Abstract
Hsc70s are constitutively synthesized members of the 70-kDa chaperone family; they are essential for viability and conserved among all organisms. When eukaryotic cells recover from stress, hsc70s accumulate in nucleoli by an unknown mechanism. Our studies were undertaken to characterize the signaling events and the targeting sequence required to concentrate hsc70 in the nucleoli of human cells. Here, we show that pharmacological inhibitors of phosphatidylinositol (PI) 3-kinase and MEK kinases as well as protein-tyrosine phosphatases abolished the stress-dependent nucleolar accumulation of hsc70. Furthermore, to identify the hsc70 nucleolar targeting sequence, green fluorescent protein-tagged fusion proteins with defined segments of hsc70 were generated and their subcellular distribution was analyzed in growing cells. These studies demonstrated that residues 225 to 297 serve as a heat-inducible nucleolar targeting signal. This segment directs green fluorescent protein to nucleoli in response to stress, but fails to do so under nonstress conditions. Fine mapping of the nucleolar targeting signal revealed that it has two separable functions. First, residues 225 to 262 direct reporter proteins constitutively to nucleoli, even without stress. Second, segment 263 to 287 functions as an autoinhibitory element that prevents hsc70 from concentrating in nucleoli when cells are not stressed. Taken together, PI 3-kinase and MEK kinase signaling as well as tyrosine dephosphorylation are essential for the accumulation of hsc70 in nucleoli of stressed cells. This process relies on a stress-dependent composite targeting signal that combines multiple functions.
Keywords: Chaperone Chaperonin, Heat Shock Protein, Nuclear Transport, Nucleolus, Nucleus, Stress
Introduction
Heat shock cognate proteins 70 (hsc70)4 are members of the 70-kDa family of chaperones, which are conserved among all organisms. Unlike cytoplasmic hsp70s, hsc70s are constitutively synthesized and essential for cell survival (1). Hsp/hsc70s are involved in a large number of cellular processes: they contribute to the proper folding of nascent polypeptides, refolding of denatured proteins, and targeting of damaged proteins to the proteasome (reviewed in Refs. 2–4). Hsp/hsc70s are essential for protein sorting to organelles and play a protective role in many human diseases and pathophysiologies, including Alzheimer and Huntington disease or ischemia of the heart and brain (reviewed in Refs. 5 and 6). Furthermore, defects in chaperone function have been linked to human disease and aging (5, 7–9).
Members of the hsp/hsc70 family are organized into three domains; the 44-kDa N-terminal ATPase domain is followed by a protein binding segment of 18 kDa and a 10-kDa variable domain at the C-terminal end (reviewed in Ref. 3). Under normal growth conditions, hsc70s shuttle between the nucleus and the cytoplasm; however, hsc70s shuttling is inhibited upon stress (10). Following heat shock, hsc70s initially accumulate in the nucleoplasm, where they associate with chaperone substrates that require refolding (10, 11). When cells recover from stress, hsc70s transiently concentrate in nucleoli possibly to restore nucleolar morphology and function (12).
The nucleolus is a specialized compartment within the nucleus, where pre-rRNA synthesis and processing take place. Together with ribosomal proteins and the assistance of multiple processing factors, these rRNAs are assembled into ribosomal subunits (13–17). Other functions have been ascribed to the nucleolus as well, including the assembly of signal recognition particle, the coordination of stress responses, virus replication, and the control of cell cycle progression and apoptosis (reviewed in Refs. 15 and 18–22). Recent proteomic studies revealed the complex composition of the mammalian nucleolus that may contain several thousand different proteins (23, 24). Importantly, the composition of nucleoli is dynamic and modulated by changes in cell physiology (25, 26). With respect to heat shock proteins, nucleoli are of particular interest, because they possibly harbor a specialized network composed of chaperones and multitasking proteins that cooperate to support cell survival under normal and stress conditions (27).
In response to stress or other stimuli, cells activate kinase signaling cascades to initiate appropriate physiological reactions. Depending on the type of stress, different non-overlapping pathways are activated, although cross-talk between these routes exists. In particular, signaling through the PI 3-kinase → Akt and MEK → ERK1/2 pathways promotes cell survival, stimulates growth as well as ATP production (28–30). Stress-induced kinase activation alters the post-translational modification of a large number of proteins; these modifications include the phosphorylation of serine, threonine, and tyrosine residues. Many such stress-induced modifications are dynamic, and a balance between phosphorylation and dephosphorylation is required to respond properly to changes in growth conditions.
Despite the vast amount of information available for heat shock proteins and the stress response, little is known about the targeting of hsc70 to nucleoli and the signaling pathways involved in hsc70 trafficking. To address these questions, we used pharmacological tools and demonstrate that PI 3-kinase and MEK kinase cascades as well as tyrosine dephosphorylation are essential for the proper accumulation of hsc70 in the nucleoli of stressed cells. Moreover, our studies define a region of hsc70 that mediates stress-dependent targeting to the nucleolus and contains two separable functions. Specifically, we identified a constitutive nucleolar targeting signal that is controlled by an autoinhibitory element, which prevents nucleolar accumulation in the absence of stress.
EXPERIMENTAL PROCEDURES
Generation of GFP-tagged Reporter Proteins
A derivative of pHM830 (31) lacking the β-galactosidase gene was used as vector. Different segments of the hsc70 coding sequence followed by a stop codon were amplified by PCR and fused in-frame to the 3′-end of the GFP gene. Alternatively, oligonucleotides were inserted into the vector to generate GFP fusions with shorter segments of hsc70. For comparison, plasmids were generated that encode GFP fused to the complete hsc70 coding sequence or hsc70 lacking codons 225–297. The expression of fusion genes is driven by a cytomegalovirus promoter. Site-directed mutagenesis was carried out to mutate residue Thr265. The correctness of all constructs was verified by DNA sequencing.
Growth, Transfection, and Heat Shock Experiments
HeLa cells where grown to ∼70% confluency, for no more than 12 passages. 24 h after transfection (10) cells were exposed to 1 h heat stress at 45.5 °C and subsequently allowed to recover at 37 °C for the times indicated.
