Abstract
Acyl-CoA-binding protein (ACBP) functions both intracellularly as part of fatty acid metabolism and extracellularly as diazepam binding inhibitor, the precursor of endozepine peptides. Two of these peptides, ODN and TTN, bind to the GABAA receptor and modulate its sensitivity to γ-aminobutyric acid (GABA). We have found that depolarization of mouse primary astrocytes induces the rapid release and processing of ACBP to the active peptides. We previously showed that ODN can trigger the rapid sporulation of the social amoeba Dictyostelium. Using this bioassay, we now show that astrocytes release the endozepine peptides within 10 min of exposure to the steroids cortisol, pregnenolone, pregnenolone sulfate, or progesterone. ACBP lacks a signal sequence for secretion through the endoplasmic reticulum/Golgi pathway and its secretion is not affected by addition of brefeldin A, a well known inhibitor of the classical secretion pathway, suggesting that it follows an unconventional pathway for secretion. Moreover, induction of autophagy by addition of rapamycin also resulted in rapid release of ACBP indicating that this protein uses components of the autophagy pathway for secretion. Following secretion, ACBP is proteolytically cleaved to the active neuropeptides by protease activity on the surface of astrocytes. Neurosteroids, such as pregnenolone sulfate, were previously shown to modulate the excitatory/inhibitory balance in brain through increased release of glutamate and decreased release of GABA. These effects of steroids in neurons will be reinforced by the release of endozepines from astrocytes shown here, and suggest an orchestrated astrocyte-neuron cross-talk that can affect a broad spectrum of behavioral functions.
Keywords: Dictyostelium, Peptide Hormones, Protein Secretion, Signal Transduction, Steroid Hormone
Introduction
Acyl-CoA-binding protein (ACBP),3 a small, highly conserved protein that plays a well defined cytoplasmic role in fatty acid metabolism, is also the precursor of peptides that signal between cells (1, 2). However, it does not have an N-terminal signal sequence that could direct it through the ER/Golgi apparatus and must use an unconventional pathway for secretion.
ACBP is proteolytically cleaved to generate the 34-amino acid peptide, TTN, as well as the 18-amino acid peptide, ODN (3–5). In the central nervous system these peptides bind to the GABAA receptor and reduce the affinity for GABA (6–8). Because they compete with benzodiazepines binding to the receptor, they have been referred to as diazepam binding inhibitor and more generally named endozepine. The peptides also induce the production of steroid hormones by binding to a separate receptor termed, peripheral benzodiazepine receptor, found both in the brain and peripheral organs (9). In the central nervous system, glial cells, specifically astrocytes, express high levels of peripheral benzodiazepine receptor and ACBP, but low expression is found in neurons (10–12). Thus, it has been proposed that astrocytes are the major source of endozepine in the central nervous system. However, little is known about how they produce the extracellular peptides or how the production of endozepine is regulated. In the social amoeba Dictyostelium discoideum, as well as the yeasts Saccharomyces cerevisiae and Pichia pastoris, the orthologs of ACBP are secreted through an unconventional pathway that includes components of the autophagy and multivesicular body pathways as well as the Golgi-associated protein GRASP (13–16).
We investigated whether the mechanisms for release of ACBP, and its processing into active peptides, in astrocytes are similar to those we previously demonstrated in Dictyostelium. In Dictyostelium, acyl-CoA-binding protein (AcbA) is released from vesicles in response to GABA (13). It is then processed extracellularly to the 34-amino acid peptide, SDF-2, which induces rapid sporulation (17). Using this non-mammalian bioassay, we found that secretion of ACBP from astrocytes and its processing into TTN occurs within a few minutes of depolarization with KCl or addition of the steroids pregnenolone sulfate and cortisol at nanomolar concentrations.
Pregnenolone sulfate, endogenously produced in the central nervous system, as well as cortisol, produced in the adrenal gland, profoundly affect central nervous system function under physiological and pathological conditions (18, 19). These effects are believed to occur through modulation of neurotransmitter release thus affecting the balance between excitatory and inhibitory neurotransmission (20–22). Specifically, pregnenolone sulfate was shown to decrease the release of GABA and thus reduce tonic inhibition (23, 24). Our results showing that nanomolar concentrations of pregnenolone sulfate produce the rapid release of endozepines from astrocytes suggest that the neurosteroid will not only diminish release of GABA from inhibitory neurons but will also ensure that transmission through postsynaptic GABAA receptors is decreased.
EXPERIMENTAL PROCEDURES
Cell Growth and Development
Primary astrocyte cultures from Swiss Webster neonatal mice were grown in glial medium at 37 °C (25). Glial cells were used when they reached confluence in 24-well plates. No variation in endozepine production was observed between different batches of glial cells with slightly different levels of confluence. Dictyostelium cells were grown in HL5 medium at 22 °C with shaking at 180 rpm (26). Exponentially growing cells at a density of 2–5 × 106/ml were harvested by centrifugation.
Molecular Biology
Standard molecular biology protocols were used for plasmid construction and manipulation. Plasmids to express the GFP-human ACBP fusion and GFP-AcbA fusion in Dictyostelium were built using the pDXA-GFP2 vectors (27). The human gene encoding ACBP was kindly provided by Dr. Inke Nitz (28). It was cloned by PCR in pGEMT-EASY and sequence verified. The EcoRI-XhoI fragments were then cloned in pDXA-GFP2 EcoRI-XhoI sites. An AcbA BamHI-XhoI fragment from previously described pGEMT-EASY-AcbA (17) was cloned in pDXA-GFP2 BamHI-XhoI sites. Recombinant AcbA was purified from Escherichia coli cells expressing pET32a-AcbA (17). A similar procedure was used to produce recombinant human ACBP from E. coli carrying pET32a-hsACBP. We recovered 50 mg of ACBP with over 95% purity as estimated by Coomassie staining of electrophoretically separated material.
