Abstract
α-Synuclein is a small presynaptic protein implicated in the pathogenesis of Parkinson disease. Nevertheless, its physiological roles and mechanisms remain incompletely understood. α-Synuclein is not only expressed in neurons but also in the vascular endothelium, which contains intracellular granules called Weibel-Palade bodies (WPBs) that contain a number of chemokines, adhesive molecules, and inflammatory cytokines. This study explored whether the exocytosis of WPB is regulated by α-synuclein. Phorbol 12-myristate 13-acetate-, thrombin-, or forskolin-induced von Willebrand factor release or translocation of P-selectin from endothelial cells were inhibited by α- and β-synuclein but not γ-synuclein. Three point mutants (A30P, A53T, and E46K) found in familial Parkinson disease also inhibited WPB exocytosis similar to that of wild-type α-synuclein. Furthermore, the negative regulation of WPB exocytosis required the N terminus or the nonamyloid β-component of Alzheimer disease amyloid region of α-synuclein, but not the C-terminal acidic tail, and α-synuclein affected WPB exocytosis through interference with RalA activation by enhancing the interaction of RalGDS-β-arrestin complexes. Immuno-EM analysis revealed that α-synuclein was localized close to WPBs. These findings imply that α-synuclein plays as a negative regulator in WPB exocytosis in endothelial cells.
Keywords: Exocytosis, Inflammation, Neurobiology, Parkinson Disease, Synuclein, Trafficking, WPB, Endothelial Cell
Introduction
α-Synuclein (α-syn)2 is a presynaptic protein that was first identified in Parkinson disease (PD) as a major component of the Lewy body (1, 2). Specific mutations (A30P, A53T, and E46K) (3–5) and multiplication of the wild-type gene (6, 7) were found in some early onset familial PD patients. Animal models with transgenic overexpression of α-syn were shown to mimic several aspects of PD such as neuronal loss and α-syn aggregation (8, 9). These findings suggest the pathological role of α-syn in several neurodegenerative diseases (9–13). Although significant progress has been made in understanding the pathological roles of α-syn, the physiological roles of α-syn remain incompletely understood. At present, several lines of evidence have been proposed for its physiological roles. It was reported that α-syn regulates the size of the presynaptic vesicular pool in primary hippocampal neurons (14) and impairs catecholamine release in PC12 cells by interfering with a late step in exocytosis (15) and that mice lacking α-syn exhibit an increased release of dopamine with paired stimuli (16), suggesting that α-syn might regulate synaptic vesicle exocytosis in the synaptic terminus. However, the molecular mechanism of how α-syn works remains unknown.
In addition to its expression in neurons, α-syn is expressed also in human platelets (17), human cerebral blood vessels (18), and hematopoietic cells (19), suggesting that its function might not be restricted only to neurons. Endothelial cells contain intracellular granules called Weibel-Palade bodies (WPBs) that contain a number of chemokines, adhesive molecules, and inflammatory cytokines that are rapidly released to the extracellular space by specific agonists (20, 21). The release to extracellular space of WPB components such as von Willebrand factor (vWF) enables the endothelium to control vascular homeostasis by participating in distinct biological processes such as inflammatory response and thrombosis (20, 21). Accumulating evidence suggests that the exocytosis of WPB is induced by a large number of different agonists that can be classified into two distinct groups as follows: those that act by elevating intracellular Ca2+ levels such as thrombin and histamine (22, 23), and those that act by raising cAMP levels in the cells such as epinephrine and forskolin (24, 25). Recently, WPB exocytosis has been reported to be induced also by phorbol esters in a Ca2+-independent manner (26, 27).
In this study, we investigated whether WPB exocytosis in human umbilical vein endothelial cells (HUVECs) is regulated by α-syn, and we attempted to elucidate the mechanism involved if it is regulated.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Isobutyl-1-methylxanthine (IBMX) and forskolin were purchased from Calbiochem. Phorbol 12-myristate 13-acetate (PMA) and thrombin from human plasma were purchased from Sigma. Antibodies raised against RalGDS, β-arrestin, GFP, vWF, α-syn, β-syn, and γ-syn were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and carboxyfluorescein diacetate (CFDA)-succinimidyl ester was purchased from Molecular Probes (Eugene, OR). Ral activation assay kit was from Upstate Biotechnology, Inc. (Lake Placid, NY); immuno-gold-conjugated mouse (H+L) antibody was from British BioCell (Cardiff, UK); and Lipofectamine, Lipofectamine Plus and Oligofectamine reagents were purchased from Invitrogen. ON-TARGET Plus SMART pool of human α-synuclein siRNA and control siRNA were purchased from Dharmacon, Inc. (Chicago). Recombinant human α-, β-, and γ-syn were purchased from ATGen (Bundang, Korea). The concentration of endotoxin in the purchased recombinant proteins, determined by chromogenic LAL end point assay according to manufacturer's instruction (Cambrex, Walkersville, MD), was <1 EU/μm (1 EU = 0.1 ng of endotoxin) (28). QuikChange site-directed mutagenesis kit was purchased from Stratagene (La Jolla, CA).
Constructs
Plasmids for α-syn, β-syn, γ-syn, A30P, E46K, and A53T were kindly provided by Dr. J. Kim, Department of Microbiology, Yonsei University College of Medicine. Using these genes as templates, pCDNA3.1- or pEGFP-α-syn, -β-syn, and -γ-syn, A30P, E46K, A53T, α-syn(1–60), -(1–95), -(61–140), and -(96–140) were constructed by PCR. Full-length human RalA cDNA was amplified from HUVEC by PCR and cloned into the pcDNA3.1 vector. The Q72L mutation (cag to ctc) was introduced by site-directed mutagenesis employing a PCR-based strategy (29). All constructs were verified by DNA sequencing and were prepared by Endo-Free plasmid Maxi kit (Qiagen, Valencia, CA) to prevent endotoxin contamination.