Pharmacological Tools
The following inhibitors were dissolved in DMSO and used at the final concentrations indicated: 25 μm PD 98059 (inhibitor of MEK, thereby preventing the activation of MAPKs ERK1/2 and ERK5), 0.1–0.6 μm phenylarsine oxide (PAO; inhibitor of protein-tyrosine phosphatases), and 50 μm LY294002 (inhibitor of PI 3-kinase). LY294002 was purchased from Cell Signaling (Danvers, MA); other inhibitors were from Calbiochem (San Diego). In all experiments DMSO concentrations were identical (0.13%), and control cells were treated with DMSO only. Cells were incubated with protein kinase inhibitors 1 h prior to heat shock, and drugs were present throughout heat shock and recovery periods. PAO was added following heat shock to analyze endogenous hsc70. For reporter proteins that can diffuse across the nuclear envelope, PAO was added immediately before heat shock.
Immunofluorescence and Detection of GFP-tagged Proteins
Immunolocalization of endogenous hsc70 was carried out essentially as described (10). Samples were incubated with 7.5 μg/ml of fluorescein isothiocyanate- or 0.4 μg/ml of Cy3-conjugated secondary anti-rat antibodies for 2 h (Jackson ImmunoResearch, West Grove, PA); all other steps followed published procedures (10). To visualize GFP-tagged reporter proteins cells were washed in phosphate-buffered saline and fixed with 3.7% formaldehyde in phosphate-buffered saline for 10 min at room temperature. DNA was stained with 4′,6-diamidino-2-phenylindole and samples were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images were acquired for 0.7-μm slices with a Zeiss LSM510 inverted microscope using a ×63 oil-immersion objective with 1.4 NA and processed in Adobe Photoshop 11.0.
Quantification of Fluorescence Signals in Nucleoli and Statistics
Pixel intensities located in nucleoli were measured with the multiwavelength cell scoring module (32). Pixel intensities/area were calculated and nucleolar/nuclear values were determined for each nucleus. For cells with more than one nucleolus, the sum of nucleolar pixel intensities/sum of the nucleolar area was used for further calculations. At least three independent experiments were carried out for each condition. Nucleolar accumulation of endogenous hsc70 was scored for a minimum of 55 cells in each experiment. For every experiment, all test results were compared with untreated control samples. Data in Fig. 1 are shown as mean ± S.E. Student's t tests (two-tailed) for unpaired samples or one-way analysis of variance was carried out to identify significant differences. For transient transfections, at least three different experiments were performed, each set with appropriate controls. Cells were visually inspected, and reporter proteins displayed similar distribution in replicate experiments. Quantification was carried out for a representative experiment, and pixel intensities were measured for 42 to 74 cells for each data point. Results are shown as mean ± S.D.
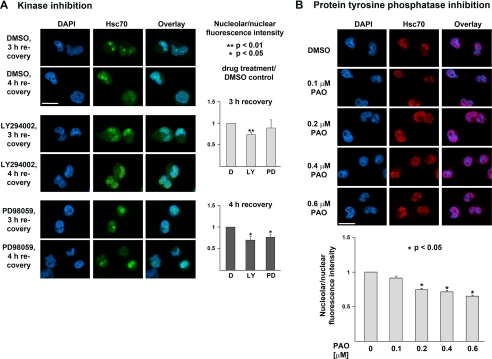
FIGURE 1.
Pharmacological inhibition of PI 3-kinase and MEK kinases or tyrosine phosphatases alters hsc70 nucleolar accumulation during recovery from stress. A, HeLa cells were incubated with DMSO, LY294002 (LY, PI3 kinase inhibitor), or PD98059 (PD, MEK inhibitor) using previously established conditions (35). Endogenous hsc70 was localized by indirect immunofluorescence and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Nucleolar accumulation was quantified after 3 and 4 h of recovery. Data are depicted as nucleolar/nuclear fluorescence intensity; results for DMSO-treated controls (D in the figure) were defined as 1. Three sets of experiments were carried out, between 57 and 86 cells were scored for each data point in every set. The graph shows mean ± S.E. Student's t test was used to determine significant differences. Size bar is 20 μm. B, HeLa cells were treated with increasing concentrations of PAO (tyrosine phosphatase inhibitor) and endogenous hsc70s were located after 3 h of recovery as described for A. Nucleolar accumulation was quantified for different PAO concentrations; the ratio of nucleolar/nuclear fluorescence of DMSO-treated control samples was defined as 1. Three sets of experiments were performed, between 55 and 106 cells were evaluated for each data point in every set. Mean ± S.E. are depicted. One-way analysis of variance was used for the statistical analysis. Size bar is 20 μm.
Western Blot Analysis and Quantification of ECL Signals
Quantitative Western blotting followed published procedures (33). Antibodies against phospho-Akt473 (1:2,000; Santa Cruz), phospho-Akt308 and total-Akt (1:1,000 and 1:2,500; Cell Signaling), hsc70 (1:5,000; Stressgen), GFP (1:500, Clontech), and actin (1:100,000; Chemicon) were used at the dilutions indicated.
RESULTS
Hsc70 and GFP-hsc70 Accumulate in Nucleoli during Recovery from Stress
Under normal growth conditions members of the hsp/hsc70 family shuttle between the nuclear and cytoplasmic compartments, but concentrate transiently in nuclei upon exposure to stress (10, 11). Upon heat shock, endogenous hsc70 initially accumulates in the nucleoplasm. However, during recovery from stress the chaperone concentrates in nucleoli (10), a process that is not understood at the molecular level. To begin to define these events we used HeLa cells to monitor the nucleolar accumulation of endogenous hsc70 in response to heat stress (supplemental Fig. S1). After a 1-h heat shock and subsequent incubation at 37 °C, hsc70 nucleolar accumulation peaked at 3 and 4 h of recovery (supplemental Fig. S1). At 5 h of recovery hsc70 remained concentrated in the nucleoli of some cells, whereas after a 15-h recovery period the distribution was similar to unstressed cells (10). Like endogenous hsc70, GFP-hsc70 in transiently transfected cells was nuclear and cytoplasmic under normal growth conditions and concentrated in the nucleus in response to heat shock. Furthermore, GFP-hsc70 accumulated in nucleoli when cells recovered from heat stress (supplemental Fig. S1); the GFP tag did not concentrate in nucleoli under any conditions (data not shown). Thus, the analysis of GFP-tagged hsc70 is a valid approach to study the nucleolar accumulation of the chaperone.