Production and Purification of Endozepine
One hour prior to the assay, the astrocytes were washed three times in 37 °C HSCC buffer (20 mm HEPES, pH 7.4, 120 mm NaCl, 5.4 mm KCl, 0.8 mm MgCl2, 1.8 mm CaCl2, 15 mm glucose) and incubated in 500 μl of fresh HSCC buffer at 37 °C. Inhibitors (mPKI, pertussis toxin, PD98059, U72122, brefeldin A, and 3-methyladenine) were added 45 min before the beginning of the assay unless otherwise stated. After induction, 400 μl of the HSCC was harvested at the indicated time and added to an Eppendorf tube containing 1 ml of MES buffer (20 mm MES, pH 6.2, 20 mm NaCl, 20 mm KCl, 1 mm MgSO4, 1 mm CaCl2) and 40 μl of A-25 anion exchange resin (Sigma A25120). After mixing, the resin was spun down, washed with 500 μl of MES buffer, followed by 500 μl of MES buffer containing 200 mm NaCl before elution with 40 μl of MES buffer containing 400 mm NaCl. The samples were kept at 4 °C until quantified by bioassay.
Strains and Bioassay
The following previously described Dictyostelium strains were use: wild-type Ax4 (29), pkaC overexpressing strain KP (30), dhkA null and pkaC overexpressing strain dhkA−/K (31), and acbA null acbA− (17). 10 μg of pDXA-GFP2-HsACBP or pDXA-GFP2-AcbA plasmids linearized with ScaI were used to transform Dictyostelium acbA− null strain by electroporation. Transformants were selected using G418 as previously described (32).
Detection of endozepine using the Dictyostelium bioassay was carried out on KP cells and their derivatives after an 18-h development in monolayers as previously described (31). Samples or defined products were added to the cells and the number of spores and undifferentiated cells were counted 1 h later unless otherwise indicated. The amounts of SDF-2 activity were determined by serial dilution of the sample before addition to KP cells. The lowest dilution giving full induction of spore formation correspond to 20 fm for SDF-2 and 20 pm for endozepine. The activity in the sample was standardized to 103 producing cells whenever applicable. The endozepine activity from astrocytes was also quantified by serial dilution but standardize per mg of soluble protein. Because the bioassay relies on 2-fold serial dilutions, we present the data with reliability range bars of ±50%. Spore viability assays were repeated at least three times as previously described (32).
Chemicals
Most restriction enzymes were from New England Biolab. 10× Eagle's medium, antibiotics, fetal calf serum, GABA, l-glutamate, cAMP, and most steroids were supplied by Sigma. Progesterone was obtained from Aldrich, corticosterone from Fluka, dexamethasone from BIOMOL International (Plymouth, MA). The AKT inhibitor IV, the phospholipase inhibitor U73122, myristylated PKI, and pertussis toxin were purchased from Calbiochem. The 34-amino acid mouse TTN peptide with the sequence TQPTDEEMLFIYSHFKQATVGDVNTDRPGLLDLK was synthesized by Chi Scientific (MA) and purified to >95%. Anti-ACBP antibodies (M-20) were from Santa Cruz Biotechnology, Inc. Antibodies to Dictyostelium AcbA and the TagC protease have been previously described (17).
RESULTS
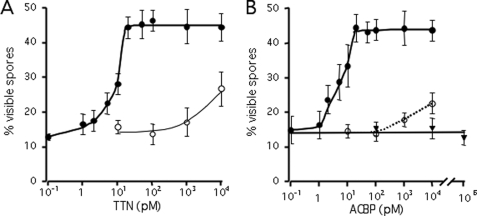
It was previously shown that depolarization by addition of 50 mm KCl can induce Muller cells, a type of astrocyte found in the retina, to release full-length ACBP in less than 30 min (33). To determine whether astrocytes in the central nervous system can be induced to release ACBP, we treated confluent cultures of mouse primary astrocytes with 50 mm KCl and collected the supernatant 15 min later. Because we had previously shown that the 18-amino acid peptide, ODN, can mimic SDF-2, a Dictyostelium peptide, which induces rapid sporulation (17), we used this bioassay because it is very sensitive to low levels of the peptide. A positive feedback loop in Dictyostelium cells amplifies threshold levels of SDF-2 several thousand-fold by releasing vesicle-stored AcbA that is rapidly processed to more SDF-2 by an extracellular protease (13, 17). Addition of as little as 0.02 pm SDF-2 to developed test cells stimulates sporulation within 15 min (17, 31). Using this bioassay, we found that synthetic TTN peptide induces maximal sporulation at 20 pm, a thousand-fold lower concentration than required for ODN (Fig. 1A). We previously showed that unrelated peptides with a similar number of negative charges do not induce sporulation (17). Addition of specific antibodies against mammalian ACBP neutralized the effects of TTN (Fig. 1A). Antibodies to Dictyostelium AcbA did not block the induction by TTN (see supplemental Fig. S1). Test cells lacking DhkA, the receptor for SDF-2, are not induced by TTN, confirming the specificity of the bioassay (supplemental Fig. S1). Addition of full-length recombinant ACBP did not induce sporulation even when added at 10 nm, but if it was treated with trypsin prior to addition to the bioassay, it was as active as TTN, giving full induction at 20 pm (Fig. 1B). Addition of antibodies to ACBP completely blocked the effects of trypsin-treated ACBP up to 100 pm but was less effective at higher concentrations as the added peptides exceeded its neutralizing activity (Fig. 1B). Full-length ACBP appears to be inactive in the bioassay and would not be expected to be processed by the Dictyostelium protease, which is not exposed until after stimulation with either SDF-2 or GABA (34).
FIGURE 1.
Detection of mammalian endozepines using the Dictyostelium bioassay. A, synthetic TTN was added to the test cells at the indicated concentrations in the absence (filled circles) or presence (open circles) of anti-ACBP antibodies (1/5,000 final dilution). The proportion of spores was scored microscopically 1 h later. Each assay was repeated 5 times and the results averaged. The error bars correspond to 1 standard deviation. B, recombinant Hs ACBP was treated with trypsin overnight followed by purification on anion exchange resin. The indicated concentration of trypsinized ACBP was added to the test cells at the indicated concentration in the absence (filled circles) or presence (open circles) of anti-ACBP antibodies (1/5,000 final dilution). Unprocessed recombinant Hs ACBP was also added to the test cells (triangles). Each assay was repeated 5 times and the results averaged.
