Abstract
Two-pore channels (TPCNs) have been proposed to form lysosomal Ca2+ release channels that are activated by nicotinic acid adenine dinucleotide phosphate. Here, we employ a glass chip-based method to record for the first time nicotinic acid adenine dinucleotide phosphate -dependent currents through a two-pore channel (TPCN2) from intact lysosomes. We show that TPCN2 is a highly selective Ca2+ channel that is regulated by intralysosomal pH. Using site-directed mutagenesis, we identify an amino acid residue in the putative pore region that is crucial for conferring high Ca2+ selectivity. Our glass chip-based method will provide electrophysiological access not only to lysosomal TPCN channels but also to a broad range of other intracellular ion channels.
Keywords: Calcium, Calcium Channels, Calcium Intracellular Release, Endosomes, Ion Channels, Lysosomes, NAADP, Calcium Release Channels, Intracellular Ion Channels, Two-pore Channels
Introduction
Nicotinic acid adenine dinucleotide phosphate (NAADP)3 is a second messenger that releases Ca2+ from intracellular stores at low nanomolar concentrations. NAADP-evoked Ca2+ release has been demonstrated in invertebrates and numerous mammalian cell types including pancreatic acinar and β-cells, cardiac and smooth muscle cells, T-lymphocytes, platelets, and neurons (1). Recent studies using NAADP binding assays and Ca2+ imaging experiments indicated that members of the two-pore channel family (TPCN1–3) constitute the native NAADP receptor (2, 3). TPCNs share sequence homology with members of the transient receptor potential (TRP) cation channel family, suggesting that they may directly form the NAADP-gated Ca2+ conductance. However, direct proof of ion channel activity of TPCNs is still missing, leaving open the possibility that another ion channel protein assembled with TPCNs could underlie the Ca2+ current. A major obstacle to address this key issue is that TPCNs are strictly localized in endolysosomal organelles, in particular acidic lysosomes that are not readily accessible to standard patch clamp measurements. Here we present a new method to record ionic currents in isolated lysosomes. Specifically, we provide direct evidence that TPCN2 is a highly selective Ca2+ channel and identify an amino acid residue in the putative pore region that is crucial for conferring Ca2+ selectivity.
EXPERIMENTAL PROCEDURES
Generation and Culture of Stable Cell Lines
Stable cell lines for enhanced GFP-tagged murine wild type TPCN2 (3) and mutant TPCN2 were generated using the Flp-InTM system (Invitrogen) according to manufacturer's protocol. Mutations in the putative selectivity filter of murine TPCN2 (N257A and E643A) were introduced using the QuikChange site-directed mutagenesis kit (Stratagene, LA Jolla, CA). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml hygromycin and kept at 37 °C, 10% CO2.
Preparation of Lysosomes
To increase the size of the lysosomes (usually <0.5 μm), cells were treated with 1 μm vacuolin for 2 h. This small compound is known to selectively increase the size of endosomes and lysosomes (4, 5). Lysosomes were prepared according to Schenkman and Cinti (6). Briefly, control HEK293 cells or HEK293 cells stably overexpressing wild type or recombinant mTPCN2 channels were homogenized in 0.25 m sucrose, 10 mm Tris-Cl, pH 7.4, on ice using a potter homogenizer to obtain the whole cell lysates. The lysates were centrifuged at 14,000 × g for 15 min at 4 °C, and the supernatant was treated with 8 mm CaCl2 (final concentration) on ice for 5 min with gentle stirring. Ca2+ causes aggregation of lysosomes. To precipitate aggregated lysosomes, a second centrifugation step at 25,000 × g for 15 min at 4 °C was used. The supernatant was collected, and the pellet was washed in 150 mm KCl, 10 mm Tris-Cl, pH 7.4, followed by a final centrifugation step at 25,000 × g for 15 min at 4 °C. The pellet containing purified lysosomes was collected and subjected to Western blotting.
Western Blots
Whole cell lysates were prepared by homogenizing HEK293 cells stably expressing wild type and mutated TPCN2 channels using a potter homogenizer. The lysates were centrifuged at 14.000 × g for 15 min at 4 °C, and the supernatant was collected. Proteins were boiled in Laemmli sample buffer for 5 min and subsequently visualized by SDS-PAGE, Western blot analysis, and ECL (GE Healthcare). The antibodies used were as follows: anti-GFP (Clontech) for detection of EGFP-TPCN2 fusion protein.
