Abstract
Angiotensin II (Ang II) stimulates thick ascending limb (TAL) O production, but the receptor(s) and signaling mechanism(s) involved are unknown. The effect of Ang II on O
production, but the receptor(s) and signaling mechanism(s) involved are unknown. The effect of Ang II on O is generally attributed to the AT1 receptor. In some cells, Ang II stimulates protein kinase C (PKC), whose α isoform (PKCα) can activate NADPH oxidase. We hypothesized that in TALs, Ang II stimulates O
is generally attributed to the AT1 receptor. In some cells, Ang II stimulates protein kinase C (PKC), whose α isoform (PKCα) can activate NADPH oxidase. We hypothesized that in TALs, Ang II stimulates O via AT1 and PKCα-dependent NADPH oxidase activation. In rat TALs, 1 nm Ang II stimulated O
via AT1 and PKCα-dependent NADPH oxidase activation. In rat TALs, 1 nm Ang II stimulated O from 0.76 ± 0.17 to 1.97 ± 0.21 nmol/min/mg (p < 0.001). An AT1 antagonist blocked the stimulatory effect of Ang II on O
from 0.76 ± 0.17 to 1.97 ± 0.21 nmol/min/mg (p < 0.001). An AT1 antagonist blocked the stimulatory effect of Ang II on O (0.87 ± 0.25 nmol/min/mg; p < 0.006), whereas an AT2 antagonist had no effect (2.16 ± 0.133 nmol/min/mg; p < 0.05 versus vehicle). Apocynin, an NADPH oxidase inhibitor, blocked Ang II-stimulated O
(0.87 ± 0.25 nmol/min/mg; p < 0.006), whereas an AT2 antagonist had no effect (2.16 ± 0.133 nmol/min/mg; p < 0.05 versus vehicle). Apocynin, an NADPH oxidase inhibitor, blocked Ang II-stimulated O by 90% (p < 0.01). Ang II failed to stimulate O
by 90% (p < 0.01). Ang II failed to stimulate O in TALs from p47phox−/− mice (p < 0.02). Monitored by fluorescence resonance energy transfer, Ang II increased PKC activity from 0.02 ± 0.03 to 0.13 ± 0.02 arbitrary units (p < 0.03). A general PKC inhibitor, GF109203X, blocked the effect of Ang II on O
in TALs from p47phox−/− mice (p < 0.02). Monitored by fluorescence resonance energy transfer, Ang II increased PKC activity from 0.02 ± 0.03 to 0.13 ± 0.02 arbitrary units (p < 0.03). A general PKC inhibitor, GF109203X, blocked the effect of Ang II on O (1.47 ± 0.21 versus 2.72 ± 0.47 nmol/min/mg with Ang II alone; p < 0.03). A PKCα- and β-selective inhibitor, Gö6976, also blocked the stimulatory effect of Ang II on O
(1.47 ± 0.21 versus 2.72 ± 0.47 nmol/min/mg with Ang II alone; p < 0.03). A PKCα- and β-selective inhibitor, Gö6976, also blocked the stimulatory effect of Ang II on O (0.59 ± 0.15 versus 2.05 ± 0.28 nmol/min/mg with Ang II alone; p < 0.001). To distinguish between PKCα and PKCβ, we used tubules expressing dominant-negative PKCα or -β. In control TALs, Ang II stimulated O
(0.59 ± 0.15 versus 2.05 ± 0.28 nmol/min/mg with Ang II alone; p < 0.001). To distinguish between PKCα and PKCβ, we used tubules expressing dominant-negative PKCα or -β. In control TALs, Ang II stimulated O by 2.17 ± 0.44 nmol/min/mg (p < 0.011). In tubules expressing dominant-negative PKCα, Ang II failed to stimulate O
by 2.17 ± 0.44 nmol/min/mg (p < 0.011). In tubules expressing dominant-negative PKCα, Ang II failed to stimulate O (change: −0.30 ± 0.27 nmol/min/mg). In tubules expressing dominant-negative PKCβ1, Ang II stimulated O
(change: −0.30 ± 0.27 nmol/min/mg). In tubules expressing dominant-negative PKCβ1, Ang II stimulated O by 2.08 ± 0.69 nmol/min/mg (p < 0.002). We conclude that Ang II stimulates TAL O
by 2.08 ± 0.69 nmol/min/mg (p < 0.002). We conclude that Ang II stimulates TAL O production via activation of AT1 receptors and PKCα-dependent NADPH oxidase.
production via activation of AT1 receptors and PKCα-dependent NADPH oxidase.
Keywords: Kidney, Protein Kinase C (PKC), Reactive Oxygen Species (ROS), Receptors, Superoxide Ion, Angiotensin, NADPH, Dominant Negative
Introduction
The reactive oxygen species superoxide (O ) plays an important role in the regulation of kidney function (1–3). O
) plays an important role in the regulation of kidney function (1–3). O decreases renal blood flow by constricting renal vessels (4), reduces glomerular filtration rate by enhancing tubuloglomerular feedback (5) and also promotes salt reabsorption along the nephron (6, 7). Excessive O
decreases renal blood flow by constricting renal vessels (4), reduces glomerular filtration rate by enhancing tubuloglomerular feedback (5) and also promotes salt reabsorption along the nephron (6, 7). Excessive O generation within the kidneys contributes to the development of hypertension (8), renal damage (9, 10), and atherosclerosis (11, 12). Thus clarifying the mechanisms that regulate O
generation within the kidneys contributes to the development of hypertension (8), renal damage (9, 10), and atherosclerosis (11, 12). Thus clarifying the mechanisms that regulate O production within the kidney may help us understand the etiology and pathophysiology of many diseases and develop new targets for treatment.
production within the kidney may help us understand the etiology and pathophysiology of many diseases and develop new targets for treatment.
O can be generated by several types of cells within the kidney (13, 14); however, it is primarily produced by the medullary thick ascending limb of the loop of Henle (TAL)2 (14). In the TAL, O
can be generated by several types of cells within the kidney (13, 14); however, it is primarily produced by the medullary thick ascending limb of the loop of Henle (TAL)2 (14). In the TAL, O production can be stimulated by several factors, including Ang II (15, 16). Ang II can activate two types of receptors: AT1 and AT2. Activation of AT1 is associated with the salt-retaining and pro-hypertensive actions of Ang II (17, 18). In the TAL, Ang II acutely stimulates O
production can be stimulated by several factors, including Ang II (15, 16). Ang II can activate two types of receptors: AT1 and AT2. Activation of AT1 is associated with the salt-retaining and pro-hypertensive actions of Ang II (17, 18). In the TAL, Ang II acutely stimulates O production (15), but neither the receptor nor the signaling cascade involved has been identified.
production (15), but neither the receptor nor the signaling cascade involved has been identified.
O can be produced by NADPH oxidase, xanthine oxidase, and the mitochondria (19). In the absence of Ang II stimulation, NADPH oxidase appears to be the main source in the renal medulla (20), particularly the TAL (14, 21, 22); however, the source of O
can be produced by NADPH oxidase, xanthine oxidase, and the mitochondria (19). In the absence of Ang II stimulation, NADPH oxidase appears to be the main source in the renal medulla (20), particularly the TAL (14, 21, 22); however, the source of O in the TAL during Ang II stimulation is still unknown.
in the TAL during Ang II stimulation is still unknown.