Heat Shock Activates PI 3-Kinase → Akt and MEK → ERK1/2 Signaling
Stress can activate different signaling pathways, and PI 3-kinase → Akt and MEK → ERK1/2 signaling cascades in particular play a role in cell survival (28–30). Quantitative Western blotting revealed that Akt phosphorylation on Thr308 and Ser473 as well as dual ERK1/2 phosphorylation increased transiently when cells were exposed to heat shock (supplemental Fig. S2). (Dual phosphorylation of Akt on residues Thr308 and Ser473 is necessary to fully activate the kinase (Ref. 30, and references therein).) As expected, levels of the constitutively synthesized hsc70 did not change drastically in response to heat shock and up to 4 h of recovery (supplemental Fig. S2).
Inhibition of PI 3-Kinase and MEK Activation Reduces the Nucleolar Accumulation of Hsc70
In stressed cells, the increase in nuclear levels of hsc70 is followed by nucleolar accumulation of the chaperone when cells recover from the insult. Therefore, we tested whether pharmacological kinase inhibitors that do not interfere with hsc70 nuclear import (Ref. 11 and data not shown) affect the concentration of hsc70 in nucleoli. PI 3-kinase inhibitor LY294002 and MEK inhibitor PD98059 were used at concentrations that prevent Akt modification on Thr308 and ERK1/2 dual phosphorylation (35). In these experiments, kinase inhibitors were present throughout the experiment. Following a 1-h preincubation, HeLa cells were heat-shocked, allowed to recover for 3 or 4 h, and monitored for hsc70 distribution. Nucleolar accumulation was quantified by measuring pixel intensities/area in nucleoli and the nucleus. Data are depicted as the nucleolar/nuclear ratio of fluorescence intensity. Results obtained for DMSO-treated control cells were defined as 1 (D in Fig. 1A). A ratio <1 indicates that hsc70 is less abundant in nucleoli when compared with DMSO-treated controls. Fig. 1A demonstrates that with LY294002 (LY) hsc70 nucleolar accumulation was reduced modestly but significantly to 87 and 85%, respectively, after 3 and 4 h of recovery. Similarly, PD98059 (PD) inhibited nucleolar accumulation at 3 and 4 h (to 95 and 89%), albeit with higher variability. These data support the idea that both PI 3-kinase and MEK-dependent signaling pathways in stressed cells are important to concentrate hsc70 in nucleoli.
In control experiments we tested the effect of pharmacological inhibitors on the phosphorylation of Akt on Thr308 and the dual phosphorylation of ERK1/2, respectively (supplemental Fig. S3). LY294002 completely abolished the phosphorylation of Akt residue Thr308, whereas PD98059 drastically diminished the heat-induced changes in ERK1/2 dual phosphorylation. These results support the idea that the inhibitors reduced the stress-dependent activation of Akt and ERK1/2. Importantly, the decreased activation of kinases correlated with a reduction in hsc70 nucleolar accumulation.
Protein-tyrosine Phosphatases Regulate the Nucleolar Targeting of Hsc70
Our previous studies demonstrated that inhibition of tyrosine phosphatases abolishes heat-induced hsc70 nuclear import in HeLa cells (11). However, it is not known whether tyrosine phosphatases also play a role in nucleolar targeting. To address this question, the tyrosine phosphatase inhibitor PAO was added to cells after heat shock, and nucleolar accumulation of endogenous hsc70 was analyzed after a 3-h recovery period. The nucleolar/nuclear fluorescence intensity in cells incubated with the solvent DMSO was defined as 1 (Fig. 1B, 0 μm PAO). Except for 0.1 μm PAO, all of the treatments significantly reduced hsc70 nucleolar accumulation when compared with control cells. These results implicate the dephosphorylation of modified tyrosine residues in nucleolar targeting of the chaperone during recovery from stress.
Residues 225 to 297 of Hsc70 Are Sufficient to Promote Stress-induced Nucleolar Accumulation
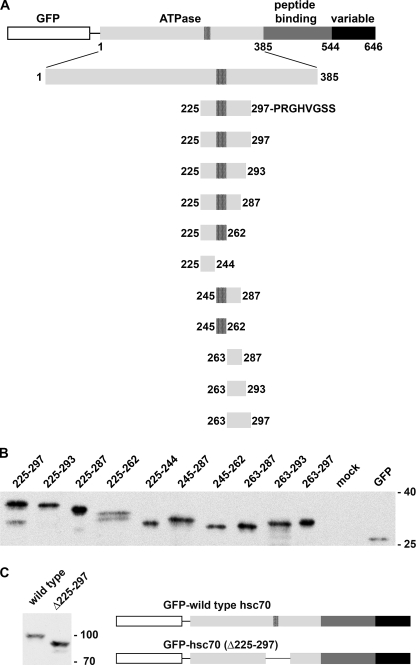
Analysis of hsc70 nucleolar accumulation is complicated by the fact that the 70-kDa chaperone exceeds the diffusion channel of the NPC. Thus, treatments that interfere with hsc70 nuclear import will also affect the subsequent concentration in nucleoli. To avoid this difficulty, we generated reporter proteins that are small enough to diffuse across the NPC. Fusion of defined hsc70 segments to a 28-kDa GFP tag produced reporter proteins with a molecular mass less than ∼40 kDa (Fig. 2, A and B). In the following, with the exception of the deletion mutant GFP-hsc70(Δ225–297), numbers in parentheses denote the residues of hsc70 that were fused to GFP. Fig. 2A shows the domain organization of hsc70 and the chaperone segments present in different constructs. Western blot analysis of crude extracts revealed that reporter proteins migrated as expected during SDS-PAGE (Fig. 2, B and C).
FIGURE 2.
Reporter proteins used in this study. A, the domain organization of hsc70 and the fusion proteins analyzed are shown. Numbers denote the first and last amino acid residues of the hsc70-derived fragment. For each construct the last codon is followed by a stop codon. A segment within the ATPase domain that fits the consensus for a bipartite nuclear localization sequence (residues 245–262) is marked with black stripes. B, HeLa cells were transiently transfected with plasmids encoding different reporter proteins that carry the indicated hsc70 residues. Crude extracts generated for transfected cells were analyzed by Western blotting. C, GFP was fused to full-length hsc70 (wild type) or a deletion mutant lacking residues 225–297. In B and C, fusion proteins were detected by Western blotting with anti-GFP antibodies. Molecular masses of marker proteins (×103) are indicated at the right margin.