Cell Culture and Transfection
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Basel, Switzerland) and grown in endothelial growth medium 2 (EGM-2) supplemented with growth factors in a kit (Bullet Kit; Lonza). HUVECs from passages 3 to 8 were used for experiments. HUVECs were transfected using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. After 24 h of transfection, the cells were used for experiments. For experiments using siRNA, HUVECs were transfected with 1 nm siRNAs of human α-synuclein or control siRNA (Dharmacon, Chicago) using Oligofectamine (Invitrogen). After transfecting for 48 h, the cells were lysed for Western blot, leukocyte adhesion assay, and ELISA analysis, unless stated otherwise.
Determination of vWF by ELISA
vWF levels in cell-free culture supernatants were determined using Imubind vWF ELISA kits (American Diagnostic Inc.) according to the manufacturer's instructions. All samples were calibrated from standards containing known concentrations of vWF.
Leukocyte Adhesion Assay
After 24 h of transfection, HUVECs were treated with 100 nm PMA for 1 h. The culture medium was then removed, and monocytic THP-1 cells that were prelabeled with CFDA were added into the culture. The cells were then allowed to adhere for 30 min. After two washes with phosphate-buffered saline (PBS), fluorescent images were immediately obtained by fluorescent microscope, Axiovert 200 M (Zeiss, Germany). Adhesion of green fluorescent cells was quantified in three randomly selected fields per dish.
Ral Activation Assay
Ral activation assay was performed according to the manufacturer's instruction. Briefly, HUVECs were lysed in Ral activation assay buffer after transfection and treatment. The cell lysates were precleared by adding 100 μl of glutathione-agarose per 1 ml of lysate and shaken for 10 min at 4 °C. The agarose beads were collected by centrifuging at 12,000 rpm for 5 s. One hundred μm GTPγS and 1 mm GDP were loaded to cell lysates as positive and negative controls, respectively. Ral assay reagent (RalBP1, agarose) was added immediately into cell lysates, and the reaction mixtures were gently shaken for 30 min at 4 °C. The agarose beads were then collected by centrifuging at 12,000 rpm for 5 s. The agarose beads were washed three times with Ral activation assay buffer and resuspended in 2× sample buffer. Proteins were resolved by SDS-PAGE and subjected to Western blot analysis using anti-RalA antibody. They were then visualized using an enhanced chemiluminescence (ECL) system (Sigma).
Confocal Microscopy
HUVECs cultured on poly-d-lysine-coated coverslips were incubated with several reagents, washed twice with PBS, and fixed in ice-cold 100% methanol. The fixed cells were then washed with PBS and incubated with PBS containing 0.1% Triton X-100 for 10 min at room temperature. After washing several times with PBS, the cells were blocked with PBS containing 5% bovine serum albumin for 30 min at room temperature and then incubated overnight with antibody for vWF and α-synuclein, respectively, at 4 °C. Preparations were then stained with fluorescence-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 2 h, mounted, and observed under a confocal microscope (Zeiss, Germany).
Western Blot and Immunoprecipitation
After transfection and treatment, HUVECs were lysed in ice-cold RIPA buffer (50 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, and 1 mm Na3VO4) containing protease inhibitors (2 mm phenylmethylsulfonyl fluoride, 100 μg/ml leupeptin, 10 μg/ml pepstatin, 1 μg/ml aprotinin, and 2 mm EDTA) for Western blot. After sonication, the lysates were centrifuged at 12,000 rpm for 10 min at 4 °C, and the supernatant was collected. Proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. For immunoprecipitation, the cells were lysed in ice-cold modified RIPA buffer (50 mm Tris-HCl, pH 7.4, 0.25% sodium deoxycholate, 300 mm NaCl, 1% Triton X-100) containing protease inhibitors, and the lysates were precleared with protein G-Sepharose for 1 h at 4 °C on an orbital shaker. Equal protein aliquots of precleared cell lysates were incubated with the indicated antibodies for 12 h and further for 4 h with protein G-Sepharose beads at 4 °C. The immune complexes were collected by centrifugation, washed five times with modified RIPA buffer, and resuspended in sample buffer. Immune complexes were subjected to SDS-PAGE and Western blot analysis using the indicated antibodies. They were then visualized using an ECL system (Sigma).
Immunoelectron Microscopy
The sections for immune electron microscopy were prepared as described previously (30). Briefly, HUVECs were grown on 24-well tissue culture plates. The cells were fixed in 2% glutaraldehyde in 0.1 m phosphate buffer, pH 7.2, on ice for 30 min. After post-fixing in 1% osmium tetroxide at room temperature for 30 min, the cells were stained with 1% uranyl acetate for 1 h and then dehydrated with a series of different concentrations of ethanol. After the samples were embedded in a gelatin capsule, the sections were prepared on carbon-coated nickel grids and stained with 1% uranyl acetate. For the immunolabeling, the sections were incubated in PBS containing 1% gelatin, followed by PBS containing 0.03 m glycine. After a rinse with PBS containing 2% bovine serum albumin, the sections were incubated overnight with α-syn antibody in PBS containing 2% bovine serum albumin at 4 °C and then with 10-nm gold-conjugated goat anti-mouse IgG antibody. The sections were then stained with 1% uranyl acetate and lead citrate and observed under an electron microscope, EM902A (Zeiss, Germany).
Statistical Analysis
All values are expressed as mean ± S.E. Statistical significance was evaluated using unpaired t test or one-way analysis of variance (GraphPad software, San Diego).
RESULTS
α-Syn Overexpression Inhibits Three Secretagogue-induced vWF Secretion in HUVECs
First, to evaluate whether PMA, thrombin, and forskolin induce vWF secretion in HUVECs, HUVECs were stimulated with 100 nm PMA, 2 units/ml thrombin, or 20 μm forskolin together with 100 μm isobutylmethylxanthine for various times. As shown in supplemental Fig. S1, three secretagogues gradually increased vWF release from HUVECs in a time-dependent manner and PMA among the three induced vWF secretion the most. When we examined the presence of intracellular vWF in HUVECs with or without stimulation using confocal microscope after staining, less intracellular vWF was detected in response to secretagogues, confirming the data by ELISA for vWF. Therefore, the result suggests that all three secretagogues induce vWF secretion in HUVECs.