In many types of cells, including TAL cells, activation of protein kinase C (PKC) has been shown to stimulate O production in response to different stimuli, including Ang II (23, 24, 25). The PKC family of serine/threonine kinases is composed of many isoforms, some of which are expressed in the TAL, including PKCα, -β, -δ, -ϵ, and -ξ (26, 27). Yang et al. have shown that in the TAL PKCα mediates the enhanced O
production in response to different stimuli, including Ang II (23, 24, 25). The PKC family of serine/threonine kinases is composed of many isoforms, some of which are expressed in the TAL, including PKCα, -β, -δ, -ϵ, and -ξ (26, 27). Yang et al. have shown that in the TAL PKCα mediates the enhanced O levels observed during diabetes (24). However, to our knowledge there have been no studies investigating whether PKC mediates the stimulatory effect of Ang II on TAL O
levels observed during diabetes (24). However, to our knowledge there have been no studies investigating whether PKC mediates the stimulatory effect of Ang II on TAL O production or the isoform(s) involved. We hypothesized that Ang II binds to the AT1 receptors, activating PKCα, which in turn stimulates NADPH oxidase activity, enhancing O
production or the isoform(s) involved. We hypothesized that Ang II binds to the AT1 receptors, activating PKCα, which in turn stimulates NADPH oxidase activity, enhancing O production by the TAL.
production by the TAL.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats (Charles River, Kalamazoo, MI) were fed a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) for at least 7 days. Wild-type and p47phox knock-out mice (Jackson Laboratories, Bar Harbor, ME), were fed regular chow for at least 7 days. On the day of the experiment, animals were anesthetized with ketamine (100 mg/kg body weight, intraperitoneally) and xylazine (20 mg/kg body weight, intraperitoneally). All protocols were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Medullary TAL Suspensions
TAL suspensions were obtained from rats weighing 150–220 g as described previously (28). This procedure yields a suspension of TALs that is >90% pure (29), so that contamination by other types of cells in our preparation was minimal or absent.
Measurement of O Production
Production
200-μl aliquots of rat TAL suspensions were placed in glass tubes, and HEPES-buffered physiological saline (130 mm NaCl, 2.5 mm NaH2PO4, 4 mm KCl, 1.2 mm MgSO4, 6 mm alanine, 1 mm Na3 citrate, 5.5 mm glucose, 2 mm Ca2+(lactate)2, and 10 mm HEPES (pH 7.4)) was added for a final volume of 1 ml. The whole suspension was used when TALs were obtained from mice. N,N′-Dimethyl-9,9′-biacridinium dinitrate (Lucigenin, Sigma-Aldrich) was added to the suspensions to give a final concentration of 5 μm. When investigating the effects of apocynin (4-hydroxy-3-methoxyacetophenone, 10 μm, Sigma-Aldrich), GF109203X (100 nm, Enzo Life Sciences, Plymouth Meeting, PA), or Gö6976 (100 nm, Enzo Life Sciences), these were added to the tube, and the volume of physiological saline was adjusted accordingly. When using the Ang II receptor antagonists PD123319 (1 μm, Parke-Davis, Ann Arbor, MI) and losartan (1 μm, Merck, Rahway, NJ), these were added to the tubules 5 min before Ang II (1 nm, Bachem, Torrance, CA). Tubules were incubated for 10 min at 37 °C and then placed in a luminometer (model FB12/Sirius, Zylux Co., Oak Ridge, TN) and maintained at 37 °C. The average of the last 3 of 10 consecutive measurements was calculated for each sample. The O scavenger 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron, Sigma-Aldrich) was added to the tube to give a final concentration of 10 mm, and measurements were repeated. The difference in average luminescence between samples with and without Tiron was used to calculate the luminescence produced by O
scavenger 4,5-dihydroxy-1,3-benzenedisulfonic acid (Tiron, Sigma-Aldrich) was added to the tube to give a final concentration of 10 mm, and measurements were repeated. The difference in average luminescence between samples with and without Tiron was used to calculate the luminescence produced by O . Measurements were normalized for protein content.
. Measurements were normalized for protein content.
Protein Content Determination
Total protein content was measured using Coomassie Plus reagent (Pierce), based on Bradford's colorimetric method.
PKC Reporter
PKC activity was measured using a fluorescence resonance energy transfer (FRET)-based PKC reporter, CKAR (generously provided by Dr. Alexandra C. Newton, Howard Hughes Medical Institute, University of California-San Diego) (30). This probe consists of the consensus sequence for PKC phosphorylation with cyan fluorescence protein (CFP) and yellow fluorescence protein (YFP) at either end. Under basal conditions CFP emission excites YFP due to its close proximity (FRET signal). Upon PKC activation, CKAR becomes phosphorylated and the probe “opens,” resulting in an increased CFP signal and decreased FRET. Thus an increase in the CFP/YFP ratio indicates heightened PKC activity. The probe was subcloned into the adenoviral shuttle vector pVQ Ad5CMV K-NpA, which contains the CMV promoter, and sent to ViraQuest (North Liberty, IA) for viral production.
Dominant Negative PKC Isoforms
The HA (hemagglutinin)-tagged dominant negative PKCα and PKCβ1 plasmids (dn-PKCα and dn-PKCβ1) were kindly provided by Dr. Jae-Won Soh, Biomedical Research Center for Signal Transduction Networks, Incheon, Korea. The dominant negatives consist of kinase-dead PKC isoforms, generated by single amino acid substitution within the kinase domain. Sequences encoding for both proteins were subcloned into a shuttle vector containing the CMV promoter. Plasmids were sent to ViraQuest for adenoviral production.
In Vivo Gene Delivery of CKAR and dn-PKC Isoforms
TALs were transduced in vivo with recombinant replication-deficient adenoviruses expressing the dn-PKCα, dn-PKCβ1, or CKAR sequence as we reported previously (31, 32). Briefly, kidneys of a 95- to 105-g rat were exposed via a flank incision, and the renal artery and vein were clamped. Four 20-μl virus injections (1 × 1012 particles/ml) were made along the longitudinal axis at a flow rate of 20 μl/min. The renal vessels were unclamped; kidneys were returned to the abdominal cavity, the muscle incision was sutured, and the skin was clipped. Because we previously found that maximum expression occurred 3–5 days after injection of the adenovirus (32, 33), all experiments were performed within these time points. Expression of the dominant negatives was confirmed by Western blots.