β-Hexosaminidase Assay
β-Hexosaminidase assay was performed as described in Ref. 7. Briefly, whole cell lysates or lysosomal preparations of HEK293 cells stably expressing GFP-TPCN2 were added to substrate buffer composed of 0.1 m sodium citrate, pH 4.6, containing 0.04% (w/v) NaN3, 0.2% (w/v) bovine serum albumin, 0.5% (w/v) Triton X-100, and 10 mm substrate (p-nitrophenyl-2-acetamido-2-deoxy-β-d-glucopyranoside; Sigma). The reaction was stopped with 5 volumes of 0.4 m glycine, pH 10.4, and A405 was measured in a spectrophotometer. Parallel experiments were performed using substrate buffer not containing Triton X-100 to measure the amount of disrupted lysosomes. Enzyme activity of intact lysosomes was calculated as ((activity in the presence of Triton X-100) − (activity in the absence of Triton X-100)).
Live Cell Imaging
HEK293 cells stably expressing wild type and mutated TPCN2 channels were grown on μ-Dishes (ibidi) in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml hygromycin and kept at 37 °C, 10% CO2. After 2, days the cells were incubated with 100 nm LysoTracker Red DND-99 (Invitrogen) for 30 min, 37 °C. To avoid background staining, the medium was changed, and the cells were immediately visualized under a Zeiss LSM 510 laser confocal microscope.
Whole Lysosome Recordings (WLR)
For whole lysosome recordings, a planar patch clamp system (Port-a-Patch, Nanion, Munich, Germany) was used. Purified lysosomes were resuspended in a washing solution containing (in mm) 150 KCl, 10 Tris-Cl, pH 7.4. Mean lysosomal capacitance was 165 ± 28 fF (n = 15). During seal formation, the bath solution contained (in mm) 120 potassium methanesulfonate, 10 EGTA, 10 HEPES, and KOH was used to adjust pH to 7.2. After seal formation, the lysosomal membrane was ruptured by applying a brief suction pulse to achieve whole lysosome configuration. Currents were recorded using an EPC-10 patch clamp amplifier (HEKA, Lambrecht, Germany) and PatchMaster acquisition software (HEKA). Data were digitized at 25 μs and filtered at 2.8 kHz. Fast and slow capacitive transients were cancelled by the compensation circuit of the EPC-10. Membrane potentials were corrected for liquid junction potential, which was calculated by pCLAMP 10 (Molecular Devices, Sunnyvale, CA). Liquid junction potentials were +4.4 mV for standard solutions, +2 mV for recording conditions of chloride currents, and 8.4 mV for bi-ionic recording conditions. For all experiments, salt-agar bridges were used to connect the reference Ag-AgCl wire to the bath solution to minimize voltage offsets. All recordings were obtained at room temperature. The membrane potential was held at −80 mV, and 500-ms voltage ramps from −100 to +100 mV were applied every 5 s. For the application of NAADP or NADP, standard external solution was completely exchanged by standard solution containing NAADP or NADP, respectively. To dissect INAADP, the current in the absence of NAADP was subtracted from the current obtained in the presence of NAADP. The current amplitude at −100 mV was extracted from individual ramp current records and used to calculate the current amplitude. To calculate PCa/PK when Ca2+ was the only permeating cation in the lysosomal solution, we used PCa/PK = γCa[K]e/(4γK×[Ca2+]i)×exp(Erev×F/RT)× (exp(Erev×F/RT)+1), where F is the Faraday constant, R is the gas constant, T is temperature, and Erev is the reversal potential. [K]e is the concentration of K+ in the external solution, and [Ca2+]i is the concentration of Ca2+ in the internal solution. The activity coefficients γCa and γK were 0.5 and 0.75, respectively.
