Abstract
In mammalian cells entry into and progression through mitosis are regulated by multiple mitotic kinases. How mitotic kinases interact with each other and coordinately regulate mitosis remains to be fully understood. Here we employed a chemical biology approach using selective small molecule kinase inhibitors to dissect the relationship between Cdk1 and Aurora A kinases during G2/M transition. We find that activation of Aurora A first occurs at centrosomes at late G2 and is required for centrosome separation independently of Cdk1 activity. Upon entry into mitosis, Aurora A then becomes fully activated downstream of Cdk1 activation. Inactivation of Aurora A or Plk1 individually during a synchronized cell cycle shows no significant effect on Cdk1 activation and entry into mitosis. However, simultaneous inactivation of both Aurora A and Plk1 markedly delays Cdk1 activation and entry into mitosis, suggesting that Aurora A and Plk1 have redundant functions in the feedback activation of Cdk1. Together, our data suggest that Cdk1, Aurora A, and Plk1 mitotic kinases participate in a feedback activation loop and that activation of Cdk1 initiates the feedback loop activity, leading to rapid and timely entry into mitosis in human cells. In addition, live cell imaging reveals that the nuclear cycle of cells becomes uncoupled from cytokinesis upon inactivation of both Aurora A and Aurora B kinases and continues to oscillate in a Cdk1-dependent manner in the absence of cytokinesis, resulting in multinucleated, polyploidy cells.
Keywords: CDK (Cyclin-dependent Kinase), Cell Cycle, Centrosome, Checkpoint Control, Mitosis, Protein Kinases, Aurora A, Plk1
Introduction
Entry into and progression through mitosis are regulated by multiple protein kinases, collectively called mitotic kinases (1). Major mitotic kinases include Cdk1, which consists of a catalytic subunit Cdc2 and a regulatory subunit cyclin B and members of the Polo-like kinase, Aurora kinase, and never in mitosis A (NIMA)-related protein kinase families. These mitotic kinases regulate various aspects of mitotic processes, such as centrosome maturation and separation, bipolar mitotic spindle formation, DNA condensation and segregation, spindle checkpoint controls, and cytokinesis. To successfully complete the complex mitotic processes and accurately segregate chromosomes into two daughter cells, activities of these mitotic kinases must be coordinated in space and time. Indeed, dysregulation of these mitotic kinases causes genome instability and is often linked to many types of human cancer (1). However, how these mitotic kinases interact and coordinate with each other is a long standing question, especially in mammalian cells.
This understanding has been stymied by observations that different organisms may employ different strategies for their mitotic regulation. For instance, in unicellular yeasts, activation of Cdk1 is necessary and sufficient to promote entry into mitosis (2). However, in multicellular filamentous fungi, such as Aspergillus nidulans, activation of both Cdk1 and never in mitosis A (NIMA) kinase is required for mitosis (3). Interestingly, the requirement for never in mitosis A (NIMA) kinase in mitotic entry is independent of Cdk1 activation and at a point after activation of Cdk1 (4). In contrast, in cycling extracts of Xenopus oocyte, activation of Plx1, a Plk1 homolog, is shown to be required for entry into mitosis through feedback activation of Cdk1 by Cdc25 (5). Immuno-depletion of Plx1 prevented both entry into mitosis and activation of Cdk1 and conversely, the addition of active Plx1 into the cycling extracts promotes premature activation of Cdk1 and entry into mitosis (5, 6). The Aurora A kinase is also shown to play a role in the activation of Cdk1 and entry into mitosis in Xenopus oocyte cycling extracts (7). However, unlike depletion of Plx1, depletion of Aurora A delays but does not block activation of Cdk1 and entry into mitosis (7).
