed.
Two different, but perhaps not mutually exclusive, mechanisms have been suggested for this process. In one mechanism, Hx is recycled to the circulation, and in the other, Hx is degraded. The recycling mechanism is based on studies with rats (15, 57), polymorphonuclear leukocytes (17), human HepG2 cells, and mouse Hepa hepatoma cells (16) in which the Hx-heme complex was found to co-localize with the transferrin-receptor complex, a complex from which transferrin is returned to circulation (58). The degradative mechanism is based on studies in COS-1 cells expressing the LRP/CD91 receptor (11) that suggest that following interaction of the Hx-heme complex with this receptor the Hx-heme complex is processed through multi-vesicular late stage endosomes (pH ≤5.5) with the Hx ultimately undergoing lysosomal degradation, similar to the fate of activated α2-macroglobulin (59). The experiments of Hvidberg et al. (11) provide no indication of the point at which heme is released from the Hx-heme complex during endosomal processing. Additionally, it is unknown if the mechanism for receptor mediated processing of Hx-heme is dependent on cell type and whether both proposed mechanisms may operate concurrently in vivo. Regardless of the endosomal mechanism of Hx-heme complex processing or the ultimate fate of the complex, the specific ion effects identified in the current work demonstrate that neither the reduction of heme iron nor low pH are required for the release of heme from the Hx-heme complex in vivo.
Metal Ion Binding Sites on the Hx-Heme Complex
Whereas the mechanism by which metal ions induce the release of heme from the Hx-heme complex may differ in bicarbonate and phosphate buffers, the proposed metal ion binding sites described previously (10) exist in both cases. Human hemopexin possesses 21 lysyl residues, so modification of at least some of these side chains in bicarbonate buffers to form negatively charged carbamino groups (30, 60, 61) will change the electrostatic potential surface of the protein, may alter the properties of metal ion binding sites present in the native protein, and may result in creation of new binding sites. Only neutral amino groups are expected to undergo carbamino group formation. As the pKa values of the lysyl residues of Hx are unknown, it is not possible to define in detail how the electrostatic properties of Hx are changed in bicarbonate buffer.
Nevertheless, this minimal modification of the protein is sufficient to promote dissociation of the Hx-heme complex in the absence of metal ions. This effect is far greater in ammonium bicarbonate than in sodium or potassium bicarbonate. Stabilization of the Hx-heme complex against metal ion-induced heme release by Na+ and K+ (relative to NH4+) may correlate with the presence of binding sites for two sodium ions in the central tunnel of each β-propeller of the N- and C-domains of the Hx structure (21, 62). The mechanism by which NH4+ promotes dissociation of the Hx-heme complex is speculative but might involve destabilization of hydrogen binding interactions between the heme propionate groups and the protein.
As little of our previous work considered the interaction of Hx with Ca2+, some comment concerning possible binding sites for this metal ion is warranted. Likely sites of calcium binding include the acidic patches at the entrances to the central tunnels of the β-propellers (62) that correspond to sites J and K that we defined previously (24). Whereas these sites, one on each domain, are similar to each other, site J (C-domain) involves three acidic residues (Asp-286, Asp-338, and Asp-381) while site K (N-domain) involves four (Asp-34, Asp-75, Asp-121, and Glu-50). This difference in the number of acidic residues should result their binding Ca2+ in an inequivalent manner, consistent with our current results. Nevertheless, it is not apparent how Ca2+ binding to these sites would stabilize heme binding to Hx. It remains possible that calcium binds elsewhere in a manner that affects the geometry of the linker region such that the stability of heme binding is increased without changing the electronic spectrum of the Hx-heme complex.
We previously found that of the metal ions we have studied, Mn2+ is unique in that it binds to apoHx with relatively high affinity (Ka = 1.5(3) × 107 m−1) but fails to bind significantly to the Hx-heme complex (29), suggesting that Mn2+ binds exclusively to the heme binding site (24). Consequently, the failure of Mn2+ to promote dissociation of the Hx-heme complex is evidence that competition of divalent metal ions for coordination to the residues that normally provide axial ligands to the heme iron is insufficient by itself to destabilize heme binding in this complex. Thus, we propose that bicarbonate promotes the dissociation of the Hx-heme complex by Cu2+, Zn2+, and Ni2+, and Co2+ because bicarbonate enables the initial binding of these four divalent metal ions to relatively low-affinity binding sites elsewhere in the Hx-heme complex in a manner that destabilizes the binding of heme sufficiently that they can also compete for binding at the heme binding site. Mn2+, on the other hand, does not bind to any low affinity sites of this type under these conditions, so it is unable to destabilize the complex sufficiently to gain access to the heme binding site.