Solutions for Electrophysiology
Supplemental Table 1S summarizes the external and internal solutions used for electrophysiological recordings of single lysosomes. Unless stated otherwise, the standard external and standard internal solutions were used for whole lysosome recordings. To investigate the pH dependence of lysosomal currents, in some experiments, the pH of the standard internal solution was adjusted to 7.2. In a subset of experiments, standard external solution containing 100 nm Ca2+ was used instead of 2 mm Ca2+. Methanesulfonate was used to replace most of the Cl− to reduce the background from an endogenous chloride conductance (supplemental Fig. 1S). For bi-ionic experiments, extralysosomal solution containing only K+ (externalK+) and internal solution containing only Ca2+ (internalCa2+) as permeant cations were used. Chloride currents were recorded using external solution containing high Cl− (externalhighCL−) and standard internal solution.
Statistics
All values are given as mean ± S.E. n is the number of experiments. An unpaired t test was performed for the comparison between two groups. Values of p < 0.05 were considered significant.
RESULTS AND DISCUSSION
To characterize currents through TPCNs in single lysosomes, we employed a direct patch clamp approach that was used in a recent study to characterize lysosomal TRP channels (8). However, all of our attempts to employ the protocol described in the latter study to characterize lysosomes of a stable cell line overexpressing TPCN2 failed.
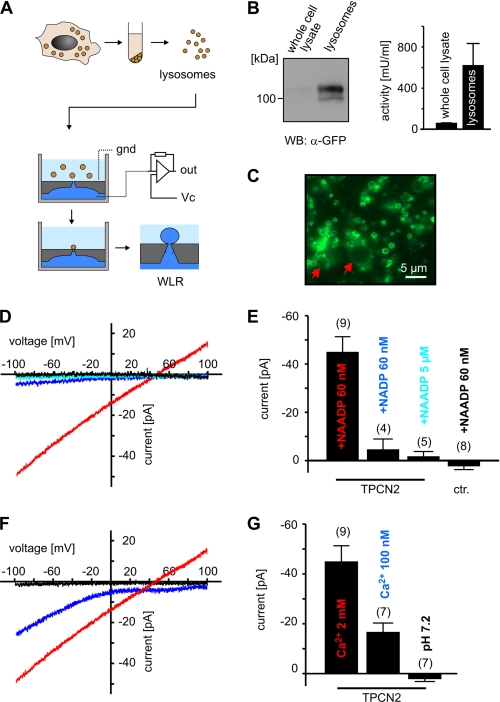
Because maintenance of lysosomal integrity was the major problem preventing current measurements with glass pipettes, we tested an alternative approach where lysosomes are attached to a small hole (<1 μm) in a microstructured planar glass chip. Fig. 1A gives an outline of our protocol. We isolated lysosomes of a stable HEK293 cell line overexpressing GFP-tagged-TPCN2. Before harvesting, the cells were treated with vacuolin to enlarge the lysosomes (8). Lysosomes obtained using this protocol contained high amounts of TPCN2 (Fig. 1B) and revealed diameters of up to 8 μm (range: 3–8 μm; Fig. 1C). The integrity of the lysosomal preparation was verified using the β-hexosaminidase assay (7, 9). Using this highly purified lysosomal preparation, we performed WLR by a planar patch clamp device.
FIGURE 1.
TPCN2-mediated currents in single lysosomes. A, workflow of lysosomal preparation and of the planar patch clamp method used for WLR. gnd, ground; Vc, command voltage; out, output of the amplifier circuitry. B, left panel, Western blot (WB) analysis of whole cell lysates (left lane) and purified lysosomes (right lane) of HEK293 cells expressing GFP-TPCN2 (12 μg of protein/lane). Right panel, activity of the lysosomal enzyme β-hexosaminidase of whole cell lysates (n = 3) and lysosomal preparations (n = 3) obtained from HEK293 cells expressing GFP-TPCN2. C, epifluorescence image of vacuolin-treated lysosomes expressing GFP-TPCN2. The red arrows mark two enlarged lysosomes. D, current-voltage relations of TPCN2 currents from single lysosomes in the presence of 60 nm NAADP (red), 60 nm NADP (dark blue), and 5 μm NAADP (light blue). Black trace, NAADP-dependent current of a wild type lysosome (control). Currents were recorded using standard external and internal solutions. Note that inward currents are currents that flow out of the lysosomes into the cytosol. E, population data for current amplitudes at −100 mV obtained from experiments shown in D. ctr., control. F, current-voltage relations of TPCN2 currents from single lysosomes in the presence of 60 nm NAADP measured with standard external solution containing 2 mm Ca2+ (red, same as in D), 100 nm Ca2+ (dark blue), and standard internal solution. Black trace, current recorded in standard external solution and standard internal solution adjusted to pH 7.2. G, population data for current amplitudes at −100 mV obtained from experiments shown in F.