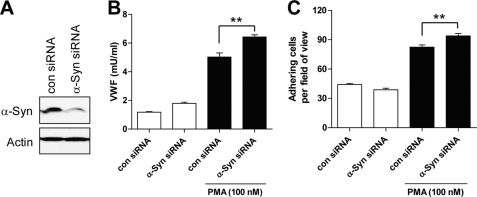
To elucidate the effect of α-syn on WPB exocytosis, we performed the following experiments. After transfection of HUVECs with α-syn, the cells were stimulated with the above described three secretagogues for 1 h, and ELISA was then performed for vWF. As shown in Fig. 1A, α-syn overexpression inhibited all three secretagogue-induced vWF secretions in HUVECs. Confocal microscopic analysis before and after stimulation revealed that more intracellular vWF was detected in the cytoplasm after stimulation when α-syn was overexpressed (Fig. 1B). Moreover, α-syn overexpression did not affect the expression level of vWF (Fig. 1C), thus suggesting that α-syn overexpression inhibited the release of vWF induced by all secretagogues. Exocytosis of WPBs is also known to induce the translocation of P-selectin, another component of WPB, from granules to the outer cellular surface, which triggers leukocyte rolling along the vessel wall (20). Therefore, to confirm the effect of α-syn on WPB exocytosis, we performed leukocyte adhesion assay. After stimulation of HUVECs with three secretagogues for 1 h, THP-1 cells that had been pre-labeled with CFDA were allowed to adhere on confluent HUVECs for 30 min. As shown in Fig. 1, D and E, leukocyte adhesion activity was also inhibited by α-syn overexpression, suggesting that the translocation of P-selectin was inhibited by α-syn overexpression. We did not observe any cellular toxicity and aggregation by α-syn overexpression (data not shown). In addition, α-syn has been known to be expressed in endothelial cells (18), and we also observed endogenous α-syn in HUVECs by Western blot (Fig. 3A). When the expression of endogenous α-syn was decreased by siRNA (Fig. 2A), the release of vWF and leukocyte adhesion were slightly but significantly increased (Fig. 2, B and C). These results indicate that α-syn specifically inhibits WPB exocytosis induced by all three different protein kinase C-, Ca2+-, and cAMP-dependent signaling pathways in endothelial cells.
FIGURE 1.
WPB exocytosis is inhibited by α-syn overexpression. HUVECs overexpressing by α-syn were stimulated with 100 nm PMA, 2 units/ml thrombin (Thr), or 20 μm forskolin (For) together with 100 μm IBMX for 1 h. A, cell-free supernatants were then analyzed for vWF by ELISA. Con, control. B, cells were stained with anti-vWF antibody (red) and observed under a confocal microscope. Blue indicates 4′,6-diamidino-2-phenylindole staining. C, after transfection for 24 h, vWF in total lysates was detected by Western blot. D and E, leukocyte adhesion assay was performed as described under “Experimental Procedures.” Green dots indicate CFDA-prelabeled THP-1 cells adhered to HUVECs. Scale bar indicates 20 μm. **, p < 0.01 against control.
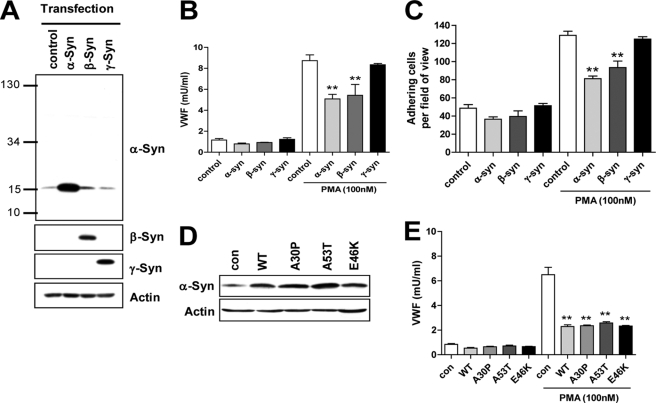
FIGURE 3.
Effects of α-, β-, γ-syn, and α-syn point mutants. A, after transfection of HUVECs with α-, β-, or γ-syn, respectively, their expression levels were detected by Western blot. B, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed by ELISA for vWF. C, leukocyte adhesion assay was performed as described under “Experimental Procedures.” D, after transfection of HUVECs with point mutants of α-syn, they were detected by Western blot. con, control; WT, wild type. E, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed for vWF by ELISA. **, p < 0.01 against control.
FIGURE 2.
Effects of α-syn siRNA on WPB exocytosis. A, after transfection of HUVECs with 1 nm control (con) and α-syn siRNA, respectively, their expression levels were detected by Western blot. B, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed by ELISA for vWF. C, leukocyte adhesion assay was performed as described under “Experimental Procedures.” **, p < 0.01 against control siRNA.
WPB Exocytosis Is Inhibited by α-Syn and β-Syn but Not by γ-Syn Overexpression
In addition to α-syn, β- and γ-syn are other members of the synuclein family that have so far been identified in humans (4, 8, 31, 32). To elucidate whether β-syn and γ-syn as well as α-syn inhibit WPB exocytosis in HUVECs, we overexpressed β- and γ-syn as well as α-syn in HUVECs, and their expression levels were examined by Western blot. As shown in Fig. 3A, endogenous α-syn expression was also detected, whereas endogenous β- and γ-syn were not detected under the present conditions. However, we were able to detect β-syn mRNA as well as α-syn mRNA by reverse transcription-PCR analysis (data not shown). Furthermore, the overexpression of β-syn as well as α-syn inhibited vWF release from HUVECs, whereas the overexpression of γ-syn did not (Fig. 3B). We also observed the similar findings in leukocyte adhesion assay (Fig. 3C). Therefore, this result, suggests that α-syn and β-syn specifically inhibit WPB exocytosis in HUVECs.
α-Syn Mutants (A30P, A53T, and E46K) Inhibit WPB Exocytosis Similarly to Wild-type α-Syn
We investigated whether the α-syn point mutants (A30P, A53T, and E46K), which are found in patients with a rare early onset familial form of Parkinson disease, have effects on WPB exocytosis in HUVECs differently from wild-type α-syn. As shown in Fig. 3D, Western blot analysis revealed that the point mutants and wild type of α-syn were overexpressed at similar levels. PMA-induced vWF release was also inhibited by the overexpression of point mutants similar to that of wild-type α-syn (Fig. 3E), suggesting that the point mutations (A30P, A53T, and E46K) do not affect the effects of wild-type α-syn on WPB exocytosis in endothelial cells.