Expression of dn-PKCα and -β
Western blots were performed as routinely done in our laboratory (28, 29). Briefly, 40 μg of TAL suspension homogenates was loaded onto an 8% polyacrylamide gel, and electrophoresis was performed for 2 h at 92 mV. After an overnight transfer, the polyvinylidene difluoride membrane was blocked in a buffer containing 20 mm Tris, 137 mm NaCl, 5% nonfat dried milk, and 0.1% Tween 20 (TBS-T) and 5% milk for 1 h at room temperature and then incubated with either a 1:1,000 dilution of a mouse monoclonal anti-HA antibody (Abgent, San Diego, CA), 1:1,000 dilution of a mouse anti-PKCα antibody (BD Biosciences, San Jose, CA) or a 1:250 dilution of a mouse anti-PKCβ antibody (BD Biosciences) for 1 h at room temperature. The membrane was washed using TBS-T and incubated for another hour with a 1:1,000 dilution of the appropriate IgG conjugated to horseradish peroxidase (Amersham Biosciences) for 1 h at room temperature. The reaction products were detected using a chemiluminescence kit (Amersham Biosciences) and by exposure to Fuji RX film.
Measurements of PKC Activity by FRET
On the day of the experiment, TAL suspensions were obtained from CKAR-transduced kidneys as indicated above and 1/5 of the suspension was seeded in a temperature-controlled chamber and warmed to 37 °C. The flow rate of the bath was 0.3 ml/min. During the 30-min equilibration period, images were acquired (100× oil objective, numerical aperture: 1.3) by alternately exciting CFP (442 nm) and YFP (514 nm) and monitoring YFP emission at 540 nm to determine expression of the FRET sensor and highlight regions of interest. During the control period, CFP/YFP emission ratios were measured by exciting CFP at 442 nm once a minute for 5 min and simultaneously monitoring CFP and YFP emissions at 440–480 (CFP) and 540–545 nm (YFP). At the end of the control period, Ang II was added to the bath and CFP/YFP monitored once every minute for 15 min. The averages corresponding to the 5-min control period and the last 5 min of the experimental period were compared. To confirm that the YFP signal was due to FRET, control experiments were performed by photobleaching CFP and measuring the decrease in YFP emission. Images were acquired using the same settings (laser intensity, detector gain and offset, resolution, and exposure time).
Statistics
All statistical analyses were performed by the Biostatistics Department of Henry Ford Hospital. Results are expressed as mean ± S.E. Data were analyzed using Student's t-tests. A version designed for unequal standard deviations was used when necessary. Some comparisons were studied using contrast statements. When multiple testing was involved, Hochberg's method was used.
RESULTS
We first investigated the effect of Ang II on TAL O production. When rat TAL suspensions were incubated for 10 min in vehicle (0.005% acetic acid in water), O
production. When rat TAL suspensions were incubated for 10 min in vehicle (0.005% acetic acid in water), O production was 0.76 ± 0.17 nmol/min/mg. However, in TALs treated with Ang II (1 nm for 10 min) it increased to 1.97 ± 0.21 nmol/min/mg (p < 0.001; n = 11), 159% stimulation (Fig. 1). These data suggested that Ang II stimulates O
production was 0.76 ± 0.17 nmol/min/mg. However, in TALs treated with Ang II (1 nm for 10 min) it increased to 1.97 ± 0.21 nmol/min/mg (p < 0.001; n = 11), 159% stimulation (Fig. 1). These data suggested that Ang II stimulates O production by TALs.
production by TALs.
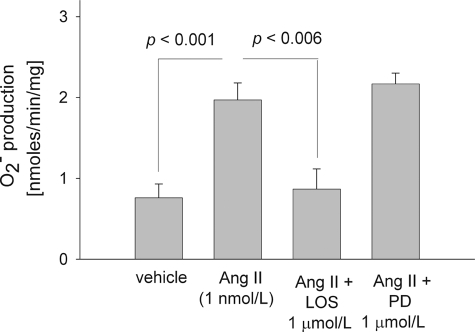
FIGURE 1.
Effect of 1 nm angiotensin II for 10 min on rat thick ascending limb O production in the absence and presence of 1 μm losartan (an AT1 receptor blocker) or 1 μm PD123319 (an AT2 receptor blocker). Ang II, angiotensin II; LOS, losartan; PD, PD123319. n = 11 for Ang II, 5 for vehicle and 6 for Ang II plus LOS and Ang II plus PD.
production in the absence and presence of 1 μm losartan (an AT1 receptor blocker) or 1 μm PD123319 (an AT2 receptor blocker). Ang II, angiotensin II; LOS, losartan; PD, PD123319. n = 11 for Ang II, 5 for vehicle and 6 for Ang II plus LOS and Ang II plus PD.
To investigate which angiotensin receptor mediates the effect of Ang II on O , we used pharmacological inhibitors of AT1 and AT2. In the presence of losartan, an AT1 receptor antagonist (1 μm), Ang II (1 nm) failed to stimulate O
, we used pharmacological inhibitors of AT1 and AT2. In the presence of losartan, an AT1 receptor antagonist (1 μm), Ang II (1 nm) failed to stimulate O production (0.87 ± 0.25 nmol/min/mg; p < 0.006 versus Ang II alone; n = 6) by rat TALs. However, when we used PD 123319, an AT2 receptor antagonist (1 μm), Ang II raised O
production (0.87 ± 0.25 nmol/min/mg; p < 0.006 versus Ang II alone; n = 6) by rat TALs. However, when we used PD 123319, an AT2 receptor antagonist (1 μm), Ang II raised O production to 2.16 ± 0.13 nmol/min/mg (p < 0.05 versus vehicle; n = 5) (Fig. 1). In different sets of experiments, neither losartan nor PD123319 changed basal O
production to 2.16 ± 0.13 nmol/min/mg (p < 0.05 versus vehicle; n = 5) (Fig. 1). In different sets of experiments, neither losartan nor PD123319 changed basal O levels (0.78 ± 0.38 nmol/min/mg for baseline versus 0.80 ± 0.06 nmol/min/mg for losartan alone; n = 3 and 1.04 ± 0.09 nmol/min/mg for baseline versus 1.05 ± 0.12 nmol/min/mg for PD123319 alone; n = 3). These data indicated that Ang II binds the AT1 receptor to stimulate O
levels (0.78 ± 0.38 nmol/min/mg for baseline versus 0.80 ± 0.06 nmol/min/mg for losartan alone; n = 3 and 1.04 ± 0.09 nmol/min/mg for baseline versus 1.05 ± 0.12 nmol/min/mg for PD123319 alone; n = 3). These data indicated that Ang II binds the AT1 receptor to stimulate O production by TALs.
production by TALs.