In Fig. 3, A–C, we analyzed the distribution of GFP-tagged fusion proteins in transiently transfected cells under different growth conditions. To this end, nucleolar and nuclear fluorescence intensities were quantified, and the ratio of nucleolar/nuclear fluorescence was calculated for each time point. Results for GFP-hsc70(225–297) under control conditions served as a reference in these studies. In each experiment, data were normalized to the nucleolar/nuclear ratio obtained for GFP-hsc70(225–297) in the absence of stress; this ratio was defined as 1.
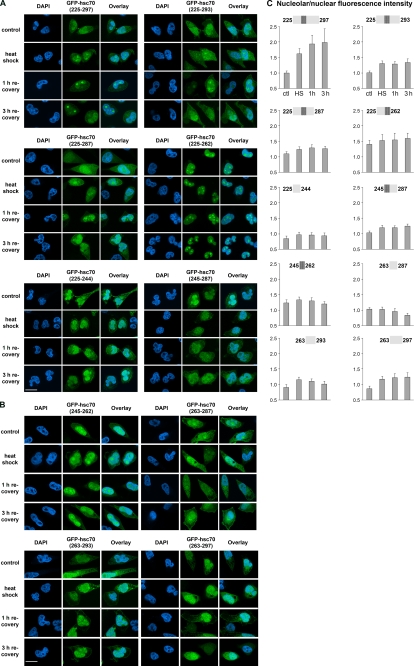
FIGURE 3.
Multiple parts of segment 225 to 297 of hsc70 control the stress-induced nucleolar accumulation of reporter proteins. A and B, GFP-tagged proteins containing different segments of hsc70 were exposed to heat stress followed by recovery for the times indicated. Fusion proteins and 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei were detected by fluorescence microscopy. Size bar is 20 μm. Note the constitutive nucleolar accumulation of GFP-hsc70(225–262) and GFP-hsc70(245–262) even in the absence of stress. C, fluorescence signals in nucleoli and nuclei were quantified for all constructs at different time points. Between 50 and 74 cells were examined for each data point. Results are shown as nucleolar/nuclear pixel intensities. Data were normalized to results obtained for GFP-hsc70(225–297) under nonstress conditions, which was defined as 1.
Fig. 3, A–C, demonstrates that, with the exception of GFP-hsc70(225–262) and GFP-hsc70(245–262), in the absence of stress all of the reporter proteins were present in the cytoplasm and nucleus, but not concentrated in nucleoli. Following heat shock, we observed nucleolar accumulation for several of these constructs, albeit with different efficiencies. The most prominent stress-dependent redistribution was observed for GFP-hsc70(225–297); the nucleolar/nuclear ratio increased from 1 in unstressed cells to ∼1.8 at 1 h and 1.9 at 3 h of recovery (Fig. 3, A and C). Addition of unrelated amino acid residues PRGHVGSS to the C-terminal end of the hsc70 fragment 225–297, referred to as GFP-hsc70(225–297aa), did not interfere with its nucleolar targeting function (see below and data not shown). Taken together, our results are consistent with the idea that residues 225–297 of hsc70 are sufficient to promote nucleolar targeting in response to heat shock. Thus, hsc70 residues 225–297 provide a stress-dependent nucleolar localization signal (NoLS).
It should be noted that for all hsc70 segments that mediated targeting to nucleoli, accumulation in the nucleolar compartment was observed at an earlier time point when compared with endogenous hsc70 or the full-length GFP-tagged chaperone. This is most likely due to the fact that the hsc70 segments analyzed by us do not contain the peptide binding domain and therefore will not be retained in the nucleoplasm by chaperone/client interactions. We have shown earlier that the hsc70/client association contributes to hsc70 retention in the nucleoplasm of stressed cells (10).
C-terminal Truncations of the Hsc70 NoLS Diminish the Stress-induced Nucleolar Localization of Reporter Proteins
To gain further insight into the functional organization of the hsc70 NoLS, we generated a panel of different truncations. Under nonstress conditions, GFP-hsc70(225–293) and GFP-hsc70(225–287) displayed a distribution that was similar to GFP-hsc(225–297); however, GFP-hsc70(225–293) and GFP-hsc70(225–287) concentrated only 1.2- to 1.3-fold in nucleoli upon heat shock or during recovery (Fig. 3, A and C). This suggests that the C-terminal portion of the hsc70 NoLS contributes to the heat-dependent nucleolar accumulation of reporter proteins, an interpretation supported by other reporter proteins (see below).
Residues 225 to 262 Promote Constitutive Nucleolar Targeting Which Is Negatively Controlled by Residues 263 to 287
Additional constructs were analyzed to characterize the hsc70 NoLS. A fragment containing residues 225–262, but not 225–244, targeted GFP to nucleoli, even under nonstress conditions. The nucleolar/nuclear ratio for GFP-hsc70(225–262) was ∼1.4 under nonstress conditions, which increased to 1.5–1.6 during heat shock and recovery. This constitutive stress-independent nucleolar accumulation was also observed, but less pronounced, for the reporter protein GFP-hsc70(245–262), a fragment rich in lysine and arginine residues (Fig. 3, B and C). By contrast, reporter proteins containing segments 245–287, 263–287, 263–293, or 263–297 showed no nucleolar accumulation in unstressed cells. After heat shock or during recovery, there was a slight nucleolar accumulation (1.1- to 1.2-fold) for segments 245–287, 263–293, and 263–297 (Fig. 3C). Together, these data support the model that residues 263–287 provide an autoinhibitory regulatory element for segment 225–262. This regulatory element prevents the concentration in nucleoli when cells have not been stressed. This is revealed by the fact that nucleolar targeting via residues 225–262 or 245–262 is abolished by the negative regulator under normal conditions. In addition, the C-terminal portion of the NoLS provides a weak stress-dependent nucleolar targeting activity on its own. These data are consistent with results described in the previous section; e.g. removal of residues 288–297 from the NoLS diminished the stress-induced nucleolar accumulation of reporter proteins.