N-terminal or NAC Region of α-Syn Is Necessary for the Inhibition of PMA-induced vWF Release in HUVECs
α-Syn consists of three distinct regions as follows: the N-terminal region contains KTKEGV repeats, which form amphipathic α-helices similar to the lipid-binding domain of apolipoproteins; the central region is a very hydrophobic NAC (non-Aβ component of Alzheimer disease) peptide; and the C-terminal region is primarily composed of acidic amino acids (31). To elucidate which region of α-syn is responsible for its negative regulation on WPB exocytosis in HUVECs, we constructed α-syn deletion mutants (supplemental Fig. S2A) and transfected HUVECs with these constructs, and we observed that PMA-induced vWF release was inhibited by syn(1–60), -(1–95), and -(61–140) as well as syn(1–140), whereas syn(96–140) was not (supplemental Fig. S2, B and C). However, we could not compare the expression levels among these proteins, because of lack of common epitope. Alternatively, we constructed α-syn deletion mutants fused with GFP (Fig. 4, C and D). As shown in Fig. 4, A and B, the overexpression of GFP-α-syn showed the effect similar to that of untagged α-syn, indicating that GFP tagging does not affect the effect of α-syn on WPB exocytosis. When these GFP-fused α-syn deletion mutants were overexpressed, PMA-induced vWF release was inhibited by GFP-syn(1–60), -(1–95), and -(61–140) as well as GFP-syn(1–140), whereas GFP-syn(96–140) did not, in agreement with those using untagged deletion mutants (Fig. 4E). In addition, we observed similar findings also in the leukocyte adhesion assay (Fig. 4F), indicating that the negative regulation of WPB exocytosis requires the N terminus or NAC region of α-syn but not the C-terminal acidic tail.
FIGURE 4.
Effects of deletion mutants of α-syn. A, after transfection of HUVECs with α-syn and GFP-α-syn, respectively, α-syn or GFP levels were detected by Western blot. con, control. B, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed for vWF by ELISA. C, schematic representation of GFP-α-syn deletion mutants. D, after transfection of HUVECs with GFP-α-syn deletion mutants for 24 h, their levels were detected by Western blot against GFP. E, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed for vWF by ELISA. F, leukocyte adhesion assay was performed as described under “Experimental Procedures.” *, p < 0.05; **, p < 0.01 against control.
Secretagogue-induced RalA Activation Is Suppressed by α-Syn Overexpression
A previous study implicated that RalA is activated by increasing intracellular Ca2+ or cAMP and that activation of RalA is a crucial step in both Ca2+- and cAMP-dependent exocytosis of WPBs (33). We therefore investigated whether RalA is activated in response to secretagogues that trigger WPB exocytosis and whether its activity is inhibited by α-syn overexpression. As shown in Fig. 5A, when HUVECs were stimulated with the three secretagogues, RalA was activated by these agents. Furthermore, when α-syn was overexpressed, all secretagogue-induced RalA activation was suppressed. When GFP-α-syn deletion mutants were overexpressed, GFP-syn(1–140), -(1–60), -(1–95), and -(61–140) suppressed thrombin-induced Ral activation, whereas GFP-syn(96–140) did not (Fig. 5B), in good agreement with the data in Fig. 4. In addition, when the active mutant of RalA (Q72L) (29) was overexpressed together with α-syn, the inhibitory effect of α-syn on WPB exocytosis was also rescued (Fig. 5, C and D). These results suggest that α-syn inhibits WPB exocytosis via suppressing secretagogue-induced RalA activation in endothelial cells.
FIGURE 5.
α-Syn overexpression inhibits small GTPase RalA activity in HUVECs. After transfection of HUVECs with GFP-α-syn (A) and GFP-α-syn deletion mutants (B), the cells were treated with 100 nm PMA, 2 units/ml thrombin, or 20 μm forskolin together with 100 μm IBMX for 1 h. GTP-bound form of RalA was then isolated from cell lysates incubated with the GST-RalBP and analyzed by Western blot with anti-RalA monoclonal antibody. The data were analyzed by the band intensities of Western blot. C, after cotransfection of HUVECs with α-syn and active RalA mutant (Q72L) and treatment with 100 nm PMA for 1 h, the expression level of α-syn and RalA was detected by Western blot. con, control. D, after stimulation with 100 nm PMA for 1 h, the cell-free supernatants were then analyzed for vWF by ELISA. *, p < 0.05; **, p < 0.01.
Interaction between RalGDS and β-Arrestin Is Enhanced by α-Syn Overexpression
Recently, both Ca2+- and cAMP-dependent RalA activation has been reported to be modulated by RalGDS, a well studied exchange factor for Ral, through dissociation of RalGDS-β-arrestin complexes upon stimulation (33). To investigate the molecular mechanism of how α-syn inhibits RalA activation, we hypothesized that RalGDS-β-arrestin complexes might be modulated by α-syn. Thus, we first investigated whether endogenous α-syn could bind to β-arrestin or RalGDS. However, we failed to detect the binding of endogenous α-syn and β-arrestin or RalGDS (data not shown), which might be due to the low expression level of α-syn or interference of binding by antibodies. However, when GFP-α-syn was overexpressed, we observed the interaction of GFP-α-syn with β-arrestin in the absence or presence of stimulation, but GFP did not (Fig. 6A). We also observed that GFP-α-syn interacted with RalGDS (Fig. 6B). In addition, we also observed that GFP-syn(1–95) interacted with both β-arrestin and RalGDS, but GFP-syn(96–140) did not (Fig. 6C), suggesting that the responsible region for interaction with both β-arrestin and RalGDS is the 1–95 region, which is in good agreement with the functional data in Fig. 4. It was reported that the activity of RalGDS is regulated by receptor activation-mediated dissociation of RalGDS-β-arrestin complexes (33, 34). We also observed that PMA induced the dissociation of RalGDS-β-arrestin complexes (supplemental Fig. S3). Interestingly, the interaction between RalGDS and β-arrestin was enhanced by GFP-α-syn overexpression, and this interaction was not altered by PMA stimulation (Fig. 6D). In addition, the overexpression of GFP-syn(1–95) and not GFP-syn(96–140) enhanced the interaction between RalGDS-β-arrestin (Fig. 6E), suggesting that α-syn inhibits RalA activation via binding RalGDS-β-arrestin complexes and enhancing the interaction between them.