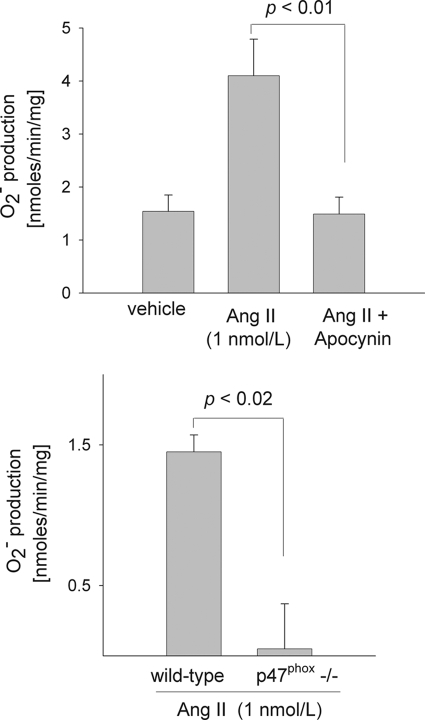
Next we tested whether NADPH oxidase is the source of Ang II-stimulated O in TALs using the NADPH oxidase inhibitor apocynin and TALs isolated from p47phox knock-out (−/−) mice. When rat TAL suspensions were incubated with vehicle (0.005% acetic acid), O
in TALs using the NADPH oxidase inhibitor apocynin and TALs isolated from p47phox knock-out (−/−) mice. When rat TAL suspensions were incubated with vehicle (0.005% acetic acid), O production was 1.54 ± 0.31nmol/min/mg. With Ang II (1 nm for 10 min) it increased to 4.10 ± 0.69 nmol/min/mg (p < 0.001 versus vehicle; n = 4). However, when we added apocynin (10 μm), Ang II failed to stimulate O
production was 1.54 ± 0.31nmol/min/mg. With Ang II (1 nm for 10 min) it increased to 4.10 ± 0.69 nmol/min/mg (p < 0.001 versus vehicle; n = 4). However, when we added apocynin (10 μm), Ang II failed to stimulate O production (1.49 ± 0.32 nmol/min/mg; p < 0.01 versus Ang II alone; n = 5) (Fig. 2A). In a different set of experiments, apocynin alone significantly reduced basal O
production (1.49 ± 0.32 nmol/min/mg; p < 0.01 versus Ang II alone; n = 5) (Fig. 2A). In a different set of experiments, apocynin alone significantly reduced basal O production by 80% (p < 0.005; n = 5). To make sure the effect of apocynin was due to specific inhibition of NADPH oxidase, we performed experiments using tubules isolated from p47phox−/− mice. In the presence of Ang II, O
production by 80% (p < 0.005; n = 5). To make sure the effect of apocynin was due to specific inhibition of NADPH oxidase, we performed experiments using tubules isolated from p47phox−/− mice. In the presence of Ang II, O production was 1.45 ± 0.12 nmol/min/mg in wild-type controls but undetectable in TALs from p47phox−/− (0.00 ± 0.32 nmol/min/mg) (p < 0.02; n = 4 for each group) (Fig. 2B). These data indicate that NADPH oxidase is the primary source of O
production was 1.45 ± 0.12 nmol/min/mg in wild-type controls but undetectable in TALs from p47phox−/− (0.00 ± 0.32 nmol/min/mg) (p < 0.02; n = 4 for each group) (Fig. 2B). These data indicate that NADPH oxidase is the primary source of O in the TAL under both basal and Ang II-stimulated conditions.
in the TAL under both basal and Ang II-stimulated conditions.
FIGURE 2.
Top, effect of 10 μm apocynin (an NADPH oxidase inhibitor) on the stimulatory effect of angiotensin II on O production by rat thick ascending limbs. Rat thick ascending limbs were incubated with 1 nm angiotensin II for 10 min in the presence or absence of apocynin and superoxide production measured. n = 5 per group. Bottom, effect of 1 nm angiotensin II for 10 min on O
production by rat thick ascending limbs. Rat thick ascending limbs were incubated with 1 nm angiotensin II for 10 min in the presence or absence of apocynin and superoxide production measured. n = 5 per group. Bottom, effect of 1 nm angiotensin II for 10 min on O production by thick ascending limbs isolated from wild-type and p47phox knock-out mice (p47phox−/−). n = 4. Ang II, angiotensin II.
production by thick ascending limbs isolated from wild-type and p47phox knock-out mice (p47phox−/−). n = 4. Ang II, angiotensin II.
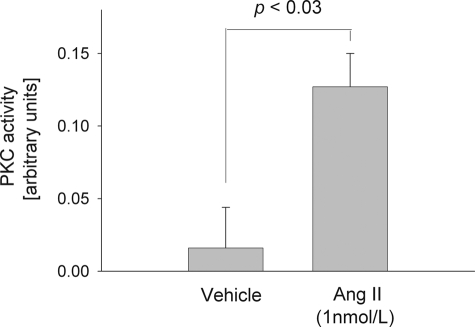
To test whether Ang II directly enhances PKC activity in the rat TAL, we measured the effect of Ang II on PKC activity using FRET. In tubules expressing CKAR and incubated with vehicle (0.005% acetic acid) the CFP/YFP ratio was 0.02 ± 0.03 arbitrary unit. Upon adding Ang II (1 nm) to the same tubules, CFP/YFP increased to 0.13 ± 0.02 arbitrary unit (p < 0.03; n = 6) (Fig. 3). These data suggested that Ang II activates PKC activity in the TAL.
FIGURE 3.
Acute effect of 1 nm Ang II on total PKC activity by FRET. Rat thick ascending limbs expressing a PKC activity reporter were used. n = 6.
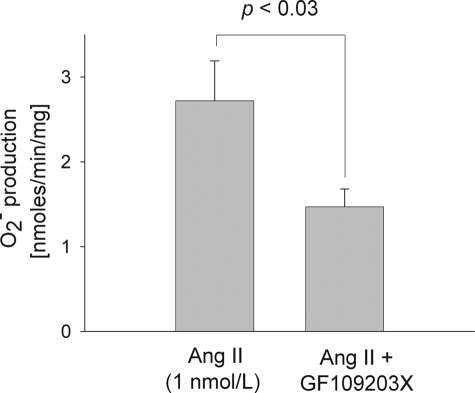
To determine whether activation of PKC is required for the stimulatory effect of Ang II on O production, we used a general PKC inhibitor, GF109203X. In rat TALs 1 nm Ang II stimulated O
production, we used a general PKC inhibitor, GF109203X. In rat TALs 1 nm Ang II stimulated O production to 2.72 ± 0.47 nmol/min/mg. However, with 100 nm GF109203X the effect of Ang II was significantly reduced (1.47 ± 0.21 nmol/min/mg; p < 0.03 versus Ang II alone; n = 6) (Fig. 4). In a different set of experiments, GF109203X did not change basal O
production to 2.72 ± 0.47 nmol/min/mg. However, with 100 nm GF109203X the effect of Ang II was significantly reduced (1.47 ± 0.21 nmol/min/mg; p < 0.03 versus Ang II alone; n = 6) (Fig. 4). In a different set of experiments, GF109203X did not change basal O production (1.13 ± 0.15 nmol/min/mg for baseline versus 0.83 ± 0.12 nmol/min/mg for GF109203X alone; n = 3). These data indicate that Ang II stimulates TAL O
production (1.13 ± 0.15 nmol/min/mg for baseline versus 0.83 ± 0.12 nmol/min/mg for GF109203X alone; n = 3). These data indicate that Ang II stimulates TAL O production by activating PKC.
production by activating PKC.
FIGURE 4.
Effect of 100 nm GF109203X (a general PKC inhibitor) on the stimulatory effect of 1 nm Ang II for 10 min on O production by rat thick ascending limbs. n = 5–6.
production by rat thick ascending limbs. n = 5–6.