Mutation of Thr265 in the NoLS Reduces the Nucleolar Accumulation in Stressed Cells
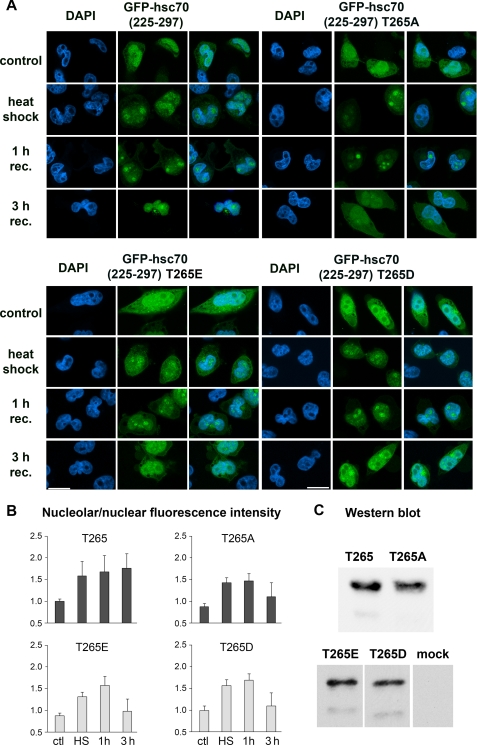
As discussed above, residues 263–287 are sufficient to regulate in a stress-dependent fashion nucleolar accumulation of the hsc70 NoLS. This portion of the chaperone contains a potential site for Akt phosphorylation at position Thr265. Indeed, mass spectrometry demonstrated the phosphorylation of Thr265 in hsp/hsc70 in vivo (PhosphoSitePlus, Cell Signaling). Because PI 3-kinase signaling and possibly Akt activity are important for endogenous hsc70 nucleolar accumulation, we mutated this residue in the reporter GFP-hsc70(225–297) to alanine, glutamic acid, or aspartic acid (Fig. 4). Subsequently, the distribution of mutant reporter proteins was quantified in transiently transfected cells. Interestingly, mutating Thr265 to alanine, glutamic acid, or aspartic acid had a similar effect on localization. When compared with the wild type segment, no drastic changes were detected immediately following heat shock or after 1 h recovery. However, upon a 3-h recovery period, each of the mutants displayed a reduced nucleolar/nuclear ratio. Control experiments showed that all of the reporter proteins migrated at the same position during SDS-PAGE (Fig. 4C).
FIGURE 4.
Mutation of Thr265 alters the distribution of GFP-hsc70(225–297) during recovery from stress. In GFP-hsc70(225–297) residue Thr265 was mutated to alanine, glutamic, or aspartic acid. A and B, in transiently transfected HeLa cells the nucleolar/nuclear distribution was quantified for different reporter proteins under normal and stress conditions. For each data point pixel values for 42 to 69 cells were determined. Results are depicted as nucleolar/nuclear pixel intensities. Data were normalized to GFP-hsc70(225–297) under control conditions, which was defined as 1. Note that nucleolar accumulation is reduced in all mutants at 3 h of recovery. Size bar is 20 μm. C, Western blot analysis of transiently synthesized reporter proteins. DAPI, 4′,6-diamidino-2-phenylindole.
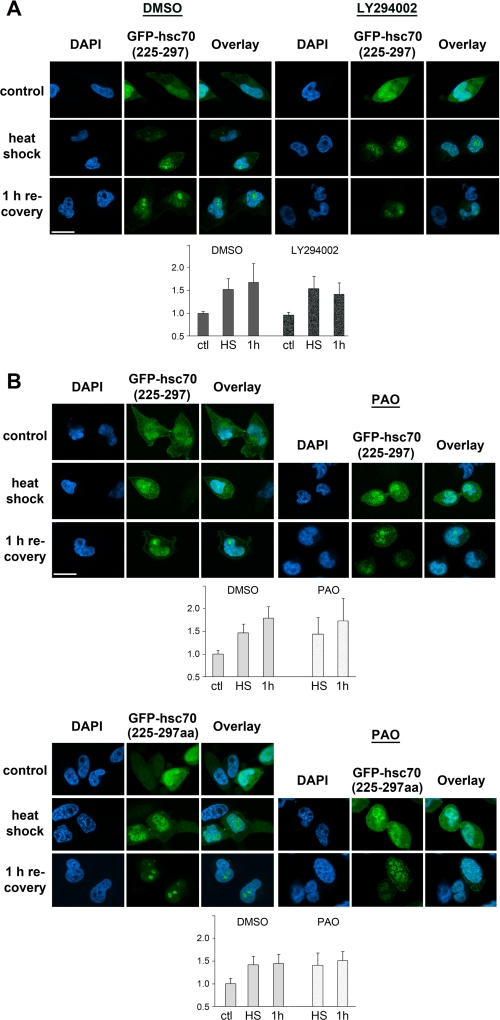
Inhibition of PI 3-Kinase Interferes with Nucleolar Accumulation Mediated by Hsc70 Segment 225–297
Because PI 3-kinase signaling and tyrosine phosphatases play a role in the nucleolar accumulation of endogenous hsc70, we next examined the effect of pharmacological inhibitors on GFP-hsc70(225–297) nucleolar accumulation in heat-stressed cells. When compared with DMSO-treated controls, incubation with LY294002 did not alter the nucleolar/nuclear ratio following heat shock. However, the ratio was reduced to 84% after a 1-h recovery period (Fig. 5A). This effect was similar to what was observed for endogenous hsc70 (Fig. 1).
FIGURE 5.
Effect of PI 3-kinase signaling and protein-tyrosine phosphatases on NoLS-mediated nucleolar accumulation of reporter proteins. A, treatment with LY294002 reduces the nucleolar accumulation of GFP-hsc70(225–297) in heat-stressed cells. Transiently transfected cells synthesizing GFP-hsc70(225–297) were incubated with DMSO or the PI 3-kinase inhibitor LY284002 (“Experimental Procedures”). B, PAO does not inhibit the nucleolar accumulation of GFP-hsc70(225–297) and GFP-hsc70(225–297aa). Transiently transfected HeLa cells were incubated with DMSO or PAO as described under “Experimental Procedures.” In A and B reporter proteins were detected in unstressed cells, after heat shock and upon 1 h recovery. Size bar is 20 μm. Quantification of nucleolar/nuclear pixel intensities was carried out as described for Fig. 3. DAPI, 4′,6-diamidino-2-phenylindole.