Mouse Astrocytes Can Be Induced to Produce Endozepines
Using the bioassay described above, we found that 50 mm KCl induced mouse astrocytes growing in culture to produce endozepine activity that reached a maximal level 15 min after induction (Fig. 2). No activity was detected when 50 mm NaCl was substituted for KCl or when the protease inhibitor TPCK was added at the same time as KCl (Fig. 2). The activity purified on the anion exchange resin was the same amount as the TTN peptide or trypsinized HsACBP (supplemental Table S1). This purification procedure allows the removal of trypsin, TPCK, and all the other chemicals added to the astrocytes prior to the detection by bioassay (see supplemental Table S1). The activity purified from astrocytes was neutralized by addition of anti-ACBP antibodies, confirming that we were observing the effects of TTN-like activity derived from processed ACBP. We previously showed that addition of anti-GABA antibodies (34) or nonspecific antibodies (35) had no inductory or inhibitory effects in this bioassay. The anti-ACBP antibodies also blocked the production of TTN-like activity when added to the astrocytes just prior to induction. Because the threshold of the bioassay is 20 pm TTN (Fig. 1A) and the supernatant of each well could be diluted 250-fold and still show activity, we calculate that astrocytes produce about 80 pmol of TTN/mg of soluble protein in 15 min. ODN, the other ACBP-derived peptide, corresponds to a fragment of TTN but is 1000-fold less efficient in the bioassay and would not be detected even if it was produced by astrocytes. However, we cannot exclude the contribution of other ACBP-derived peptides and the general term of endozepine corresponding to all the active peptides derived from ACBP will be used to describe the activity in this study.
FIGURE 2.
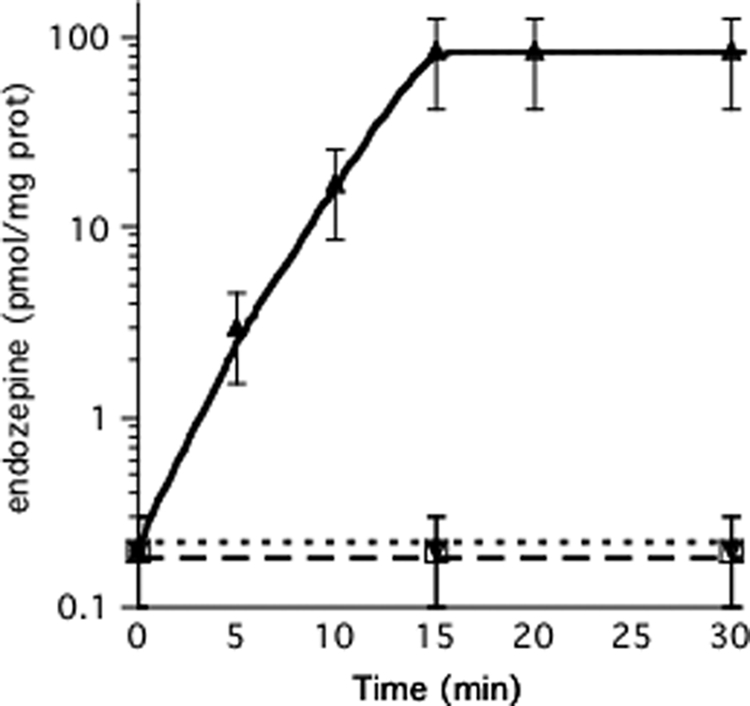
Induction of endozepine production by KCl. Astrocytes in 24-well plates were washed twice with HSCC buffer and incubated in 500 μl of HSCC buffer at 37 °C. Endozepine production was induced by adding 50 mm KCl to the astrocytes (triangles). 2 μm TPCK (open squares) or anti-ACBP antibodies (diamonds) diluted 1/5,000 were added just prior to induction by 50 mm KCl. At the indicated times, 400 μl of buffer was harvested to quantify the amount of endozepines using the Dictyostelium bioassay. Each experiment was repeated at least three times. To standardize the experiments, cells from a well in each series were lysed using Triton X-100 and the amount of soluble protein measured. The bars indicate the reliability range.
The induction of AcbA secretion by low levels of SDF-2 in Dictyostelium is inhibited by glutamate and stimulated by GABA (34). We tested whether glutamate or GABA would induce ACBP secretion and processing from astrocytes but found that neither had any effect. We previously showed that steroids induce release of GABA from Dictyostelium leading to the induction of AcbA secretion (36). Therefore, we tested a series of steroids for ACBP secretion and processing from astrocytes. We found that cortisol, pregnenolone, pregnenolone sulfate, and progesterone were active, whereas other steroids tested had little or no effect (Table 1). Cortisol and pregnenolone sulfate were the most efficient and able to induce maximal levels of endozepine production at concentrations of 5 and 10 nm, respectively (Fig. 3A), whereas pregnenolone and progesterone required far higher concentrations (Fig. 3C). Endozepine activity appeared within 5 min after addition of 100 nm cortisol and reached maximal levels at 10 min (Fig. 3B). Addition of anti-ACBP antibodies just prior to induction with cortisol completely blocked the ability of material secreted from the astrocytes from inducing sporulation in the bioassay indicating that the steroid was inducing endozepine release (Fig. 3B). Similar results were found following induction with the other steroids tested. To estimate the proportion of ACBP secreted by astrocytes, we treated cell lysates with trypsin and purified the resulting endozepine activity on anion exchange resin before testing various dilutions in the bioassay. Total astrocyte lysates generated about 3 nmol of endozepine activity per mg of soluble protein. Because we recover 80 pmol of endozepine activity per mg of protein from the supernatant after induction, roughly 3% is secreted.
TABLE 1.