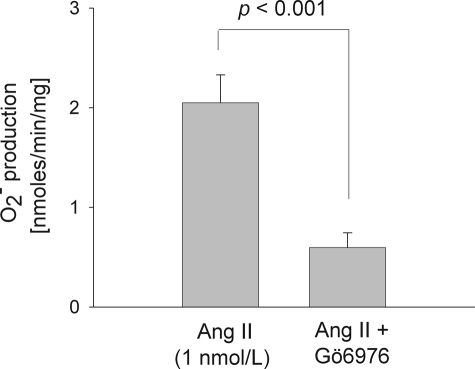
To find out which PKC isoform(s) mediates Ang II-stimulated O production, we used Gö6976, a PKCα- and β1-selective inhibitor. In rat TAL suspensions, 1 nm Ang II raised O
production, we used Gö6976, a PKCα- and β1-selective inhibitor. In rat TAL suspensions, 1 nm Ang II raised O production to 2.05 ± 0.28 nmol/min/mg. However, when we added Gö6976 (100 nm) to the preparation, Ang II failed to stimulate O
production to 2.05 ± 0.28 nmol/min/mg. However, when we added Gö6976 (100 nm) to the preparation, Ang II failed to stimulate O production (0.59 ± 0.15 nmol/min/mg; p < 0.001; n = 6) (Fig. 5). In a different set of experiments, Gö6976 did not change basal O
production (0.59 ± 0.15 nmol/min/mg; p < 0.001; n = 6) (Fig. 5). In a different set of experiments, Gö6976 did not change basal O production (1.17 ± 0.10 nmol/min/mg for baseline versus 1.10 ± 0.07 nmol/min/mg for Gö6976 alone; n = 3). These results suggested that Ang II stimulates TAL O
production (1.17 ± 0.10 nmol/min/mg for baseline versus 1.10 ± 0.07 nmol/min/mg for Gö6976 alone; n = 3). These results suggested that Ang II stimulates TAL O production by activating PKCα and/or PKCβ1.
production by activating PKCα and/or PKCβ1.
FIGURE 5.
Effect of 100 nm Gö6976 (a selective PKCα and β1 inhibitor) on the stimulatory effect of 1 nm Ang II for 10 min on O production by rat thick ascending limbs. n = 6.
production by rat thick ascending limbs. n = 6.
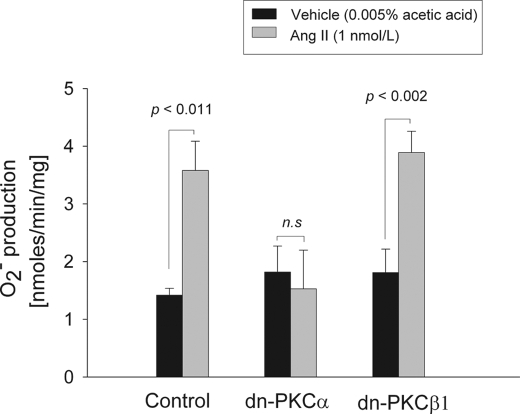
To clarify the PKC isoform(s) involved, we transduced rat TALs in vivo so that they expressed either control DNA, dominant negative PKCα (dn-PKCα) or dominant negative PKCβ1 (dn-PKCβ1). Expression of the dominant negatives was maximal 3–5 days after adenoviral injection as assessed by Western blots. The dominant negatives are HA-tagged, kinase-dead mutants generated by a single point mutation within the kinase domain. Thus, their expression can be monitored by the presence of HA and also by an increase in total PKCα or -β (because the antibodies used for Western blot also recognize the mutants). We found >500% increase of HA expression compared with the non-injected kidney (n = 4 for dn-PKCα and n = 3 for dn-PKCβ1). In addition, total PKCα increased by 250% in dn-PKCα-injected versus non-injected kidney (p < 004; n = 4) and PKCβ by 293% dn-PKCβ1-injected versus non-injected kidney (p < 0.08; n = 3). All experiments were performed 3–5 days after adenoviral transduction. In control rat TALs incubated with vehicle (0.005% acetic acid), O production was 1.42 ± 0.12 nmol/min/mg, and with 1 nm Ang II it rose to 3.58 ± 0.51 nmol/min/mg of protein (p < 0.011, n = 5) (Fig. 6). In contrast, in tubules expressing dn-PKCα, Ang II failed to stimulate O
production was 1.42 ± 0.12 nmol/min/mg, and with 1 nm Ang II it rose to 3.58 ± 0.51 nmol/min/mg of protein (p < 0.011, n = 5) (Fig. 6). In contrast, in tubules expressing dn-PKCα, Ang II failed to stimulate O (1.53 ± 0.67 nmol/min/mg; n.s. versus vehicle), whereas in tubules expressing dn-PKCβ1 Ang II raised O
(1.53 ± 0.67 nmol/min/mg; n.s. versus vehicle), whereas in tubules expressing dn-PKCβ1 Ang II raised O production to 3.89 ± 0.37 nmol/min/mg (p < 0.002 versus vehicle; n = 6) (Fig. 6). Neither dn-PKC isoform had any effect on basal O
production to 3.89 ± 0.37 nmol/min/mg (p < 0.002 versus vehicle; n = 6) (Fig. 6). Neither dn-PKC isoform had any effect on basal O (vehicle-treated suspensions; black bars in Fig. 6). These data indicated that PKCα mediates the stimulatory effect of Ang II on O
(vehicle-treated suspensions; black bars in Fig. 6). These data indicated that PKCα mediates the stimulatory effect of Ang II on O production by TALs.
production by TALs.
FIGURE 6.
Effect of vehicle or 1 nm Ang II for 10 min on O production by rat thick ascending limbs expressing control, dn-PKCα, or dn-PKCβ1. n = 5–6.
production by rat thick ascending limbs expressing control, dn-PKCα, or dn-PKCβ1. n = 5–6.
DISCUSSION
We hypothesized that Ang II acts on the AT1 receptor to stimulate O production by the TAL, and that this process involves stimulation of PKCα, which in turn activates NADPH oxidase. We found that: 1) Ang II stimulated rat TAL O
production by the TAL, and that this process involves stimulation of PKCα, which in turn activates NADPH oxidase. We found that: 1) Ang II stimulated rat TAL O production, and this process was halted by blocking AT1 but not AT2; 2) the NADPH oxidase inhibitor apocynin blocked the stimulatory effect of Ang II on O
production, and this process was halted by blocking AT1 but not AT2; 2) the NADPH oxidase inhibitor apocynin blocked the stimulatory effect of Ang II on O production; 3) Ang II-induced O
production; 3) Ang II-induced O production was blunted in TALs isolated from p47phox−/− mice; 4) in rat TALs Ang II increased PKC activity as measured by FRET; 5) in rat TALs, Ang II-induced O
production was blunted in TALs isolated from p47phox−/− mice; 4) in rat TALs Ang II increased PKC activity as measured by FRET; 5) in rat TALs, Ang II-induced O production was blocked by a general PKC inhibitor as well as by an inhibitor of both PKC α and β1; and finally 6) Ang II-induced O
production was blocked by a general PKC inhibitor as well as by an inhibitor of both PKC α and β1; and finally 6) Ang II-induced O production was reduced in rat TALs expressing dn-PKCα but intact in tubules expressing dn-PKCβ1.
production was reduced in rat TALs expressing dn-PKCα but intact in tubules expressing dn-PKCβ1.