FIGURE 6.
α-Syn binds to both β-arrestin and RalGDS, and the interaction of β-arrestin and RalGDS is enhanced by α-syn overexpression. A, after transfection of HUVECs with GFP or GFP-α-syn and treatment with 100 nm PMA for 1 h, the cells were lysed and immunoprecipitated with anti-GFP and anti-β-arrestin antibodies. B, anti-GFP and anti-RalGDS antibodies; D, anti-RalGDS and anti-β-arrestin antibodies. con, control. After transfection of HUVECs with GFP, GFP-syn(1–95), or GFP-syn(96–140) and treatment with 100 nm PMA for 1 h, the cells were lysed and immunoprecipitated with anti-GFP (C) or anti-β-arrestin antibodies (E), and the immunoprecipitated proteins were analyzed by Western blot for the indicated antibodies.
α-Syn Is Localized Close to WPB in HUVECs
Although α-syn was reported to be expressed in endothelial cells (18), evidence for its exact cellular distribution is lacking. To elucidate the localization of α-syn in endothelial cells, we first performed immunofluorescence assay. Unfortunately, however, we could not define the localization of endogenous α-syn in HUVECs due to very low expression level of endogenous α-syn. Moreover, when α-syn was overexpressed, it showed dispersed staining pattern in the cytoplasm (supplemental Fig. S4). To elucidate it more clearly, we performed immunoelectron microscopic analysis. Interestingly, when endothelial cells were incubated with α-syn antibody followed by gold-conjugated secondary antibody, most labelings were detected close to WPB (Fig. 7, A–C). However, when the cells were incubated without primary antibody, we did not detect any labeling (Fig. 7, D–F), suggesting that α-syn is localized specifically close to WPB and may function there.
FIGURE 7.
α-Syn is localized close to WPB in HUVECs. HUVECs were prepared as described under “Experimental Procedures,” and the sections were incubated overnight in the presence (A–C) or absence (D and E) of α-syn antibody at 4 °C and then with 10-nm gold conjugated goat anti-mouse IgG antibody. The sections were observed under an electron microscope. The regions of A and D in the boxes were magnified in the images of B, C and E, F, respectively. Note that α-syn was close to WPB in HUVECs. Arrow indicates the cluster of gold particles. Scale bar indicates 0.6 μm.
Exogenously Added Recombinant α-Syn Also Inhibits vWF Release in HUVECs
In previous studies, α-syn has been detected in extracellular biological fluids, including human cerebrospinal fluid and blood plasma, of both healthy subjects and patients with PD (35, 36). Moreover, α-syn was shown to be rapidly secreted from neuronal cells (37), and it penetrates into a variety of cells, including neurons, platelets, fibroblasts, and microglia, and affects the function of these cells (28, 38, 39). Therefore, to investigate whether extracellular α-syn also affects WPB exocytosis in endothelial cells, we incubated HUVECs with recombinant α-syn and stimulated the cells with the three secretagogues. As shown in Fig. 8A, exogenously added α-syn also inhibited vWF release in HUVECs in a dose-dependent manner. We also observed similar findings in leukocyte adhesion assay (Fig. 8B). Furthermore, when HUVECs were treated with recombinant β- and γ-syn as well as α-syn, findings similar to the data shown in Fig. 3B were observed (Fig. 8C), suggesting that both exogenously added as well as endogenously expressed α-syn have influence on WPB exocytosis in endothelial cells.
FIGURE 8.
Exogenously added recombinant α-syn also inhibits vWF release in HUVECs. A, after treatment of HUVECs with the indicated dose of recombinant α-syn for 1 h, the cells were stimulated with 100 nm PMA, 2 units/ml thrombin, or 20 μm forskolin together with 100 μm IBMX for 1 h. The cell-free supernatants were then analyzed for vWF by ELISA. B, after treatment of HUVECs with 1 μm recombinant α-syn for 1 h, the cells were stimulated with 100 nm PMA. Leukocyte adhesion assay was then performed. C, after treatment of HUVECs with 1 μm recombinant α-, β-, or γ-syn for 1 h, the cells were stimulated with 100 nm PMA. The cell-free supernatants were then analyzed for vWF by ELISA. **, p < 0.01 against control (con).
DISCUSSION
Accumulating evidence indicates that α-syn may negatively function in vesicular trafficking (14–16, 40, 41). However, the molecular mechanisms of how α-syn functions still remain incompletely understood. In this study, we focused on elucidating the molecular mechanism of how α-syn functions.
Endothelial cells, which contain intracellular granules called WPBs, possess advantages for elucidating this type of studies due to the following reasons. First, the exocytosis of WPBs in endothelial cells involves a series of discrete stages as follows: loading of cargo into the nascent granule; granule budding; targeting of the granule to the endothelial membrane; priming of the granule; fusion of the granule and plasma membranes, and finally granule recycling, which is similar to that of synaptic vesicle in neurons (20). Second, the exocytosis of WPBs is well known to be induced by two distinct groups of secretagogues that act through different signaling pathways (20, 21), indicating that we could decipher at which level α-syn functions by using both distinct groups of secretagogues. Finally, they normally express low levels of endogenous α-syn (18), indicating that α-syn functions in endothelial cells under physiological conditions.