In TPCN2-expressing lysosomes, NAADP-dependent currents (INAADP) could be recorded using voltage ramps from −100 mV to +100 mV (Fig. 1D). INAADP was activated by low nanomolar concentrations of NAADP (60 nm), whereas it was not present at low micromolar NAADP (5 μm) and was insensitive to NADP (Fig. 1, D and E). These findings were consistent with the activation profile of TPCN2 as determined in Ca2+ imaging experiments (2, 3). The current-voltage relationship of INAADP was non-rectifying and exhibited a reversal potential of 44.4 ± 5 mV (n = 5) that closely matches the calculated reversal potential for Ca2+ (42.5 mV). This indicated that TPCN2-mediated currents are highly selective for Ca2+ and strongly argued against a relevant permeability for K+ (Erev: −14 mV) in the presence of Ca2+. In agreement with this hypothesis, lowering the [Ca2+]cytosol to 100 mm shifted the reversal potential to >100 mV (Fig. 1F). Current recordings using bi-ionic conditions revealed that the average permeability ratio for PCa/PK was >1000 (Erev >100 mV; Fig. 2F). Increasing intralysosomal pH to 7.2 completely abolished TPCN2-mediated currents, indicating that intralysosomal protons are required for NAADP-dependent channel opening (Fig. 1F).
FIGURE 2.
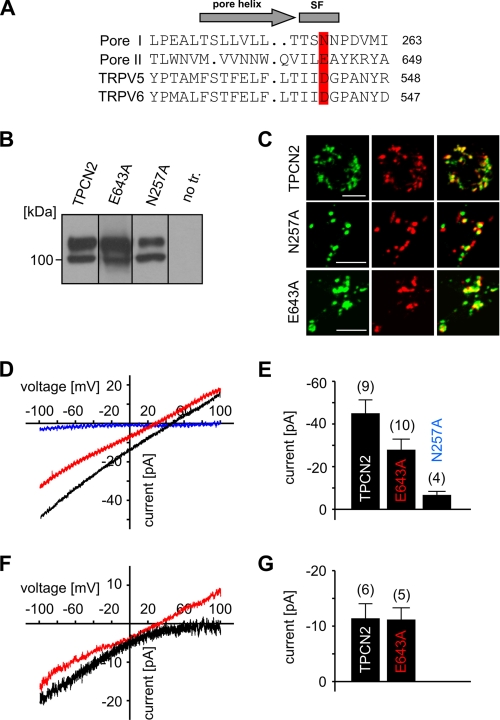
Mutation E643A alters ion selectivity of TPCN2. A, alignment of the putative pore region of TPCN2 with that of TRPV5 and TRPV6 channels. Highlighted residues in TPCN2 were mutated to generate N257A and E643A constructs, respectively. SF, putative selectivity filter. B, Western blots of HEK293 cells stably expressing wild type and mutated TPCN2 channels (30 μg of protein/lane obtained from whole cell lysates). no tr., no transfection. C, confocal microscopy of HEK293 cells expressing wild type and mutated TPCN2 channels indicates that wild type and mutant TPCN2 are targeted to the lysosomes (green, TPCN2; red, LysoTracker; yellow, overlay; scale bar, 5 μm). D, effect of pore mutations on Ca2+ permeability of TPCN2. Current-voltage relations of TPCN2 (black), N257A (dark blue), and E643A (red)-mediated currents in the presence of 60 nm NAADP. E, population data for current amplitudes obtained in similar experiments as shown in D. Currents were recorded in standard internal and external solution. F, current-voltage relation obtained for TPCN2 (black) and E643A (red) using bi-ionic conditions (externalK+ and internalCa2+ solution). G, population data for similar experiments as shown in F.