Hemopexin has been reported to bind heme with an affinity greater than that of hemoglobin (19) and is frequently referred to as having a greater affinity for heme than other b-type heme proteins. It is remarkable, therefore, that relatively mild solution conditions can result in release of heme from the Hx-heme complex and that this binding equilibrium is highly influenced by the ionic composition of solution and the presence of divalent metal ions. For now, the true physiological consequences of the processes we have identified in this study cannot be evaluated satisfactorily until the ionic composition of endosomes and perhaps other cellular compartments are better characterized. These results also raise the possibility that some pathogenic bacteria may use related means to release heme from the Hx-heme complex and other heme proteins to help fulfill their metabolic requirement for iron.
Acknowledgment
The Cary 6000 spectrophotometer was purchased with funds from the Canada Foundation for Innovation and the B.C. Knowledge Development Fund.
This work was supported by a CBS-CIHR Partnership grant and by a Canada Research Chair (to A. G. M.).
ApoHx refers to the hemopexin glycoprotein without ferriprotoporphyrin IX bound at a site equivalent to that identified in the rabbit Hx structure (21) and implies nothing concerning the binding of metal ions.
- Hx
- hemopexin
- MW
- molecular weight.
REFERENCES
- 1.Delanghe J. R., Langlois M. R. (2001) Clin. Chim. Acta 312, 13–23 [DOI] [PubMed] [Google Scholar]
- 2.Tolosano E., Altruda F. (2002) DNA Cell Biol. 21, 297–306 [DOI] [PubMed] [Google Scholar]
- 3.Tolosano E., Fagoonee S., Morello N., Vinchi F., Fiorito V. (2010) Antioxid. Redox. Signal. 12, 305–320 [DOI] [PubMed] [Google Scholar]
- 4.Tolosano E., Hirsch E., Patrucco E., Camaschella C., Navone R., Silengo L., Altruda F. (1999) Blood 94, 3906–3914 [PubMed] [Google Scholar]
- 5.Holt S., Reeder B., Wilson M., Harvey S., Morrow J. D., Roberts L. J., 2nd, Moore K. (1999) Lancet 353, 1241. [DOI] [PubMed] [Google Scholar]
- 6.Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G. M., Eaton J. W., Balla G. (2002) Blood 100, 879–887 [DOI] [PubMed] [Google Scholar]
- 7.Tolosano E., Fagoonee S., Hirsch E., Berger F. G., Baumann H., Silengo L., Altruda F. (2002) Blood 100, 4201–4208 [DOI] [PubMed] [Google Scholar]
- 8.Tolosano E., Cutufia M. A., Hirsch E., Silengo L., Altruda F. (1996) Biochem. Biophys. Res. Commun. 218, 694–703 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K., Kobayashi N., Doi T., Hijikata T., Machida I., Namiki H. (2003) Cell Struct. Funct. 28, 243–253 [DOI] [PubMed] [Google Scholar]
- 10.Mauk M. R., Rosell F. I., Mauk A. G. (2007) Nat. Prod. Rep. 24, 523–532 [DOI] [PubMed] [Google Scholar]
- 11.Hvidberg V., Maniecki M. B., Jacobsen C., Højrup P., Møller H. J., Moestrup S. K. (2005) Blood 106, 2572–2579 [DOI] [PubMed] [Google Scholar]
- 12.Smith A., Rish K. R., Lovelace R., Hackney J. F., Helston R. M. (2009) Biometals 22, 421–437 [DOI] [PubMed] [Google Scholar]