Steroids tested for endozepine production
The concentration of steroids necessary to induce production of 80 pmol of endozepine/mg of protein is indicated. Weak induction corresponds to production of 8 pmol/mg of protein of endozepine or less.
| Steroids | Minimal concentration required for induction |
|---|---|
| Cortisol | 5 nm |
| Pregnenolone-sulfate | 10 nm |
| Pregnenolone | 50 nm |
| Progesterone | 1000 nm |
| 17α-Progesterone | No effect up to 10 μm |
| 11α-Progesterone | No effect up to 10 μm |
| Aldosterone | No effect up to 10 μm |
| 11-Deoxycortisol | Weak induction at 1 μm |
| Dexamethasone | Weak induction at 1 μm |
FIGURE 3.
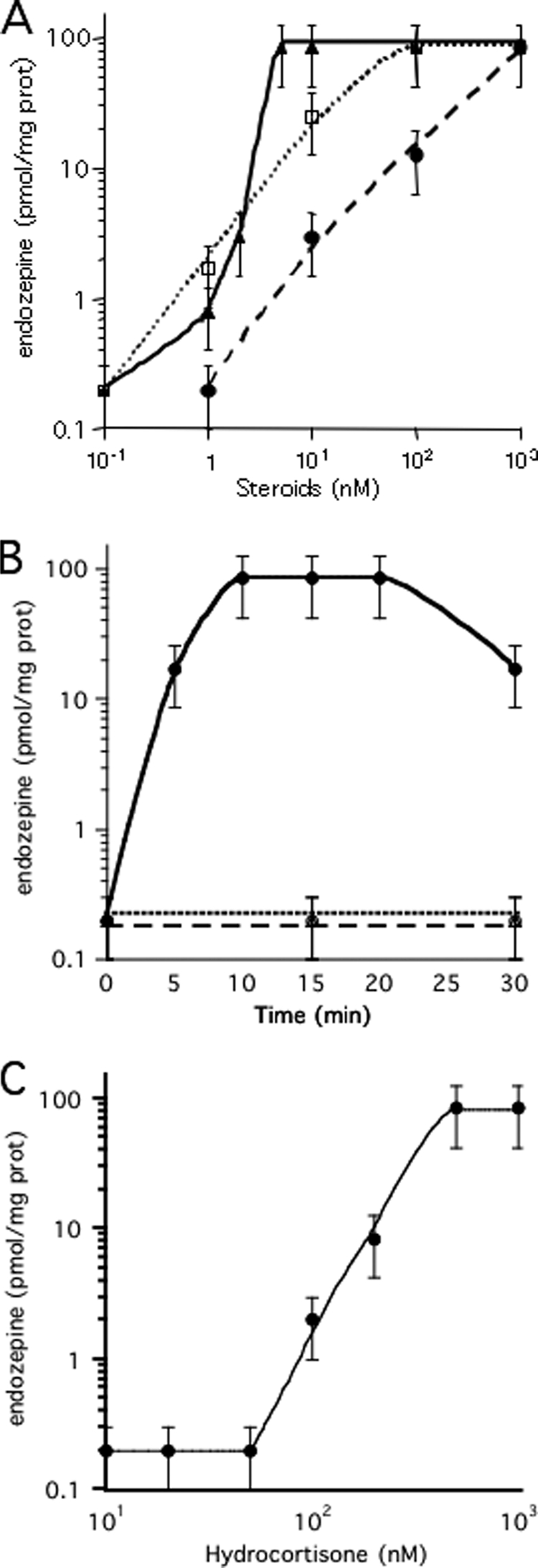
Induction of endozepine production by steroids. A, induction by pregnenolone sulfate and cortisol in the presence or absence of mifepristone. The indicated concentrations of cortisol (filled triangles) or pregnenolone sulfate (open circle) were added to astrocytes. For competition assay, 1 μm mifepristone was added to astrocytes just prior to endozepine induction with the indicated amount of cortisol (open circle) or pregnenolone sulfate (filled circle). The cells were incubated 15 min at 37 °C before harvesting 400 μl of the buffer to quantify the amount of endozepines with the Dictyostelium bioassay. The experiments were repeated at least three times. The bars indicate the reliability range. B, time course of endozepine production. Endozepine production was induced by addition of 100 nm cortisol to the astrocytes (circles) incubated at 37 °C for the indicated time. 2 μm TPCK (squares) or anti-ACBP antibodies (triangles) diluted 1/5,000 were added just prior to induction by cortisol. The amount of endozepine produced in a well was quantified. The experiments were repeated at least three times. The bars indicate the reliability range. C, induction by pregnenolone and progesterone. The indicated concentrations of pregnenolone (filled triangles) or progesterone (open square) were added to astrocytes. The amount of endozepine was quantified from samples harvested after 15 min incubation at 37 °C. The experiments were repeated at least three times. The bars indicate the reliability range.
Addition of 1 μm mifepristone (RU-486), a synthetic steroid inhibitor, to astrocytes just prior to stimulation by cortisol or pregnenolone sulfate acts as a competitive inhibitor, increasing the steroid concentration required for endozepine production by 50–100-fold (Fig. 3A). Addition of 1 μm mifepristone fully blocked the induction of endozepine production by 1 μm of either pregnenolone or progesterone (supplemental Fig. S2). As expected, mifepristone did not block the effect of KCl (Table 2). To test whether a G-coupled receptor might be involved in response to the steroid as it is in Dictyostelium (36), astrocytes were incubated with pertussis toxin that blocks downstream G proteins. However, pertussis toxin did not block endozepine production in response to KCl or cortisol (Table 2). Inhibition of phospholipase C by U73122, ERK2 by PD59059, or AKT protein kinase with AKT inhibitor IV also failed to inhibit endozepine production following induction by cortisol or KCl. On the other hand, myristoylated PKI, a specific inhibitor of the cAMP-dependent protein kinase (PKA), blocked endozepine production (Table 2). To confirm the direct involvement of PKA, the cell permeant PKA activator 8-Br-cAMP was added to astrocytes. 1 mm 8-Br-cAMP resulted in production of 80 pmol of endozepines/mg of protein within 90 min.
TABLE 2.