If TPCN2 itself forms the ion-conducting pore, it should be possible to change the ion selectivity of INAADP by introducing mutations into the pores of TPCN2. The two putative pore loops of TPCN2 share substantial homology with members of the TRP channel family (Fig. 2A). In particular, an acidic residue, which confers Ca2+/Mg2+ selectivity in TRPV5 and TRPV6 (10, 11), is conserved in pore loop II (Glu-643 in mTPCN2 and Asp-660 in hTPCN2). This residue aligns in pore loop I with an asparagine that is also conserved in TPCNs (Asn-257 in mTPCN2). We mutated both residues individually to alanine and analyzed the mutants functionally. Although N257A protein was produced and expressed in lysosomes (Fig. 2, B and C), no INAADP was detected for this mutant (Fig. 2, D and E). By contrast, the E643A mutant gave rise to substantial INAADP (Fig. 2D). Importantly, the reversal potentials of E643A-mediated current was significantly less positive (22.1 ± 4.0 mV (n = 9); p < 0.01) than that of wild type TPCN2 (44.4 ± 5 mV (n = 5); Fig. 2, D and E). This change implied that mutant TPCN2 had lost some of its very high selectivity for Ca2+ over monovalent cations. In support of this interpretation, current recordings using bi-ionic conditions revealed that the average permeability ratio for PCa/PK changed from >1000 in wild type TPCN2 to a permeability ratio of 8 in mutant TPCN2 (Fig. 2F; Erev: 36 ± 6.5 mV (n = 4)).
In summary, we report for the first time NAADP-mediated ionic currents in single lysosomes expressing TPCN2. We show that TPCN2 forms a highly selective Ca2+ conductance and identify an acidic residue in pore loop II of the protein that is a key determinant of ion selectivity. In conjunction with previous biochemical and imaging studies (2, 3), our results establish TPCN2 as an NAADP-gated Ca2+ channel. The planar patch clamp method used in this study was by far superior to conventional patch clamp methods, allowing us to reliably characterize currents from a large number of isolated lysosomes. There is initial evidence that this technique will also allow the characterization of other lysosomal ion channels (e.g. chloride current; see supplemental Fig. 1S) that could not be directly studied so far. Finally, we expect that our method will be very useful to characterize ionic channels in other types of organelles and intracellular compartments (e.g. the endoplasmic reticulum or the nuclear envelope).
Supplementary Material
This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Bayerische Forschungsstiftung.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1S and Table 1S.
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- NADP
- nicotinamide adenine dinucleotide phosphate
- TPCN
- two-pore channel
- TRP
- transient receptor potential
- GFP
- green fluorescent protein
- WLR
- whole lysosome recordings
- F
- farad
- m
- murine
- h
- human.
REFERENCES
- 1.Zhu M. X., Ma J., Parrington J., Galione A., Evans A. M. (2010) FEBS Lett. 584, 1966–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) Pflugers. Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerny J., Feng Y., Yu A., Miyake K., Borgonovo B., Klumperman J., Meldolesi J., McNeil P. L., Kirchhausen T. (2004) EMBO Rep. 5, 883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huynh C., Andrews N. W. (2005) EMBO Rep. 6, 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenkman J. B., Cinti D. L. (1978) Methods Enzymol. 52, 83–89 [DOI] [PubMed] [Google Scholar]
- 7.Kasper D., Planells-Cases R., Fuhrmann J. C., Scheel O., Zeitz O., Ruether K., Schmitt A., Poët M., Steinfeld R., Schweizer M., Kornak U., Jentsch T. J. (2005) EMBO J. 24, 1079–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong X. P., Cheng X., Mills E., Delling M., Wang F., Kurz T., Xu H. (2008) Nature 455, 992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornak U., Kasper D., Bösl M. R., Kaiser E., Schweizer M., Schulz A., Friedrich W., Delling G., Jentsch T. J. (2001) Cell 104, 205–215 [DOI] [PubMed] [Google Scholar]
- 10.Voets T., Janssens A., Droogmans G., Nilius B. (2004) J. Biol. Chem. 279, 15223–15230 [DOI] [PubMed] [Google Scholar]
- 11.Voets T., Prenen J., Vriens J., Watanabe H., Janssens A., Wissenbach U., Bödding M., Droogmans G., Nilius B. (2002) J. Biol. Chem. 277, 33704–33710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.