- 13.Otterbein L. E., Soares M. P., Yamashita K., Bach F. H. (2003) Trends Immunol. 24, 449–455 [DOI] [PubMed] [Google Scholar]
- 14.Davies D. M., Smith A., Muller-Eberhard U., Morgan W. T. (1979) Biochem. Biophys. Res. Commun. 91, 1504–1511 [DOI] [PubMed] [Google Scholar]
- 15.Smith A., Morgan W. T. (1979) Biochem. J. 182, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith A., Hunt R. C. (1990) Eur. J. Cell Biol. 53, 234–245 [PubMed] [Google Scholar]
- 17.Okazaki H., Taketani S., Kohno H., Tokunaga R., Kobayashi Y. (1989) Cell Struct. Funct. 14, 129–140 [DOI] [PubMed] [Google Scholar]
- 18.Potter D., Chroneos Z. C., Baynes J. W., Sinclair P. R., Gorman N., Liem H. H., Muller-Eberhard U., Thorpe S. R. (1993) Arch. Biochem. Biophys. 300, 98–104 [DOI] [PubMed] [Google Scholar]
- 19.Hrkal Z., Vodrázka Z., Kalousek I. (1974) Eur. J. Biochem. 43, 73–78 [DOI] [PubMed] [Google Scholar]
- 20.Miller Y. I., Shaklai N. (1999) Biochim. Biophys. Acta 1454, 153–164 [DOI] [PubMed] [Google Scholar]
- 21.Paoli M., Anderson B. F., Baker H. M., Morgan W. T., Smith A., Baker E. N. (1999) Nat. Struct. Biol. 6, 926–931 [DOI] [PubMed] [Google Scholar]
- 22.Morgan W. T., Sutor R. P., Muller-Eberhard U. (1976) Biochim. Biophys. Acta 434, 311–323 [DOI] [PubMed] [Google Scholar]
- 23.Deeb R. S., Muller-Eberhard U., Peyton D. H. (1994) Biochim. Biophys. Acta 1200, 161–166 [DOI] [PubMed] [Google Scholar]
- 24.Rosell F. I., Mauk M. R., Mauk A. G. (2007) Biochemistry 46, 9301–9309 [DOI] [PubMed] [Google Scholar]
- 25.Rosell F. I., Mauk M. R., Mauk A. G. (2005) Biochemistry 44, 1872–1879 [DOI] [PubMed] [Google Scholar]
- 26.Mauk M. R., Rosell F. I., Mauk A. G. (2007) Biochemistry 46, 15033–15041 [DOI] [PubMed] [Google Scholar]
- 27.Flaherty M. M., Rish K. R., Smith A., Crumbliss A. L. (2008) Biometals 21, 239–248 [DOI] [PubMed] [Google Scholar]
- 28.Shipulina N. V., Smith A., Morgan W. T. (2001) J. Protein Chem. 20, 145–154 [DOI] [PubMed] [Google Scholar]
- 29.Mauk M. R., Rosell F. I., Lelj-Garolla B., Moore G. R., Mauk A. G. (2005) Biochemistry 44, 1864–1871 [DOI] [PubMed] [Google Scholar]
- 30.Mauk M. R., Rosell F. I., Mauk A. G. (2009) J. Am. Chem. Soc. 131, 16976–16983 [DOI] [PubMed] [Google Scholar]
- 31.Seery V. L., Hathaway G., Eberhard U. M. (1972) Arch. Biochem. Biophys. 150, 269–272 [DOI] [PubMed] [Google Scholar]
- 32.Linder R. E., Records R., Barth G., Bunnenberg E., Djerassi C., Hedlund B. E., Rosenberg A., Benson E. S., Seamans L., Moscowitz A. (1978) Anal. Biochem. 90, 474–480 [DOI] [PubMed] [Google Scholar]
- 33.Antonini E., Brunori M. (1971) Hemoglobin and Myoglobin in Their Reactions with Ligands, North-Holland Publishing Co., London [Google Scholar]
- 34.Mauk M. R., Ferrer J. C., Mauk A. G. (1994) Biochemistry 33, 12609–12614 [DOI] [PubMed] [Google Scholar]
- 35.Kuzelová K., Mrhalová M., Hrkal Z. (1997) Biochim. Biophys. Acta 1336, 497–501 [DOI] [PubMed] [Google Scholar]
- 36.O'Neil M. J. (ed) (2006) Merck Index, Twelfth Ed., Merck & Co., White House Station [Google Scholar]