Pharmacological inhibition of endozepine production
Astrocytes in HSCC were preincubated for 1 h with the indicated inhibitors prior to induction by either 50 mm KCl, 100 nm cortisol or 2 μg/μl of rapamycin. The amount of endozepine produced was quantified from 400 μl of buffer harvested after induction for 15 (potassium chloride, cortisol) or 30 min (rapamycin). The following concentration of inhibitors were used: 10 μg/ml of brefeldin A, 200 ng/ml of pertussis toxin, 20 μm mPKI, 100 nm Akt inhibitor IV, 10 μm PD59059, 1 μm U73122, 1 μm Mifepristone, 2 mm 3-methyladenine. Each experiment was repeated at least three times.
| Potassium chloride | Cortisol | Rapamycin | |
|---|---|---|---|
| Brefeldin A | No inhibitiona | No inhibition | No inhibition |
| Pertussis toxin | No inhibition | No inhibition | No inhibition |
| mPKI | 100% inhibition | 100% Inhibition | 100% Inhibition |
| Akt inhibitor IV | No inhibition | No inhibition | 100% Inhibition |
| PD 98059 | No inhibition | No inhibition | 90% Inhibition |
| U72122 | No inhibition | No inhibition | No inhibition |
| Mifepristone | No inhibition | 98% Inhibition | Not done |
| 3-Methyladenine | Not done | 95% Inhibition | 95–98% Inhibition |
a No inhibition indicates that the endozepine detected was indistinguishable from maximal level of 80 pmol/mg of protein, whereas 100% inhibition indicates the activity was under the detection limit of 0.5 pmol of endozepine/mg of protein.
Expression of human ACBP linked to GFP in Dictyostelium cells lacking endogenous AcbA rescues the sporulation defect in acbA null cells and generates SDF-2 activity in the fruiting bodies (Table 3). Expression of Dictyostelium AcbA linked to GFP in an acbA− null strain also rescues the sporulation defects and generates higher levels of SDF-2 activity in the fruiting bodies (Table 3). The difference in the apparent SDF-2 activities in these transformants is likely to result from the higher affinity of the test cells to Dictyostelium SDF-2 peptide compared with TTN. Because human ACBP expressed in Dictyostelium can generate active peptides, it is clear that ACBP is secreted and processed. To confirm that ACBP can be converted into the active peptide by the Dictyostelium protease, we added recombinant ACBP to Dictyostelium cells (supplemental Table S2). Upon activation of the test cells with GABA, abundant endozepine activity was generated. Inhibition of the protease with TPCK blocked the production of active peptides as did addition of antibodies to the specific protease.
TABLE 3.
Dictyostelium AcbA null is rescued by human ACBP expression
107 cells of the indicated strains were developed 24 h on nitrocellulose filter saturated with PDF buffer. Fruiting bodies were dissociated in 1 ml of PDF. After centrifugation, an aliquot of the supernatant was harvested to purify and quantify SDF-2 activity, whereas the spore viability was tested by resuspending spores in PDF containing 0.5% Triton X-100 before dilution and plating on bacterial lawns. The spore viability was normalized to that of wild-type spores. The SDF-2 activity was calculated per 103 producing cells.
| Strain | Spore viability | SDF-2 activity |
|---|---|---|
| Wild-type | 100% | 10,000 units |
| AcbA− | 12 ± 8% | <0.02 units |
| AcbA−/GFP-AcbA | 102 ± 10% | 200–1,000 units |
| AcbA−/GFP-HsACBP | 95 ± 10% | 10–50 units |
Extracellular Conversion of ACBP into Endozepines by Astrocytes
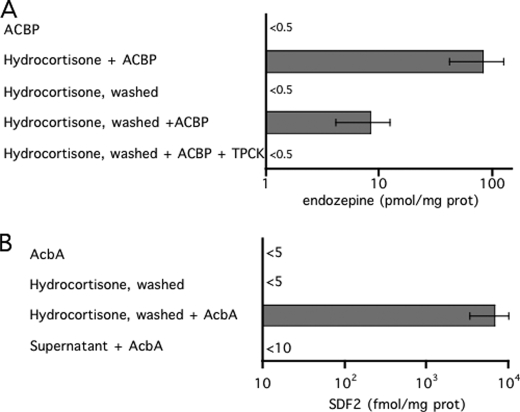
Because production of endozepine was blocked when the protease inhibitor TPCK was added at the same time as KCl (Fig. 2) or cortisol (Fig. 3B), it seemed likely that the proteolytic processing necessary for conversion of ACBP to TTN was occurring extracellularly. To determine whether the protease activity is constitutively present or appears only after induction, we added recombinant ACBP to astrocytes treated or not with 100 nm cortisol. No activity was observed when ACBP was added to untreated cells, even after 3 h of incubation (Fig. 4A), indicating that there is no or little background trypsin-like protease activity in these conditions. When ACBP was added to cells treated with cortisol, the level of endozepine produced was in the same range as cells treated with cortisol alone. However, it was not possible to distinguish endogenous production of endozepine from the conversion of the recombinant ACBP. To solve this problem, astrocytes were washed 15 min after the induction by cortisol, followed by addition of the recombinant ACBP. Washed cells generated levels of endozepine from added ACBP corresponding to about 10% of the endogenous level. Addition of the protease inhibitor TPCK together with recombinant ACBP blocked the production of endozepines by cortisol-induced astrocytes (Fig. 4A). Most of the proteolytic activity appeared to be cell associated because washed cells still processed exogenous ACBP to endozepines.
FIGURE 4.
Processing of recombinant ACBP or ACBA by astrocytes. A, astrocytes were incubated with the indicated components: 20 pmol of ACBP for 1 h; 100 nm cortisol and 20 pmol of ACBP for 15 min; 100 nm cortisol for 15 min followed by two buffer changes (washed) and an additional 15-min incubation; 100 nm cortisol for 15 min followed by washing then incubation for 15 min in the presence of 20 pmol of ACBP with or without 2 μm TPCK. Endozepine activity was then quantified. The experiments were repeated at least three times, the bars indicate the reliability range. B, astrocytes were incubated with the indicated components: 20 pmol of Dictyostelium AcbA for 1 h; 100 nm cortisol for 15 min followed by two changes of buffer (washed) and an additional 15-min incubation in the presence or absence of 20 pmol of AcbA. The supernatant of astrocytes activated by cortisol was incubated for 15 min with 20 pmol of AcbA before determination of the level of SDF-2. The experiments were repeated at least three times.