In this study, we observed that the overexpression of α-syn inhibited and α-syn siRNA transfection enhanced vWF release and leukocyte adhesion, which were induced by three different types of secretagogues, suggesting that α-syn may function as a negative regulator of WPB exocytosis. Interestingly, we also observed that the overexpression of α-syn slightly inhibited vWF release without secretagogues. In the present condition, it is not clear whether α-syn also regulates the constitutive secretion of vWF or not. Further studies are needed to elucidate them in more detail. In addition, the overexpression of β-syn, another protein of the synuclein family, as well as α-syn inhibited WPB exocytosis. It is in good agreement with our previous study using platelets (39), suggesting that α-syn and β-syn may have redundant functions. The N-terminal amphipathic regions of synuclein family members are well conserved; however, the C-terminal acidic tails are very diverse in both size and sequence (42). In particular, the N-terminal amphipathic region of β-syn is similar to that of α-syn, showing a 90% amino acid sequence homology (43). Interestingly, however, the overexpression of γ-syn did not inhibit WPB exocytosis, even though the N-terminal region of γ-syn is highly homologous to those of α- and β-syn. Furthermore, we could detect α- and β-syn mRNA but not γ-syn mRNA by reverse transcription-PCR (data not shown). Therefore, these results might be due to their physiological expression in endothelial cells. We also observed that α-syn point mutants A30P, A53T, and E46K, which are found in early onset familial Parkinson disease, inhibited WPB exocytosis as much as wild-type α-syn, although these mutant forms of α-syn appear to have properties slightly different from the wild type with respect to in vitro aggregation patterns and cellular cytotoxicity (44–46). These data are also in good agreement with previous reports that demonstrated the physiological role of α-syn (15, 28, 39), suggesting that α-syn point mutants found in familial Parkinson disease may not affect the physiological role of α-syn.
The result of β-syn led us to speculate that the responsible region of α-syn might be the N-terminal amphipathic region. The deletion mutants containing the N-terminal amphipathic region of α-syn (syn(1–60) and syn(1–95)) efficiently inhibited WPB exocytosis like wild-type α-syn. In addition, the deletion mutant containing the NAC region (syn(61–140)) also inhibited it efficiently. However, the deletion mutant containing only C-terminal acidic tail (syn(96–140)) did not, indicating that the N-terminal region containing the N-terminal amphipathic region or NAC region may be responsible for the effect of α-syn on WPB exocytosis.
Because the overexpression of α-syn inhibited WPB exocytosis induced by three different types of secretagogues, it is highly likely that α-syn may function as a negative regulator of WPB exocytosis at the level of common pathway induced by three different types of secretagogues. RalA activation has previously been demonstrated as a crucial step in both Ca2+- and cAMP-dependent WPB exocytosis (24, 47), and RalGDS plays a vital role in the regulation of RalA-dependent WPB exocytosis via receptor activation-mediated dissociation of RalGDS-β-arrestin complexes (33, 34). We also observed that both thrombin and forskolin induced RalA activation. In addition, PMA also induced RalA activation. Interestingly, the overexpression of α-syn inhibited RalA activation induced by all the above three secretagogues. GFP-α-syn deletion mutants containing the N-terminal or NAC region inhibited RalA activation, whereas GFP-α-syn deletion mutants containing only the C-terminal acidic tail did not, in good agreement with the data of vWF release and leukocyte adhesion activity. In addition, the overexpression of active RalA (Q72L) also rescued the effect of α-syn on WPB exocytosis. These data suggest that α-syn regulates WPB exocytosis through modulating RalA activation. In this study, we also observed that α-syn bound to RalGDS and β-arrestin and enhanced RalGDS-β-arrestin complexes. Enhancing RalGDS-β-arrestin complexes by α-syn overexpression may inhibit secretagogue-induced RalA activation. α-Syn has been known to exhibit chaperone activity and has many intracellular binding partners and regulates their function (43, 48–50), implying that α-syn may function through interaction with several other proteins. In agreement with other studies, we also observed that α-syn regulated WPB exocytosis in endothelial cells by binding to RalGDS and/or β-arrestin and by enhancing the interaction between them, although we could not exclude the possibility that α-syn is able to bind to one of two proteins, RalGDS and β-arrestin or to RalGDS-β-arrestin complexes as a linker.
Immunoelectron microscopic analysis revealed that α-syn was localized close to WPB in endothelial cells. Although α-syn lacks a transmembrane domain or lipid anchor, it associates with synaptic vesicles in neuronal cells (31) and artificial membranes (51), suggesting that α-syn may function in synaptic transmission. In addition, it was reported that α-syn loosely associates with plasma membrane, endoplasmic reticulum, and the membrane of α-granules in platelets (17), and we also reported that α-syn functions as a negative regulator of Ca2+-dependent α-granule release in platelets (39). Our present observations together with previous reports imply that α-syn may play a role in the regulation of vesicle release by being localized near vesicles or membrane.
Recently, extracellular α-syn has been focused on elucidating the relationship between α-syn and the pathogenesis of PD. Many researchers observed that exogenously added α-syn shows different effects, depending on cell types (11, 52–54), and we also earlier observed that exogenously added α-syn regulates α-granule release in platelets (39) and phagocytic function of microglia (28), which is associated with its internalization into cells. In this study, exogenously added α-syn was found to inhibit WPB exocytosis in endothelial cells, similar to endogenously overexpressed α-syn, suggesting that α-syn could function outside the cells as well as in the cytosol.
In this study, α-syn was found to regulate WPB exocytosis by modulating RalA activity. However, there are some other possibilities about the mechanism of how α-syn regulates WPB exocytosis in endothelial cells. α-Syn has been known to inhibit phospholipase D activity (55, 56), and phospholipase D is reported to regulate the secretory machinery in endothelial cells as those in neuron or endocrine cells (57), thus indicating that α-syn may inhibit WPB exocytosis through the inhibition of phospholipase D activity. In addition, α-syn overexpression induces oxidative stress in neuronal cells (9, 58), and mild oxidative stress inhibits endothelial exocytosis by inhibiting N-ethylmaleimide-sensitive factor (59), suggesting that α-syn may inhibit WPB exocytosis by regulation of intracellular oxidative stress. Further studies are needed to elucidate in more detail how α-syn works in endothelial cells.