- 37.Krezel A., Mylonas M., Kopera E., Bal W. (2006) Acta Biochim. Pol. 53, 721–727 [PubMed] [Google Scholar]
- 38.Krezel A., Kopera E., Protas A. M., Poznański J., Wyslouch-Cieszyńska A., Bal W. (2010) J. Am. Chem. Soc. 132, 3355–3366 [DOI] [PubMed] [Google Scholar]
- 39.Kamboh M. I., Ferrell R. E. (1987) Am. J. Hum. Genet. 41, 645–653 [PMC free article] [PubMed] [Google Scholar]
- 40.Muller-Eberhard U. (1988) Methods Enzymol. 163, 536–565 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi N., Takahashi Y., Heiny M. E., Putnam F. W. (1985) J. Chromatogr. 326, 373–385 [DOI] [PubMed] [Google Scholar]
- 42.Wu A. H. B. (2006) Tietz Clinical Guide to Laboratory Tests, 4th Ed., Elsevier Saunders, New York [Google Scholar]
- 43.McLean F. C., Hastings A. B. (1934) J. Biol. Chem. 107, 337–350 [Google Scholar]
- 44.Bowers G. N., Jr., Brassard C., Sena S. F. (1986) Clin. Chem. 32, 1437–1447 [PubMed] [Google Scholar]
- 45.Casey J. R., Grinstein S., Orlowski J. (2010) Nat. Rev. Mol. Cell Biol. 11, 50–61 [DOI] [PubMed] [Google Scholar]
- 46.Cartwright G. E., Markowitz H., Shields G. S., Wintrobe M. M. (1960) Am. J. Med. 28, 555–563 [DOI] [PubMed] [Google Scholar]
- 47.Rózga M., Sokolowska M., Protas A. M., Bal W. (2007) J. Biol. Inorg. Chem. 12, 913–918 [DOI] [PubMed] [Google Scholar]
- 48.Vallee B. L., Gibson J. G., 2nd. (1948) J. Biol. Chem. 176, 445–457 [PubMed] [Google Scholar]
- 49.Caroli S., Alimonti A., Coni E., Petrucci F., Senofonte O., Violante N. (1994) Crit. Rev. Anal. Chem. 24, 363–398 [Google Scholar]
- 50.Blindauer C. A., Harvey I., Bunyan K. E., Stewart A. J., Sleep D., Harrison D. J., Berezenko S., Sadler P. J. (2009) J. Biol. Chem. 284, 23116–23124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonawane N. D., Thiagarajah J. R., Verkman A. S. (2002) J. Biol. Chem. 277, 5506–5513 [DOI] [PubMed] [Google Scholar]
- 52.Sonawane N. D., Verkman A. S. (2003) J. Cell Biol. 160, 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dyke R. W. (1995) Am. J. Physiol. 269, C943–C954 [DOI] [PubMed] [Google Scholar]
- 54.Gerasimenko J. V., Tepikin A. V., Petersen O. H., Gerasimenko O. V. (1998) Curr. Biol. 8, 1335–1338 [DOI] [PubMed] [Google Scholar]
- 55.Mellman I., Fuchs R., Helenius A. (1986) Annu. Rev. Biochem. 55, 663–700 [DOI] [PubMed] [Google Scholar]
- 56.Zak O., Aisen P. (2003) Biochemistry 42, 12330–12334 [DOI] [PubMed] [Google Scholar]
- 57.Smith A., Morgan W. T. (1978) Biochem. Biophys. Res. Commun. 84, 151–157 [DOI] [PubMed] [Google Scholar]
- 58.Clague M. J. (1998) Biochem. J. 336, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldenthal K. L., Hedman K., Chen J. W., August J. T., Vihko P., Pastan I., Willingham M. C. (1988) J. Histochem. Cytochem. 36, 391–400 [DOI] [PubMed] [Google Scholar]
- 60.Morrow J. S., Keim P., Gurd F. R. (1974) J. Biol. Chem. 249, 7484–7494 [PubMed] [Google Scholar]
- 61.Wittmann B., Gros G. (1981) J. Biol. Chem. 256, 8332–8340 [PubMed] [Google Scholar]
- 62.Faber H. R., Groom C. R., Baker H. M., Morgan W. T., Smith A., Baker E. N. (1995) Structure 3, 551–559 [DOI] [PubMed] [Google Scholar]