The cell-associated proteolytic activity that appears following cortisol treatment of astrocytes is also able to process Dictyostelium AcbA into SDF-2. Addition of AcbA to astrocytes only resulted in SDF-2 if the cells had been previously induced with cortisol (Fig. 4B). Washing the cells after induction did not reduce their processing ability and no activity was found in the material washed off the cells (Fig. 4B). Therefore, it appears that the proteolytic processing of AcbA is mediated by the same activity that generates endogenous TTN.
Unconventional Secretion of ACBP
Like Dictyostelium AcbA, ACBP lacks a signal sequence targeting the protein to the ER/Golgi network and extracellular secretion mechanisms. To determine whether ACBP is secreted unconventionally, astrocytes were incubated with brefeldin A, a potent inhibitor of classical secretion machinery. Preincubation of the astrocytes with 10 μg/ml of brefeldin A failed to inhibit the production of endozepines upon stimulation by cortisol or KCl (Fig. 2 and Table 2). In Dictyostelium, we have shown that AcbA is secreted by a novel vesicular mechanism (13, 15). It has been suggested that autophagy might be involved in the formation of such secretory vesicles (37). To test that hypothesis, we added rapamycin, an inducer of autophagy, to astrocytes and found that it induces the production of endozepines within 30 min (Fig. 5). 3-Methyladenine has been shown to be a potent autophagy inhibitor for animal cells, including astrocytes (38). Addition of 3-methyladenine prior to induction dramatically reduces the production of endozepine upon rapamycin or steroid induction (Table 2). Not surprisingly, the induction of endozepine production by rapamycin is not affected by brefeldin A, pertussis toxin, or U73122. On the other hand, mPKI and Akt inhibitor IV were found to block endozepine induction by rapamycin and the ERK 1/2 inhibitor PD98059 was found to reduce the amount of endozepine released by 90% (Table 2). It appears that rapamycin induction of autophagy and endozepine production involves PKA, AKT, and ERK protein kinases in some way.
FIGURE 5.
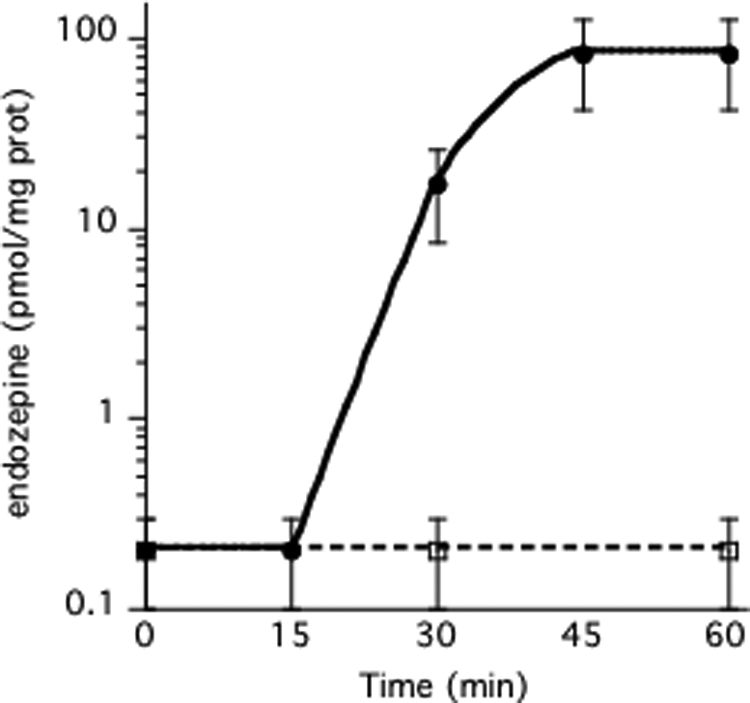
Induction of endozepine production by rapamycin. 2 μg/μl of rapamycin was added to astrocytes incubated at 37 °C in the absence (filled circles) or presence (open squares) of anti-ACBP antibodies. At the indicated time, 400 μl of buffer was harvested to quantify the amount of endozepines using the Dictyostelium bioassay. Each experiment was repeated at least three times. The bars indicate the reliability range.
DISCUSSION
Most proteins that are secreted have an N-terminal signal sequence that targets them to the ER/Golgi pathway (39). It is often assumed that proteins lacking such a signal will not be secreted. However, exceptions to this rule have been found in increasing number and recent proteomic studies of animal cells suggest that 16% of the secreted proteins lack a signal sequence (40). Surprisingly, over half of the proteins secreted by Leishmania braziliensis follow non-classical secretion mechanisms (41). Over 20 mammalian proteins have now been well characterized as being secreted through one of the multiple unconventional secretion mechanisms (37). A proteomic study showed that ACBP is one of many proteins secreted from brefeldin A-treated astrocytes indicating that secretion occurred through an unconventional mechanism (40). The protein was detected when astrocytes were incubated for 18 h without any specific induction. Our results not only confirm that ACBP is secreted through a brefeldin A-insensitive unconventional pathway but avoid long term nonspecific effects of the drug by rapid induction of secretion by KCl or steroids. The mechanism of ACBP secretion was not previously confronted because it was assumed that it was processed to peptides inside the cells and that these peptides could pass through the cell membrane (8). We have recently shown in the yeast S. cerevisiae that acyl-CoA-binding protein (ACB1) is secreted in response to nitrogen starvation (14). Yeast mutants lacking components of the classical ER/Golgi secretion pathway were found to release ACB1 normally, but mutants lacking autophagy genes failed to secrete ACB1. Autophagy genes were also shown to be essential for release of ACB1 from the distantly related yeast P. pastoris where the protein is processed into peptides that are necessary for sporulation (16). In Dictyostelium, AcbA is found in vesicles that accumulate below the plasma membrane just prior to secretion (13). This vesicular accumulation does not occur in mutants lacking key autophagy proteins. The autophagy inducer rapamycin triggers production of endozepine-like activity in P. pastoris (16), Dictyostelium (13), and astrocytes, suggesting that autophagy dependence is conserved.