We found that AT1 mediated the stimulatory effect of Ang II on O production in the rat TAL but AT2 did not, consistent with several studies conducted with other tissues. Fu et al. (33) recently reported that, in freshly isolated macula densa cells, Ang II stimulated O
production in the rat TAL but AT2 did not, consistent with several studies conducted with other tissues. Fu et al. (33) recently reported that, in freshly isolated macula densa cells, Ang II stimulated O production, and this effect was blocked by the AT1 antagonist losartan. Jaimes et al. (13) found that Ang II stimulated O
production, and this effect was blocked by the AT1 antagonist losartan. Jaimes et al. (13) found that Ang II stimulated O production in cultured mesangial cells, and this effect was blocked by an AT1 antagonist. Plumb et al. (35) reported that Ang II stimulated O
production in cultured mesangial cells, and this effect was blocked by an AT1 antagonist. Plumb et al. (35) reported that Ang II stimulated O production in human platelets, and this effect was blunted by an AT1 receptor antagonist. Although we recently reported that Ang II acts on AT2 receptors to activate other signaling events in the TAL (34), in the present study the AT2 antagonist PD123319 had no effect on Ang II-stimulated O
production in human platelets, and this effect was blunted by an AT1 receptor antagonist. Although we recently reported that Ang II acts on AT2 receptors to activate other signaling events in the TAL (34), in the present study the AT2 antagonist PD123319 had no effect on Ang II-stimulated O production, suggesting that AT2 receptors do not play a role in AT1-stimulated O
production, suggesting that AT2 receptors do not play a role in AT1-stimulated O production in the TAL. Thus it seems likely that activation of each receptor subtype leads to stimulation of independent signaling pathways.
production in the TAL. Thus it seems likely that activation of each receptor subtype leads to stimulation of independent signaling pathways.
We also questioned whether NADPH oxidase is the source of O in Ang II-stimulated TALs. NADPH oxidase is an enzymatic complex that comprises five components: p40phox, p47phox, p67phox, p22phox, and NOX (35). Under basal conditions p40phox, p47phox and p67phox are located in the cytosol as a complex. Upon stimulation, p47phox becomes phosphorylated and the cytosolic complex translocates to the cell membrane, where it assembles with p22phox and NOX and generates O
in Ang II-stimulated TALs. NADPH oxidase is an enzymatic complex that comprises five components: p40phox, p47phox, p67phox, p22phox, and NOX (35). Under basal conditions p40phox, p47phox and p67phox are located in the cytosol as a complex. Upon stimulation, p47phox becomes phosphorylated and the cytosolic complex translocates to the cell membrane, where it assembles with p22phox and NOX and generates O (36). Thus p47phox is essential for activation of NADPH oxidase. We found that apocynin, which inhibits translocation of p47phox to the plasma membrane, completely inhibited Ang II-stimulated O
(36). Thus p47phox is essential for activation of NADPH oxidase. We found that apocynin, which inhibits translocation of p47phox to the plasma membrane, completely inhibited Ang II-stimulated O production, suggesting that in the TAL all Ang II-stimulated O
production, suggesting that in the TAL all Ang II-stimulated O is generated by NADPH oxidase. In addition, we found that apocynin alone significantly reduced basal levels of O
is generated by NADPH oxidase. In addition, we found that apocynin alone significantly reduced basal levels of O in the rat TAL suggesting that NADPH oxidase generates basal O
in the rat TAL suggesting that NADPH oxidase generates basal O levels. We recognize that the basal level of O
levels. We recognize that the basal level of O on Fig. 2 is higher than what we found for Fig. 1. The explanation for such discrepancy is unknown; however, it should be mentioned that experiments were done during different times of the year, and this may influence production of O
on Fig. 2 is higher than what we found for Fig. 1. The explanation for such discrepancy is unknown; however, it should be mentioned that experiments were done during different times of the year, and this may influence production of O by the rat TAL.
by the rat TAL.
To make sure the effect of apocynin was specifically due to inhibition of NADPH oxidase, we tested TALs isolated from p47phox−/− mice and found that they had low basal levels of O , which were not stimulated by Ang II, indicating that: 1) p47phox is required for Ang II-stimulated O
, which were not stimulated by Ang II, indicating that: 1) p47phox is required for Ang II-stimulated O production in TALs and 2) it maintains basal TAL levels of O
production in TALs and 2) it maintains basal TAL levels of O . We were unable to uncover any compensatory mechanism that enables O
. We were unable to uncover any compensatory mechanism that enables O to be generated under both basal and stimulated conditions in these mice. We recognize that the results obtained in mice cannot necessarily be extrapolated to rats. In fact, the degree of Ang II-stimulated O
to be generated under both basal and stimulated conditions in these mice. We recognize that the results obtained in mice cannot necessarily be extrapolated to rats. In fact, the degree of Ang II-stimulated O production was lower in mice compared with rats. However, the p47phox−/− mice were used as a tool to investigate the involvement of NADPH oxidase so that we did not rely only on pharmacological inhibition. These findings are consistent with data from Li et al. (14) showing that in unstimulated TALs NADPH oxidase is the major source of O
production was lower in mice compared with rats. However, the p47phox−/− mice were used as a tool to investigate the involvement of NADPH oxidase so that we did not rely only on pharmacological inhibition. These findings are consistent with data from Li et al. (14) showing that in unstimulated TALs NADPH oxidase is the major source of O production. In addition, a recent report from our laboratory demonstrated that luminal flow stimulated TAL O
production. In addition, a recent report from our laboratory demonstrated that luminal flow stimulated TAL O production via activation of NADPH oxidase (37). Thus both mechanical and humoral factors are capable of activating NADPH oxidase and thereby enhancing O
production via activation of NADPH oxidase (37). Thus both mechanical and humoral factors are capable of activating NADPH oxidase and thereby enhancing O production by the TAL. Taken together, these data confirm that NADPH oxidase is the main source of O
production by the TAL. Taken together, these data confirm that NADPH oxidase is the main source of O in the TAL under both basal and stimulated conditions.
in the TAL under both basal and stimulated conditions.