In an additional set of experiments we tested whether PAO alters the distribution of GFP-hsc70(225–297) and GFP-hsc70(225–297aa). Because GFP-tagged fusion proteins do not have to be imported into nuclei and show some nucleolar accumulation immediately after heat shock, we added PAO before stressing the cells. Under these conditions, PAO did not diminish the nucleolar/nuclear ratio of GFP-hsc70(225–297) or GFP-hsc70(225–297aa) when compared with DMSO-treated cells (Fig. 5B).
Taken together, our results support the idea that PI 3-kinase signaling contributes to the regulation of nucleolar accumulation of the reporter protein GFP-hsc70(225–297). By contrast, tyrosine phosphatases do not seem to play a significant role in this process.
Residues 225 to 297 Are Necessary, but Not Sufficient, for Nuclear Import of Hsc70
Given the importance of fragment 225–297 in hsc70 nucleolar accumulation, we next determined the role of residues 225–297 in hsc70 nuclear import. A GFP-tagged reporter protein lacking residues 225–297 (GFP-hsc70(Δ225–297)) did not concentrate in nuclei under control or heat shock conditions, demonstrating that removal of this segment interferes with heat-induced nuclear accumulation (supplemental Fig. S4).
Fragment 225–297 contains clusters of positively charged amino acid residues that fit the consensus sequence of a classical NLS (37). To test whether this segment promotes transport into the nucleus, we generated GFP-hsc70(225–297)-β-galactosidase, a reporter protein that contains GFP-hsc70(225–297) fused to β-galactosidase. With a molecular mass of >150 kDa for the monomer, this reporter protein cannot diffuse across the nuclear envelope. GFP-hsc70(225–297)-β-galactosidase did not accumulate in nuclei, neither in unstressed cells nor following heat shock (supplemental Fig. S4), suggesting that at least in the context of this construct residues 225–297 do not mediate import into the nucleus. Thus, fragment 225–297 does not function as a constitutive or heat-inducible NLS.
DISCUSSION
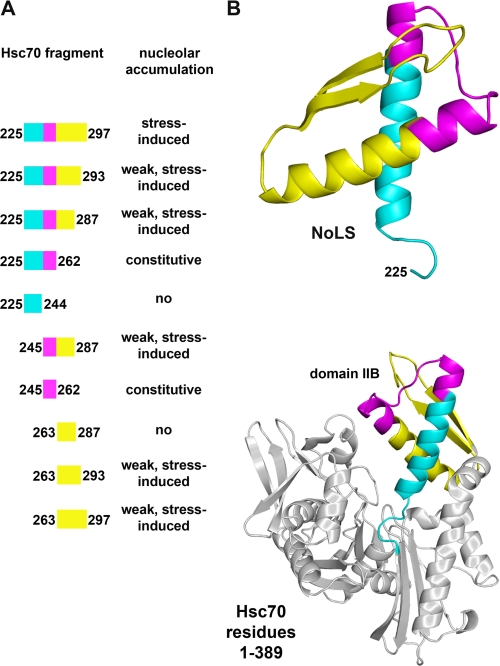
The experiments described here characterize the mechanisms that underlie the nucleolar targeting of hsc70 in cells recovering from stress. Our studies define the minimal region of hsc70 that promotes nucleolar accumulation upon heat shock. In particular, we demonstrate that residues 225–297 are sufficient to concentrate the passenger protein GFP in nucleoli. Importantly, a prerequisite for this nucleolar accumulation is the exposure to stress. Taken together, our data show that residues 225–297 function as a NoLS, which contains all the information required to promote stress-regulated nucleolar targeting (summarized in Fig. 6). Within the tertiary structure of the N-terminal hsc70 ATPase domain, this segment is located in the well defined domain B of lobe II, a region that comprises residues 229–306 (38, 39).
FIGURE 6.
Simplified model for the hsc70 NoLS. A, results obtained for the nucleolar localization of GFP-tagged fusion proteins are summarized. B, the secondary structure of the N-terminal residues 1–389 of hsc70 and different segments of the NoLS are shown (generated with Polyview-3D; Ref. 34). Within the N-terminal ATPase domain the NoLS is located in domain IIB of lobe II (38, 39). Residues 225–244 are depicted in blue, segment 245–262 is magenta, and the C-terminal portion including the autoinhibitory region (residues 263–297) is yellow.
We further dissected the function of hsc70 fragment 225–297 and identified residues 225–262 as a constitutive signal that promotes nucleolar targeting even in the absence of stress (Fig. 6A). The same applies to residues 245–262; however, nucleolar targeting by segment 245–262 was always less pronounced when compared with residues 225–262. This suggests that residues 225–244 enhance nucleolar localization, although they have no nucleolar targeting activity per se.
With our systematic analysis of the hsc70 NoLS we demonstrate that segment 263–287 diminishes the constitutive nucleolar targeting function that is provided by residues 225–262. This negative control operates in unstressed cells, thereby preventing the association with nucleoli under normal conditions. Interestingly, residues 263–287 interfered with the constitutive nucleolar targeting mediated by residues 245–262, even under stress conditions (Fig. 6). This indicates that segment 225–244 plays a crucial role in overcoming the autoinhibitory effect of region 263–287 upon stress, but is not sufficient on its own to promote nucleolar accumulation. Furthermore, the quantitative analysis of truncated forms of the NoLS revealed that residues 263–297 also contribute to the stress-induced nucleolar accumulation promoted by the NoLS.
On the basis of the results described here we propose a simplified model for the nucleolar targeting mediated by fragment 225–297 of hsc70 (Fig. 6). Stress-dependent nucleolar accumulation requires the combined action of different parts of this fragment. Under normal growth conditions, nucleolar targeting is prevented by the autoinhibitory element located in residues 263–287. During recovery from stress, this negative regulator will be inactivated, and the nucleolar targeting function of residues 225–262 prevails. Furthermore, segment 263–297 provides an additional, albeit weak, stress-inducible signal for nucleolar targeting.