In summary, α-syn negatively regulated secretagogue-induced WPB exocytosis in endothelial cells, and this effect was not affected by three point mutations of α-syn. β-Syn also showed the same effect on WPB exocytosis as α-syn. The N terminus and NAC of α-syn were required for the effect of α-syn on WPB exocytosis. The inhibitory effect of α-syn appeared to involve its specific binding to RalGDS and/or β-arrestin and enhancement of RalGDS-β-arrestin complexes. The small GTPase RalA activity, which is downstream of RalGDS, was also inhibited by α-syn overexpression. Furthermore, α-syn was localized close to WPB, and exogenously added α-syn inhibited WPB exocytosis similar to endogenously overexpressed α-syn. These observations indicate that α-syn functions as a negative regulator of WPB exocytosis in endothelial cells. Finally, the present observations would be very helpful for elucidating the physiological functions of α-syn in endothelial cells.
Supplementary Material
Acknowledgment
We thank Dr. J. Kim (Department of Microbiology, Yonsei University College of Medicine) for providing plasmids for α-syn, β-syn, γ-syn, A30P, E46K, and A53T.
This work was supported in part by National Research Foundation of Korea Grant KRF-2008-331-E00016 funded by the Korean Government and the Korea Science and Engineering Foundation through Chronic Inflammatory Disease Research Center Ajou University Grant R13-2003-019.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- α-syn
- α-synuclein
- WPB
- Weibel-Palade body
- vWF
- von Willebrand factor
- PMA
- phorbol 12-myristate 13-acetate
- PD
- Parkinson disease
- HUVEC
- human umbilical vein endothelial cell
- CFDA
- carboxyfluorescein diacetate
- IBMX
- isobutylmethylxanthine
- ELISA
- enzyme-linked immunosorbent assay
- GFP
- green fluorescent protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- siRNA
- small interfering RNA
- PBS
- phosphate-buffered saline
- NAC
- nonamyloid β-component of Alzheimer disease.
REFERENCES
- 1.Gai W. P., Yuan H. X., Li X. Q., Power J. T., Blumbergs P. C., Jensen P. H. (2000) Exp. Neurol. 166, 324–333 [DOI] [PubMed] [Google Scholar]
- 2.Baba M., Nakajo S., Tu P. H., Tomita T., Nakaya K., Lee V. M., Trojanowski J. Q., Iwatsubo T. (1998) Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 3.Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 5.Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 6.Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destée A. (2004) Lancet 364, 1167–1169 [DOI] [PubMed] [Google Scholar]
- 7.Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 8.Feany M. B., Bender W. W. (2000) Nature 404, 394–398 [DOI] [PubMed] [Google Scholar]
- 9.Hsu L. J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., Wong J., Takenouchi T., Hashimoto M., Masliah E. (2000) Am. J. Pathol. 157, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Agnaf O. M., Jakes R., Curran M. D., Middleton D., Ingenito R., Bianchi E., Pessi A., Neill D., Wallace A. (1998) FEBS Lett. 440, 71–75 [DOI] [PubMed] [Google Scholar]
- 11.Sung J. Y., Kim J., Paik S. R., Park J. H., Ahn Y. S., Chung K. C. (2001) J. Biol. Chem. 276, 27441–27448 [DOI] [PubMed] [Google Scholar]
- 12.Saha A. R., Ninkina N. N., Hanger D. P., Anderton B. H., Davies A. M., Buchman V. L. (2000) Eur. J. Neurosci. 12, 3073–3077 [DOI] [PubMed] [Google Scholar]
- 13.da Costa C. A., Ancolio K., Checler F. (2000) J. Biol. Chem. 275, 24065–24069 [DOI] [PubMed] [Google Scholar]
- 14.Murphy D. D., Rueter S. M., Trojanowski J. Q., Lee V. M. (2000) J. Neurosci. 20, 3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen K. E., Schmitz Y., Troyer M. D., Mosharov E., Dietrich P., Quazi A. Z., Savalle M., Nemani V., Chaudhry F. A., Edwards R. H., Stefanis L., Sulzer D. (2006) J. Neurosci. 26, 11915–11922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E., Shinsky N., Verdugo J. M., Armanini M., Ryan A., Hynes M., Phillips H., Sulzer D., Rosenthal A. (2000) Neuron 25, 239–252 [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M., Yoshimoto M., Sisk A., Hsu L. J., Sundsmo M., Kittel A., Saitoh T., Miller A., Masliah E. (1997) Biochem. Biophys. Res. Commun. 237, 611–616 [DOI] [PubMed] [Google Scholar]
- 18.Tamo W., Imaizumi T., Tanji K., Yoshida H., Mori F., Yoshimoto M., Takahashi H., Fukuda I., Wakabayashi K., Satoh K. (2002) Neurosci. Lett. 326, 5–8 [DOI] [PubMed] [Google Scholar]
- 19.Shin E. C., Cho S. E., Lee D. K., Hur M. W., Paik S. R., Park J. H., Kim J. (2000) Mol. Cells 10, 65–70 [DOI] [PubMed] [Google Scholar]
- 20.Lowenstein C. J., Morrell C. N., Yamakuchi M. (2005) Trends Cardiovasc. Med. 15, 302–308 [DOI] [PubMed] [Google Scholar]
- 21.Rondaij M. G., Bierings R., Kragt A., van Mourik J. A., Voorberg J. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1002–1007 [DOI] [PubMed] [Google Scholar]
- 22.Hamilton K. K., Sims P. J. (1987) J. Clin. Invest. 79, 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine J. D., Harlan J. M., Harker L. A., Joseph M. L., Counts R. B. (1982) Blood 60, 531–534 [PubMed] [Google Scholar]
- 24.Rondaij M. G., Sellink E., Gijzen K. A., ten Klooster J. P., Hordijk P. L., van Mourik J. A., Voorberg J. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1315–1320 [DOI] [PubMed] [Google Scholar]