Processing of AcbA in Dictyostelium and yeast has been directly shown to occur by cell-associated protease following secretion (14, 16, 17). We have now shown that this is also the case for ACBP in astrocytes (Fig. 4). ACBP thus appears to undergo unconventional processing by extracellular trypsin-like protease activity that is exposed following induction. In fact, added trypsin can convert recombinant ACBP into active endozepine (Fig. 1) and AcbA into SDF-2 (17). Human ACBP protein can also be secreted and converted into active peptides by Dictyostelium cells (Table 2 and supplemental Figs. S1), whereas astrocytes can convert AcbA into SDF-2. The protease activities thus appear to be of relatively low specificity because they can convert substrates that share only 55% identity at the amino acid level. This mechanism for secretion of ACBP and processing into endozepine probably occurs in all mammalian astrocytes because we obtained identical results when using mouse or rat astrocytes. The secretion and processing of ACBP into endozepines in the extracellular environment by Dictyostelium, yeasts, and mammals shows a surprising conservation in organisms that diverged over 1 billion years ago.
The induction of endozepine production by cortisol and progesterone sulfate is a fast non-genomic response. Although cortisol is not produced in the central nervous system, it is a potent neuroactive steroid that can modify neurotransmitter release through binding to the membranes receptors (19). Acute cortisol effects include increased neuronal excitability through modulation of ion channels such as voltage-gated calcium and potassium channels, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor and N-methyl-d-aspartate (NMDA) receptors as well as through modulation of neurotransmitter release (19, 42). The increased neuronal excitability seems to occur through binding of cortisol to the high affinity membrane-bound mineralocorticoid receptor, whereas binding to the lower affinity membrane glucocorticoid receptors reduces neuronal excitability. The low nanomolar concentration of cortisol needed to trigger the release and processing of ACBP from astrocytes suggests that mineralocorticoid receptors may be involved. However, the lack of induction by aldosterone, which has the highest affinity for mineralocorticoid receptors, argues against it. The relatively high concentration of pregnenolone and progesterone are required to induce endozepine production and it is possible that they act only after being metabolized into pregnenolone sulfate by astrocytes. The lack of response to metabolic intermediates between pregnenolone and cortisol suggest that different receptors are used. This, together with the absence of inhibition by pertussis toxin, suggests that these receptors are not GPCRs either, but could belong to the recently characterized PAQR or PGRMC families of steroid receptors (43, 44).
Pregnenolone sulfate is produced in the central nervous system and plays major roles in the modulation of neurotransmitter receptors as well as neurotransmitter release (reviewed in Ref. 20). Pregnenolone sulfate can inhibit GABAA receptors (45), increase glutamate release (46), and decrease GABA release (23, 24). Endozepines are also known to decrease the affinity of GABAA receptors for GABA. Physiological increases of pregnenolone sulfate or cortisol will promote the glial-neuronal cross-talk through release of endozepine and affect neurotransmitter release, resulting into a net increase in central nervous system excitability as observed, for example, in conditions of acute stress (19, 42).
PKA appears to play a central role in the induction of endozepine production because its inhibition prevents induction by KCl, steroids, or rapamycin. PKA was also shown to be required for production of ODN, one of the endozepines, in response to the pituitary adenylate cyclase-activating polypeptide (47). On the other hand, the Akt and ERK 1/2 protein kinases appear to be required only for the response to rapamycin. These protein kinases are frequently involved in the same signaling pathways as the target of rapamycin (reviewed in Ref. 48). However, the interactions between these components are complex and a more detailed study will be required to understand this signaling pathway. It is likely that induction by pituitary adenylate cyclase-activating polypeptide, KCl, rapamycin, and steroids converge at the level of PKA regulation.
Supplementary Material
Acknowledgment
The plasmid with human ACBP cDNA was kindly provided by Dr. Inke Nitz (University of Kiel, Germany).
This work was supported, in whole or in part, by National Institutes of Health Grant GM78175.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1 and S2.
- ACBP
- acyl-CoA-binding protein
- AcbA
- acyl-CoA-binding protein A
- GABA
- γ-aminobutyric acid
- TPCK
- l-1-tosylamido-2-phenylethyl chloromethyl ketone
- GFP
- green fluorescent protein
- MES
- 4-morpholineethanesulfonic acid
- PKI
- protein kinase A inhibitor
- PKA
- protein kinase A
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Faergeman N. J., Wadum M., Feddersen S., Burton M., Kragelund B. B., Knudsen J. (2007) Mol. Cell. Biochem. 299, 55–65 [DOI] [PubMed] [Google Scholar]
- 2.Knudsen J., Mandrup S., Rasmussen J. T., Andreasen P. H., Poulsen F., Kristiansen K. (1993) Mol. Cell. Biochem. 123, 129–138 [DOI] [PubMed] [Google Scholar]
- 3.Ferrero P., Santi M. R., Conti-Tronconi B., Costa E., Guidotti A. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 827–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarneri P., Berkovich A., Guidotti A., Costa E. (1990) Neuropharmacology 29, 419–428 [DOI] [PubMed] [Google Scholar]