Interestingly, within the hsc70 ATPase domain all of the functional components of the hsc70 NoLS are present in domain B of lobe II (38, 39). Domain IIB consists of two α-helical regions that are followed by two β-strands. Residues 225–262 of domain IIB, sufficient for constitutive nucleolar targeting (Fig. 6, blue and magenta), contribute to two α-helical regions that are connected by a loop (Fig. 6, magenta, and supplemental Movie M1). The constitutively active NoLS is followed by its negative regulator, located within residues 263–297 (Fig. 6, yellow), which gives rise to a helical region followed by a segment, predicted to be organized in antiparallel β-strands. Several segments of domain IIB are conserved (38), and the C-terminal portion of the first β-strand in particular is highly conserved. Notably, this segment is part of the negative regulator identified by us.
It is tempting to speculate how the hsc70 NoLS controls stress-dependent nucleolar localization. We propose that this process combines separate functions contributed by different parts of the NoLS. In one scenario, stress might trigger conformational changes in the NoLS, thereby masking sites on hsc70 that interact with components in the nucleoplasm. Such masking of interacting sites would promote the release of hsc70 from anchors in the nucleoplasm. Alternatively, stress could expose sites on hsc70 that are recognized by binding partners in the nucleolus, but hidden under non-stress conditions. In this case, nucleolar retention of the chaperone would be favored. Given the complex contributions of different parts of the NoLS to nucleolar accumulation, we favor the idea that the combination of multiple interactions, possibly with different binding partners, determines the ultimate distribution of reporter proteins. At the same time, it is likely that not only hsc70, but also its binding partners, both in the nucleolus and the nucleoplasm, are regulated by stress. Ultimately, these associations could be altered by post-translational modification of hsc70 and/or its interacting components. It is feasible that these modifications are regulated by kinases and phosphatases that depend on signaling through PI 3-kinase and MEK kinase pathways.
Our results for mutations introduced at Thr265 are in line with this speculation. Thr265 has been shown to be phosphorylated in vivo; it is also a potential target for the protein kinase Akt. Because mutations to alanine as well as glutamic or aspartic acid reduced the nucleolar accumulation of reporter proteins, it is feasible that Thr265 cycles between the unmodified and phosphorylated state. Both forms might interact with different binding partners, all of which could regulate hsc70 nucleolar accumulation. Future experiments will have to address these questions.
The complex regulation of hsc70 nucleolar targeting is consistent with recent studies for other components of the nucleolus. For example, the movement of individual proteins or ribosomal subunits between nucleoli and the nucleoplasm occurs in a highly organized fashion (Refs. 15, 16, and 18, and references therein). The same applies to the intracellular trafficking of hsc70, which is, in part, dictated by its interactions with polypeptides that need to be folded. In support of this model, we demonstrated earlier that in heat-stressed cells binding of hsc70 to chaperone substrates controls its retention in the nucleoplasm (10). However, chaperone/client interactions are not the sole contributor to hsc70 nucleolar accumulation. Our previous results showed that hsc70 binding to nucleoli is at least partially ATP-insensitive and thus likely to be unrelated to chaperone function (10). This idea is further substantiated by our current study, as the reporter proteins used here offer the advantage that they do not carry the peptide binding domain. Consequently, interactions with chaperone clients did not interfere with their localization and enabled us to dissect the functional organization of the NoLS.
One of the limiting factors for the analysis of nucleolar targeting is that molecules larger than the diffusion channel of the NPC require active nuclear import. As transport in and out of the nucleus is sensitive to stress (Refs. 10 and 41 and references therein), we used reporter proteins that are small enough to diffuse across NPCs. Interestingly, fragment 225–297, when fused to GFP and β-galactosidase, did not support nuclear import, neither under normal nor heat shock conditions. This suggests that the stress-dependent hsc70 NoLS does not operate as an NLS. Our results are in line with Tsukahara and Maru (40), who reported that the protein binding domain of hsc70 plays a role in hsc70 nuclear import. These results, however, do not explain why GFP-hsc70(Δ225–297) failed to accumulate in nuclei of heat-shocked cells. One possibility is that two regions present in separate domains of hsc70 are simultaneously required to import hsc70 into the nucleus. Alternatively, deletion of residues 225–297 might generate a mutant protein, whose altered structure prevents an NLS located in the peptide binding domain from functioning.
Dang and Lee (42) previously described that residues 250–267 in hsp70 are similar to an NLS. This portion of the chaperone, when fused to pyruvate kinase, promoted constitutive nuclear and nucleolar localization in unstressed cells. Another study described a nucleolar retention signal for hsc70, comprised of nine mostly basic residues of the chaperone (36). When fused to hydrophobic repeats, which are not present in authentic hsc70, nucleolar targeting of a GFP reporter protein was observed at pH 6.3. Collectively, these data support our idea that a short segment of hsp70 or hsc70, when removed from its normal context, could serve as a nucleolar targeting signal. However, the complex regulation of NoLS function is likely to be revealed only within the proper domain organization of the chaperone.
The experiments presented here analyzed for the first time the signaling events that participate in the nucleolar accumulation of hsc70. We showed that PI 3-kinase and MEK kinases as well as protein-tyrosine phosphatase activities play a part in the heat-dependent concentration of endogenous hsc70 in nucleoli. Importantly, PI 3-kinase controls also the distribution of a reporter protein that contains only the NoLS. By contrast, tyrosine phosphatases have an effect on the nucleolar targeting of full-length hsc70, whereas the NoLS is not affected. This points to the possibility that additional residues, located outside of the NoLS, participate in the regulation of hsc70 nucleolar accumulation.
Taken together, our studies defined the signaling events implicated in hsc70 nucleolar targeting and identified a novel stress-inducible NoLS that is composed of multiple functional elements. In particular, the combination of a constitutive nucleolar targeting signal with an autoinhibitory segment provides a sophisticated mechanism to control the localization of hsc70 under normal and stress conditions.
Supplementary Material
This work was supported in part by grants from Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Heart and Stroke Foundation of Canada (to U. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Movie M1.
- Hsc70
- heat shock cognate protein 70
- PI
- phosphatidylinositol
- DMSO
- dimethyl sulfoxide
- GFP
- humanized green fluorescent protein
- NPC
- nuclear pore complex
- NLS
- nuclear localization sequence
- NoLS
- nucleolar localization sequence
- PAO
- phenylarsine oxide
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- ERK
- extracellular signal-regulated kinase.