- 25.Vischer U. M., Wollheim C. B. (1997) Thromb. Haemost. 77, 1182–1188 [PubMed] [Google Scholar]
- 26.Brose N., Rosenmund C. (2002) J. Cell Sci. 115, 4399–4411 [DOI] [PubMed] [Google Scholar]
- 27.Silinsky E. M., Searl T. J. (2003) Br. J. Pharmacol. 138, 1191–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J. Y., Paik S. R., Jou I., Park S. M. (2008) Glia 56, 1215–1223 [DOI] [PubMed] [Google Scholar]
- 29.Maehama T., Tanaka M., Nishina H., Murakami M., Kanaho Y., Hanada K. (2008) J. Biol. Chem. 283, 35053–35059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouley D. M., Ghori N., Mercer K. L., Falkow S., Ramakrishnan L. (2001) Infect. Immun. 69, 7820–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maroteaux L., Campanelli J. T., Scheller R. H. (1988) J. Neurosci. 8, 2804–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surguchov A., Surgucheva I., Solessio E., Baehr W. (1999) Mol. Cell. Neurosci. 13, 95–103 [DOI] [PubMed] [Google Scholar]
- 33.Rondaij M. G., Bierings R., van Agtmaal E. L., Gijzen K. A., Sellink E., Kragt A., Ferguson S. S., Mertens K., Hannah M. J., van Mourik J. A., Fernandez-Borja M., Voorberg J. (2008) Blood 112, 56–63 [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharya M., Anborgh P. H., Babwah A. V., Dale L. B., Dobransky T., Benovic J. L., Feldman R. D., Verdi J. M., Rylett R. J., Ferguson S. S. (2002) Nat. Cell Biol. 4, 547–555 [DOI] [PubMed] [Google Scholar]
- 35.Borghi R., Marchese R., Negro A., Marinelli L., Forloni G., Zaccheo D., Abbruzzese G., Tabaton M. (2000) Neurosci. Lett. 287, 65–67 [DOI] [PubMed] [Google Scholar]
- 36.El-Agnaf O. M., Salem S. A., Paleologou K. E., Cooper L. J., Fullwood N. J., Gibson M. J., Curran M. D., Court J. A., Mann D. M., Ikeda S., Cookson M. R., Hardy J., Allsop D. (2003) FASEB J. 17, 1945–1947 [DOI] [PubMed] [Google Scholar]
- 37.Lee H. J., Patel S., Lee S. J. (2005) J. Neurosci. 25, 6016–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn K. J., Paik S. R., Chung K. C., Kim J. (2006) J. Neurochem. 97, 265–279 [DOI] [PubMed] [Google Scholar]
- 39.Park S. M., Jung H. Y., Kim H. O., Rhim H., Paik S. R., Chung K. C., Park J. H., Kim J. (2002) Blood 100, 2506–2514 [DOI] [PubMed] [Google Scholar]
- 40.Lee S. J., Jeon H., Kandror K. V. (2008) Acta Neurobiol. Exp. 68, 509–515 [DOI] [PubMed] [Google Scholar]
- 41.Stein A., Radhakrishnan A., Riedel D., Fasshauer D., Jahn R. (2007) Nat. Struct. Mol. Biol. 14, 904–911 [DOI] [PubMed] [Google Scholar]
- 42.Lavedan C. (1998) Genome Res. 8, 871–880 [DOI] [PubMed] [Google Scholar]
- 43.Lücking C. B., Brice A. (2000) Cell. Mol. Life Sci. 57, 1894–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conway K. A., Harper J. D., Lansbury P. T. (1998) Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 45.Li J., Uversky V. N., Fink A. L. (2001) Biochemistry 40, 11604–11613 [DOI] [PubMed] [Google Scholar]
- 46.Ostrerova-Golts N., Petrucelli L., Hardy J., Lee J. M., Farer M., Wolozin B. (2000) J. Neurosci. 20, 6048–6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Leeuw H. P., Fernandez-Borja M., Reits E. A., Romani de Wit T., Wijers-Koster P. M., Hordijk P. L., Neefjes J., van Mourik J. A., Voorberg J. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 899–904 [DOI] [PubMed] [Google Scholar]
- 48.Kim T. D., Paik S. R., Yang C. H., Kim J. (2000) Protein Sci. 9, 2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrerova N., Petrucelli L., Farrer M., Mehta N., Choi P., Hardy J., Wolozin B. (1999) J. Neurosci. 19, 5782–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souza J. M., Giasson B. I., Lee V. M., Ischiropoulos H. (2000) FEBS Lett. 474, 116–119 [DOI] [PubMed] [Google Scholar]
- 51.Davidson W. S., Jonas A., Clayton D. F., George J. M. (1998) J. Biol. Chem. 273, 9443–9449 [DOI] [PubMed] [Google Scholar]
- 52.Klegeris A., Pelech S., Giasson B. I., Maguire J., Zhang H., McGeer E. G., McGeer P. L. (2008) Neurobiol. Aging 29, 739–752 [DOI] [PubMed] [Google Scholar]
- 53.Lee S. B., Park S. M., Ahn K. J., Chung K. C., Paik S. R., Kim J. (2009) Biochem. Biophys. Res. Commun. 381, 39–43 [DOI] [PubMed] [Google Scholar]
- 54.Su X., Maguire-Zeiss K. A., Giuliano R., Prifti L., Venkatesh K., Federoff H. J. (2008) Neurobiol. Aging 29, 1690–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenco J. M., Rawlingson A., Daniels B., Morris A. J. (1998) Biochemistry 37, 4901–4909 [DOI] [PubMed] [Google Scholar]
- 56.Ahn B. H., Rhim H., Kim S. Y., Sung Y. M., Lee M. Y., Choi J. Y., Wolozin B., Chang J. S., Lee Y. H., Kwon T. K., Chung K. C., Yoon S. H., Hahn S. J., Kim M. S., Jo Y. H., Min D. S. (2002) J. Biol. Chem. 277, 12334–12342 [DOI] [PubMed] [Google Scholar]
- 57.Disse J., Vitale N., Bader M. F., Gerke V. (2009) Blood 113, 973–980 [DOI] [PubMed] [Google Scholar]
- 58.Parihar M. S., Parihar A., Fujita M., Hashimoto M., Ghafourifar P. (2009) Int. J. Biochem. Cell Biol. 41, 2015–2024 [DOI] [PubMed] [Google Scholar]
- 59.Matsushita K., Morrell C. N., Mason R. J., Yamakuchi M., Khanday F. A., Irani K., Lowenstein C. J. (2005) J. Cell Biol. 170, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.