To test the involvement of PKC in Ang II-stimulated O production, we first measured total PKC activation in real-time by FRET. We found that Ang II acutely increased PKC activity within 3 min after adding Ang II. When we applied the general PKC pharmacological inhibitor GF109203X, we found that it inhibited Ang II-induced O
production, we first measured total PKC activation in real-time by FRET. We found that Ang II acutely increased PKC activity within 3 min after adding Ang II. When we applied the general PKC pharmacological inhibitor GF109203X, we found that it inhibited Ang II-induced O production, indicating that PKC activity is necessary for Ang II to stimulate O
production, indicating that PKC activity is necessary for Ang II to stimulate O production by TALs. These data are consistent with recent reports from other investigators suggesting that PKC contributes to enhanced O
production by TALs. These data are consistent with recent reports from other investigators suggesting that PKC contributes to enhanced O production in the kidney. Zhang et al. (38) showed that Ang II constricted pericytes within the vasa recta via a mechanism requiring activation of PKC. Yang et al. reported that PKC activation is responsible for the increased O
production in the kidney. Zhang et al. (38) showed that Ang II constricted pericytes within the vasa recta via a mechanism requiring activation of PKC. Yang et al. reported that PKC activation is responsible for the increased O in diabetic rat TALs (24). More recently, we have shown that luminal flow stimulates O
in diabetic rat TALs (24). More recently, we have shown that luminal flow stimulates O production via activation of PKC in isolated perfused TALs (37). Thus activation of PKC appears to be an important mechanism leading to enhanced O
production via activation of PKC in isolated perfused TALs (37). Thus activation of PKC appears to be an important mechanism leading to enhanced O within the kidney.
within the kidney.
The PKC protein family is composed of at least eight members, of which five have been shown to be expressed in the TAL: PKCα, -β, -δ, -ϵ, and -ξ (26, 27). To find out which isoform(s) might be involved in Ang II-induced O production, we used Gö6976, which inhibits both PKCα and -β. We found that Gö6976 completely blocked Ang II-induced O
production, we used Gö6976, which inhibits both PKCα and -β. We found that Gö6976 completely blocked Ang II-induced O production. Because we know of no pharmacological inhibitor specific enough to target only PKCα or -β1, we used adenoviral-mediated transduction of dn-PKCα or -β. We found that Ang II stimulated O
production. Because we know of no pharmacological inhibitor specific enough to target only PKCα or -β1, we used adenoviral-mediated transduction of dn-PKCα or -β. We found that Ang II stimulated O production both in controls and in dn-PKCβ1-transduced TALS; however, it had no effect on TALs transduced with dn-PKCα. Taken together, these data indicate that Ang II stimulates NADPH oxidase-derived O
production both in controls and in dn-PKCβ1-transduced TALS; however, it had no effect on TALs transduced with dn-PKCα. Taken together, these data indicate that Ang II stimulates NADPH oxidase-derived O production in the TAL by activating PKCα.
production in the TAL by activating PKCα.
We conclude that in the TAL, Ang II acts on the AT1 receptors, activating PKCα, which in turn stimulates first NADPH oxidase and ultimately O production, although the exact signaling pathway remains unknown. The AT1 receptors are coupled to Gq and Gi proteins. Activation of Gq enhances diacylglycerol production and stimulates intracellular Ca2+ (39, 40), either of which is capable of activating the classic PKC isoforms α and β (41). In other cells, O
production, although the exact signaling pathway remains unknown. The AT1 receptors are coupled to Gq and Gi proteins. Activation of Gq enhances diacylglycerol production and stimulates intracellular Ca2+ (39, 40), either of which is capable of activating the classic PKC isoforms α and β (41). In other cells, O stimulation by AT1 activation has been attributed to increased diacylglycerol generation and subsequent activation of PKC. In addition, AT1-stimulated O
stimulation by AT1 activation has been attributed to increased diacylglycerol generation and subsequent activation of PKC. In addition, AT1-stimulated O production is mediated by increases in intracellular Ca2+ (42). Because PKC is stimulated by diacylglycerol, and both NADPH oxidase and PKC are sensitive to increases in intracellular Ca2+ (42), both of these pathways could mediate AT1-dependent activation of PKCα in the TAL. In addition, Ang II has been reported to activate the small GTPase Rac (43), whose trafficking and translocation to the plasma membrane play an important role in activation of NADPH oxidase (44). In the TAL, Rac mediates NaCl-induced O
production is mediated by increases in intracellular Ca2+ (42). Because PKC is stimulated by diacylglycerol, and both NADPH oxidase and PKC are sensitive to increases in intracellular Ca2+ (42), both of these pathways could mediate AT1-dependent activation of PKCα in the TAL. In addition, Ang II has been reported to activate the small GTPase Rac (43), whose trafficking and translocation to the plasma membrane play an important role in activation of NADPH oxidase (44). In the TAL, Rac mediates NaCl-induced O production (45). Thus Rac could also participate in both Ang II-induced NADPH oxidase activation and O
production (45). Thus Rac could also participate in both Ang II-induced NADPH oxidase activation and O production in the TAL.
production in the TAL.
In this study we report that activation of PKCα is required for Ang II to stimulate O production in the TAL. However, we have shown previously that O
production in the TAL. However, we have shown previously that O activates PKCα in this segment (26). According to our data, PKCα also enhances O
activates PKCα in this segment (26). According to our data, PKCα also enhances O production via NADPH oxidase assembly with the p47phox subunit. Therefore it is possible that Ang II initiates a cycle whereby small increases in Ang II increase O
production via NADPH oxidase assembly with the p47phox subunit. Therefore it is possible that Ang II initiates a cycle whereby small increases in Ang II increase O production, which in turn overstimulates PKCα and ultimately heightens oxidative stress.
production, which in turn overstimulates PKCα and ultimately heightens oxidative stress.
In summary, in TALs Ang II acts on the AT1 receptor to activate PKCα, which in turn stimulates NADPH oxidase and enhances O production. This could be an important regulatory mechanism whereby Ang II modulates O
production. This could be an important regulatory mechanism whereby Ang II modulates O levels in the renal medulla under physiological conditions. In addition, defects in the Ang II/PKC/NADPH/O
levels in the renal medulla under physiological conditions. In addition, defects in the Ang II/PKC/NADPH/O pathway in the TAL could play a role in the development of hypertension, renal damage, and atherosclerosis.
pathway in the TAL could play a role in the development of hypertension, renal damage, and atherosclerosis.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-70985 and HL-028982 (to J. L. G.).
- TAL
- thick ascending limb of the loop of Henle
- Ang II
- angiotensin II
- PKC
- protein kinase C
- FRET
- fluorescence resonance energy transfer
- CFP
- cyan fluorescent protein
- YFP
- yellow fluorescent protein
- CMV
- cytomegalovirus
- HA
- hemagglutinin
- dn
- dominant negative.