REFERENCES
- 1.Rohde M., Daugaard M., Jensen M. H., Helin K., Nylandsted J., Jäättelä M. (2005) Genes Dev. 19, 570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daugaard M., Rohde M., Jäättelä M. (2007) FEBS Lett. 581, 3702–3710 [DOI] [PubMed] [Google Scholar]
- 3.Vos M. J., Hageman J., Carra S., Kampinga H. H. (2008) Biochemistry 47, 7001–7011 [DOI] [PubMed] [Google Scholar]
- 4.Morimoto R. I., Cuervo A. M. (2009) J. Gerontol. A Biol. Sci. Med. Sci. 64, 167–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hishiya A., Takayama S. (2008) Oncogene 27, 6489–6506 [DOI] [PubMed] [Google Scholar]
- 6.Giffard T. G., Yenari M. A. (2004) J. Neurosurg. Anesthesiol. 16, 53–61 [DOI] [PubMed] [Google Scholar]
- 7.Barral J. M., Broadley S. A., Schaffar G., Hartl F. U. (2004) Semin. Cell. Dev. Biol. 15, 17–29 [DOI] [PubMed] [Google Scholar]
- 8.Macario A. J., Conway de Macario E. (2005) New Engl. J. Med. 353, 1489–1501 [DOI] [PubMed] [Google Scholar]
- 9.Morimoto R. I. (2008) Genes Dev. 22, 1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodiha M., Chu A., Lazrak O., Stochaj U. (2005) Am. J. Physiol. Cell Physiol. 289, C1034–1041 [DOI] [PubMed] [Google Scholar]
- 11.Chu A., Matusiewicz N., Stochaj U. (2001) FASEB J. 15, 1478–1480 [DOI] [PubMed] [Google Scholar]
- 12.Pelham H. R. B. (1984) EMBO J. 3, 3095–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam Y. W., Trinkle-Mulcahy L., Lamond A. I. (2005) J. Cell Sci. 118, 1335–1337 [DOI] [PubMed] [Google Scholar]
- 14.Lo S. J., Lee C. C., Lai H. J. (2006) Cell Res. 16, 530–538 [DOI] [PubMed] [Google Scholar]
- 15.Boisvert F. M., van Koningsbruggen S., Navascués J., Lamond A. I. (2007) Nat. Rev. Mol. Cell Biol. 8, 574–585 [DOI] [PubMed] [Google Scholar]
- 16.Henras A. K., Soudet J., Gérus M., Lebaron S., Caizergues-Ferrer M., Mougin A., Henry Y. (2008) Cell Mol. Life Sci. 65, 2334–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirri V., Urcuqui-Inchima S., Roussel P., Hernandez-Verdun D. (2008) Histochem. Cell Biol. 129, 13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson M. O., Dundr M. (2005) Histochem. Cell Biol. 123, 203–216 [DOI] [PubMed] [Google Scholar]
- 19.Mayer C., Grummt I. (2005) Cell Cycle 4, 1036–1038 [DOI] [PubMed] [Google Scholar]
- 20.Hiscox J. A. (2007) Nat. Rev. Microbiol. 5, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeown P. C., Shaw P. J. (2009) Chromosoma 118, 11–23 [DOI] [PubMed] [Google Scholar]
- 22.Pederson T., Tsai R. Y. (2009) J. Cell Biol. 184, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung A. K., Andersen J. S., Mann M., Lamond A. I. (2003) Biochem. J. 376, 553–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad Y., Boisvert F. M., Gregor P., Cobley A., Lamond A. I. (2009) Nucleic Acids Res. 37, D181–D184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen J. S., Lam Y. W., Leung A. K., Ong S. E., Lyon C. E., Lamond A. I., Mann M. (2005) Nature 433, 77–83 [DOI] [PubMed] [Google Scholar]
- 26.Lam Y. W., Evans V. C., Heesom K. J., Lamond A. I., Matthews D. A. (2010) Mol. Cell. Proteomics 9, 117–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bański P., Kodiha M., Stochaj U. (2010) Trends Biol. Sci., in press [DOI] [PubMed] [Google Scholar]
- 28.Shaw R. J., Cantley L. C. (2006) Nature 441, 424–430 [DOI] [PubMed] [Google Scholar]
- 29.Shaul Y. D., Seger R. (2007) Biochim. Biophys. Acta 1773, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 30.Sale E. M., Sale G. J. (2008) Cell Mol. Life Sci. 65, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorg G., Stamminger T. (1999) BioTechniques 26, 858–862 [DOI] [PubMed] [Google Scholar]
- 32.Kodiha M., Brown C. M., Stochaj U. (2008) Sci. Signal. 1, pl2. [DOI] [PubMed] [Google Scholar]
- 33.Kodiha M., Rassi J. G., Brown C. M., Stochaj U. (2007) Am. J. Physiol. Cell. Physiol. 293, C1427–C1436 [DOI] [PubMed] [Google Scholar]
- 34.Porollo A., Meller J. (2007) BMC Bioinformatics 8, 316; doi:10.1186/1471-2105-8-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodiha M., Bański P., Stochaj U. (2009) FEBS Lett. 583, 1987–1993 [DOI] [PubMed] [Google Scholar]
- 36.Mekhail K., Rivero-Lopez L., Al-Masri A., Brandon C., Khacho M., Lee S. (2007) Mol. Biol. Cell 18, 3966–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall K. S., Zhang Z., Curran J., Derbyshire S., Mymryk J. S. (2007) BMC Mol. Biol. 8, 6.doi:10.1186/1471-2199-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flaherty K. M., DeLuca-Flaherty C., McKay D. B. (1990) Nature 346, 623–628 [DOI] [PubMed] [Google Scholar]
- 39.Jiang J., Prasad K., Lafer E. M., Sousa R. (2005) Mol. Cell. 20, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsukahara F., Maru Y. (2004) J. Biol. Chem. 279, 8867–8872 [DOI] [PubMed] [Google Scholar]
- 41.Kodiha M., Chu A., Matusiewicz N., Stochaj U. (2004) Cell Death Differ. 11, 862–874 [DOI] [PubMed] [Google Scholar]
- 42.Dang C. V., Lee W. M. F. (1989) J. Biol. Chem. 264, 18019–18023 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.