- 5.Slobodyansky E., Guidotti A., Wambebe C., Berkovich A., Costa E. (1989) J. Neurochem. 53, 1276–1284 [DOI] [PubMed] [Google Scholar]
- 6.Berkovich A., McPhie P., Campagnone M., Guidotti A., Hensley P. (1990) Mol. Pharmacol. 37, 164–172 [PubMed] [Google Scholar]
- 7.Do-Rego J. L., Mensah-Nyagan A. G., Beaujean D., Leprince J., Tonon M. C., Luu-The V., Pelletier G., Vaudry H. (2001) J. Neurochem. 76, 128–138 [DOI] [PubMed] [Google Scholar]
- 8.Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 3531–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos V., Berkovich A., Krueger K. E. (1991) Neuropharmacology 30, 1417–1423 [DOI] [PubMed] [Google Scholar]
- 10.Masmoudi O., Gandolfo P., Tokay T., Leprince J., Ravni A., Vaudry H., Tonon M. C. (2005) J. Neurochem. 94, 561–571 [DOI] [PubMed] [Google Scholar]
- 11.Tonon M. C., Désy L., Nicolas P., Vaudry H., Pelletier G. (1990) Neuropeptides 15, 17–24 [DOI] [PubMed] [Google Scholar]
- 12.Yanase H., Shimizu H., Yamada K., Iwanaga T. (2002) Arch. Histol. Cytol. 65, 27–36 [DOI] [PubMed] [Google Scholar]
- 13.Cabral M., Anjard C., Malhotra V., Fuller D., Loomis W., Kuspa A. (2010) Eukaryotic Cell, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duran J. M., Anjard C., Stefan C., Loomis W. F., Malhotra V. (2010) J. Cell Biol. 188, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinseth M. A., Anjard C., Fuller D., Guizzunti G., Loomis W. F., Malhotra V. (2007) Cell 130, 524–534 [DOI] [PubMed] [Google Scholar]
- 16.Manjithaya R., Anjard C., Loomis W. F., Subramani S. (2010) J. Cell Biol. 188, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anjard C., Loomis W. F. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 7607–7611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Ovejero D., Azcoitia I., Doncarlos L. L., Melcangi R. C., Garcia-Segura L. M. (2005) Brain Res. Rev. 48, 273–286 [DOI] [PubMed] [Google Scholar]
- 19.Prager E. M., Johnson L. R. (2009) Sci. Signal. 2, re5. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs T. T., Russek S. J., Farb D. H. (2006) Pharmacol. Biochem. Behav. 84, 555–567 [DOI] [PubMed] [Google Scholar]
- 21.Zheng P. (2009) Prog. Neurobiol. 89, 134–152 [DOI] [PubMed] [Google Scholar]
- 22.Zinder O., Dar D. E. (1999) Acta Physiol. Scand. 167, 181–188 [DOI] [PubMed] [Google Scholar]
- 23.Mtchedlishvili Z., Kapur J. (2003) Mol. Pharmacol. 64, 857–864 [DOI] [PubMed] [Google Scholar]
- 24.Teschemacher A., Kasparov S., Kravitz E. A., Rahamimoff R. (1997) Brain Res. 772, 226–232 [DOI] [PubMed] [Google Scholar]
- 25.Kinney J. W., Davis C. N., Tabarean I., Conti B., Bartfai T., Behrens M. M. (2006) J. Neurosci. 26, 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sussman M. (1987) Methods Cell Biol. 28, 9–29 [DOI] [PubMed] [Google Scholar]
- 27.Levi S., Polyakov M., Egelhoff T. T. (2000) Plasmid 44, 231–238 [DOI] [PubMed] [Google Scholar]
- 28.Nitz I., Döring F., Schrezenmeir J., Burwinkel B. (2005) Int. J. Biochem. Cell Biol. 37, 2395–2405 [DOI] [PubMed] [Google Scholar]
- 29.Knecht D. A., Loomis W. F. (1987) Science 236, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 30.Anjard C., Pinaud S., Kay R. R., Reymond C. D. (1992) Development 115, 785–790 [DOI] [PubMed] [Google Scholar]
- 31.Anjard C., Zeng C., Loomis W. F., Nellen W. (1998) Dev. Biol. 193, 146–155 [DOI] [PubMed] [Google Scholar]
- 32.Anjard C., Loomis W. F. (2008) Development 135, 819–827 [DOI] [PubMed] [Google Scholar]
- 33.Qian Z., Bilderback T. R., Barmack N. H. (2008) J. Neurochem. 105, 1287–1299 [DOI] [PubMed] [Google Scholar]
- 34.Anjard C., Loomis W. F. (2006) Development 133, 2253–2261 [DOI] [PubMed] [Google Scholar]
- 35.Wang N., Söderbom F., Anjard C., Shaulsky G., Loomis W. F. (1999) Mol. Cell. Biol. 19, 4750–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjard C., Su Y., Loomis W. F. (2009) Development 136, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickel W., Rabouille C. (2009) Nat. Rev. Mol. Cell Biol. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 38.Hwang J., Lee S., Lee J. T., Kwon T. K., Kim D. R., Kim H., Park H. C., Suk K. (2010) Br. J. Pharmacol. 159, 586–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schatz G., Dobberstein B. (1996) Science 271, 1519–1526 [DOI] [PubMed] [Google Scholar]
- 40.Lafon-Cazal M., Adjali O., Galéotti N., Poncet J., Jouin P., Homburger V., Bockaert J., Marin P. (2003) J. Biol. Chem. 278, 24438–24448 [DOI] [PubMed] [Google Scholar]
- 41.Cuervo P., De Jesus J. B., Saboia-Vahia L., Mendonça-Lima L., Domont G. B., Cupolillo E. (2009) J. Proteomics 73, 79–92 [DOI] [PubMed] [Google Scholar]
- 42.Yuen E. Y., Liu W., Karatsoreos I. N., Feng J., McEwen B. S., Yan Z. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14075–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinton R. D., Thompson R. F., Foy M. R., Baudry M., Wang J., Finch C. E., Morgan T. E., Pike C. J., Mack W. J., Stanczyk F. Z., Nilsen J. (2008) Front. Neuroendocrinol. 29, 313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith J., Kupchak B., Garitaonandia I., Hoang L., Maina A., Regalla L., Lyons T. (2008) Steroids 73, 1060–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majewska M. D., Mienville J. M., Vicini S. (1988) Neurosci. Lett. 90, 279–284 [DOI] [PubMed] [Google Scholar]
- 46.Dong Y., Fu Y. M., Sun J. L., Zhu Y. H., Sun F. Y., Zheng P. (2005) Cell. Mol. Life Sci. 62, 1003–1014 [DOI] [PubMed] [Google Scholar]
- 47.Masmoudi O., Gandolfo P., Leprince J., Vaudry D., Fournier A., Patte-Mensah C., Vaudry H., Tonon M. C. (2003) FASEB J. 17, 17–27 [DOI] [PubMed] [Google Scholar]
- 48.Memmott R. M., Dennis P. A. (2009) Cell. Signal. 21, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.