REFERENCES
- 1.Cowley A. W., Jr. (2008) Hypertension 52, 777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor N. E., Glocka P., Liang M., Cowley A. W., Jr. (2006) Hypertension 47, 692–698 [DOI] [PubMed] [Google Scholar]
- 3.Evans R. G., Fitzgerald S. M. (2005) Curr. Opin. Nephrol. Hypertens. 14, 9–15 [DOI] [PubMed] [Google Scholar]
- 4.Kopkan L., Majid D. S. (2006) Hypertension 47, 568–572 [DOI] [PubMed] [Google Scholar]
- 5.Liu R., Ren Y., Garvin J. L., Carretero O. A. (2004) Kidney Int. 66, 268–274 [DOI] [PubMed] [Google Scholar]
- 6.Juncos R., Hong N. J., Garvin J. L. (2006) Am. J. Physiol. Regul Integr. Comp. Physiol. 290, R79–R83 [DOI] [PubMed] [Google Scholar]
- 7.Juncos R., Garvin J. L. (2005) Am. J. Physiol. Renal Physiol. 288, F982–F987 [DOI] [PubMed] [Google Scholar]
- 8.Makino A., Skelton M. M., Zou A. P., Roman R. J., Cowley A. W., Jr. (2002) Hypertension 39, 667–672 [DOI] [PubMed] [Google Scholar]
- 9.Peixoto E. B., Pessoa B. S., Biswas S. K., Lopes de Faria J. B. (2009) Am. J. Nephrol. 29, 309–318 [DOI] [PubMed] [Google Scholar]
- 10.Manning R. D., Jr., Tian N., Meng S. (2005) Am. J. Nephrol. 25, 311–317 [DOI] [PubMed] [Google Scholar]
- 11.Costa C. A., Amaral T. A., Carvalho L. C., Ognibene D. T., da Silva A. F., Moss M. B., Valenca S. S., de Moura R. S., Resende A. C. (2009) Am. J. Hypertens. 22, 1242–1249 [DOI] [PubMed] [Google Scholar]
- 12.Manning R. D., Jr., Meng S., Tian N. (2003) Acta Physiol. Scand. 179, 243–250 [DOI] [PubMed] [Google Scholar]
- 13.Jaimes E. A., Galceran J. M., Raij L. (1998) Kidney International 54, 775–784 [DOI] [PubMed] [Google Scholar]
- 14.Li N., Yi F. X., Spurrier J. L., Bobrowitz C. A., Zou A. P. (2002) Am. J. Physiol. Renal Physiol. 282, F1111–F1119 [DOI] [PubMed] [Google Scholar]
- 15.Mori T., Cowley A. W., Jr. (2003) Hypertension 42, 588–593 [DOI] [PubMed] [Google Scholar]
- 16.Silva G. B., Garvin J. L. (2008) Hypertension 52, 1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y., Knox F. G. (1995) Am. J. Physiol. 269, F40–F46 [DOI] [PubMed] [Google Scholar]
- 18.Navar L. G., Harrison-Bernard L. M., Imig J. D., Cervenka L., Mitchell K. D. (2000) Am. J. Hypertens. 13, 45S–54S [DOI] [PubMed] [Google Scholar]
- 19.Wilcox C. S. (2002) Curr. Hypertens. Rep. 4, 160–166 [DOI] [PubMed] [Google Scholar]
- 20.Zou A. P., Li N., Cowley A. W., Jr. (2001) Hypertension 37, 547–553 [DOI] [PubMed] [Google Scholar]
- 21.Li N., Zhang G., Yi F. X., Zou A. P., Li P. L. (2005) Am. J. Physiol. Renal Physiol. 289, F1048–F1056 [DOI] [PubMed] [Google Scholar]
- 22.Hong N. J., Garvin J. L. (2007) Am. J. Physiol. Renal Physiol. 292, F993–F998 [DOI] [PubMed] [Google Scholar]
- 23.Inoguchi T., Sonta T., Tsubouchi H., Etoh T., Kakimoto M., Sonoda N., Sato N., Sekiguchi N., Kobayashi K., Sumimoto H., Utsumi H., Nawata H. (2003) J. Am. Soc. Nephrol. 14, S227–S232 [DOI] [PubMed] [Google Scholar]
- 24.Yang J., Lane P. H., Pollock J. S., Carmines P. K. (2009) Am. J. Physiol. Renal Physiol. 297, F1220–F1228 [DOI] [PubMed] [Google Scholar]
- 25.Amlal H., LeGoff C., Vernimmen C., Soleimani M., Paillard M., Bichara M. (1998) Am. J. Physiol. 274, C1047–C1056 [DOI] [PubMed] [Google Scholar]
- 26.Silva G. B., Ortiz P. A., Hong N. J., Garvin J. L. (2006) Hypertension 48, 467–472 [DOI] [PubMed] [Google Scholar]
- 27.Aristimuño P. C., Good D. W. (1997) Am. J. Physiol. 272, F624–F631 [DOI] [PubMed] [Google Scholar]
- 28.Herrera M., Garvin J. L. (2005) Am. J. Physiol. Renal Physiol. 288, F58–F64 [DOI] [PubMed] [Google Scholar]
- 29.Herrera M., Garvin J. L. (2004) Am. J. Physiol. Renal Physiol. 287, F231–F235 [DOI] [PubMed] [Google Scholar]
- 30.Violin J. D., Zhang J., Tsien R. Y., Newton A. C. (2003) J. Cell Biol. 161, 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz P. A., Hong N. J., Plato C. F., Varela M., Garvin J. L. (2003) Kidney Int. 63, 1141–1149 [DOI] [PubMed] [Google Scholar]
- 32.Ortiz P. A., Hong N. J., Wang D., Garvin J. L. (2003) Hypertension 42, 674–679 [DOI] [PubMed] [Google Scholar]
- 33.Fu Y., Zhang R., Lu D., Liu H., Chandrashekar K., Juncos L. A., Liu R. (2010) Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R707–R712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrera M., Garvin J. L. (2010) J. Biol. Chem. 285, 14932–14940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babior B. M. (1999) Blood 93, 1464–1476 [PubMed] [Google Scholar]
- 36.Shiose A., Kuroda J., Tsuruya K., Hirai M., Hirakata H., Naito S., Hattori M., Sakaki Y., Sumimoto H. (2001) J. Biol. Chem. 276, 1417–1423 [DOI] [PubMed] [Google Scholar]
- 37.Hong N. J., Silva G. B., Garvin J. L. (2010) Am. J. Physiol. Renal. Physiol. 298, F885–F891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z., Rhinehart K., Kwon W., Weinman E., Pallone T. L. (2004) Am. J. Physiol. Heart Circ. Physiol. 287, H773–H781 [DOI] [PubMed] [Google Scholar]
- 39.Higuchi S., Ohtsu H., Suzuki H., Shirai H., Frank G. D., Eguchi S. (2007) Clin. Sci. 112, 417–428 [DOI] [PubMed] [Google Scholar]
- 40.Balla T., Varnai P., Tian Y., Smith R. D. (1998) Endocr. Res. 24, 335–344 [DOI] [PubMed] [Google Scholar]
- 41.Mizuno N., Itoh H. (2009) Neurosignals 17, 42–54 [DOI] [PubMed] [Google Scholar]
- 42.Spitaler M., Cantrell D. A. (2004) Nat. Immunol. 5, 785–790 [DOI] [PubMed] [Google Scholar]
- 43.Seshiah P. N., Weber D. S., Rocic P., Valppu L., Taniyama Y., Griendling K. K. (2002) Circ. Res. 91, 406–413 [DOI] [PubMed] [Google Scholar]
- 44.Hordijk P. L. (2006) Circ. Res. 98, 453–462 [DOI] [PubMed] [Google Scholar]
- 45.Silva G. B., Garvin J. L. (2010) Am. J. Physiol. Renal Physiol. 298, F421–F425 [DOI] [PMC free article] [PubMed] [Google Scholar]