Contrary to Xenopus oocyte cycling extract, depletion of Plk1 by RNAi in human cells resulted in activation of Cdk1 and a mitotic arrest (8), suggesting that Plk1 is not essential for Cdk1 activation of human cells. Consistent with this, inactivation of Plk1 with small molecule kinase inhibitor also results in a mitotic arrest (9). On the other hand, depletion of Aurora A kinase in synchronized HeLa cells by RNAi was reported to prevent Cdk1 activation and entry into mitosis (10). It was proposed that during G2/M transition of human cells, Aurora A kinase functions upstream of Cdk1 and is required for mitotic commitment and initial activation of Cdk1 (10). However, RNAi-mediated inactivation of Aurora A kinase in the randomly growing, non-synchronized HeLa cells did not arrest in G2 as would be expected for a role in Cdk1 activation, but instead the cells were arrested in mitosis with monopolar spindles (11–13). Selective inhibition of Aurora A activity with small molecule kinase inhibitor also gives rise to a similar mitotic phenotype as siRNA2 (14). Mitotic arrest in the absence of Aurora A kinase activity, on the other hand, would indicate that Aurora A kinase does not play an essential role in promoting entry into mitosis or activation of Cdk1. However, these seemly contradictory observations could be explained with a mechanism by which Cdk1, Aurora A, and Plk1 are all part of a feedback activation loop to promote rapid G2/M transition (15).
In this study we further dissected the relationship between Cdk1 and Aurora A kinases during G2/M cell cycle progression by a chemical biology approach. We selectively inactivated Cdk1 and Aurora kinases, respectively, using potent and selective small molecule kinase inhibitors during a synchronized cell cycle of HeLa cells and analyzed the consequences of selective inactivation of the mitotic kinases on G2/M progression and activation of each other. We find that Aurora A kinase is first activated at centrosomes and is required for centrosome separation at late G2 independent of Cdk1 activity. However, inactivation of Aurora A kinase alone shows no apparent effect on Cdk1 activation and entry into mitosis and instead leads to a mitotic arrest with monopolar spindles. On the contrary, inactivation of Cdk1 kinase prevents entry into mitosis and mitotic activation of Aurora A, thus suggesting that Cdk1 acts upstream of Aurora A kinase during G2/M transition. We further show that Aurora A and Plk1 have redundant functions in feedback activation of Cdk1 to promote timely entry into mitosis.
EXPERIMENTAL PROCEDURES
Cell Culture, Cell Synchronization, RNAi, and Compound Treatment
HeLa cells, HeLa S3, U2OS, CaLu-6, NIH-3T3, and BJ cells were obtained from ATCC and maintained as per ATCC instructions. Human umbilical vein endothelial cells were obtained from Lonza and maintained after Lonza instructions. HeLa S3 suspension cells were synchronized by elutriation using a Beckman Avanti J-25i centrifuge with a Sanderson chamber in a JE-6B rotor and a Masterflex peristaltic pump by Cole Parmer (Vernon Hills, IL), basically following the previously established procedures (16). The adherent culture of HeLa cells was synchronized by double thymidine block/release as described by Hirota et al. (10). A human Cdc2 siRNA smart pool was purchased from Dharmacon and used as previously described (13). All kinase inhibitors were prepared as 10 mm DMSO stock and were sequentially diluted in the growth medium. Cells were treated with the kinase inhibitors by medium exchange.
Immunoblotting, Antibodies, and Chemical Reagents
Protein lysates from cultured cells were prepared and analyzed by immunoblotting on nitrocellulose membranes as previously described (13). The following antibodies were used in this study: rabbit anti-human pericentrin from Abcam (Cambridge, UK), mouse anti-human α-tubulin and mouse anti-human γ-tubulin from Sigma, rabbit anti-human phosphohistone H3 (Ser-10) and mouse anti-human cyclin B1 from UBI (Charlottesville, VA), mouse anti-human cyclin B1 from Upstate Biotechnology (Temecula, CA), rabbit anti-human phospho-Cdc2 (Tyr-15) and rabbit anti-human Cdc2 from Cell Signaling (Boston, MA), mouse anti-human cyclin B1 (GNS1) from Santa Cruz (Santa Cruz, CA), and anti-human IAK1/Aurora A kinase antibody from BD Transduction. Several rabbit anti-human phosphohistone H1 antibodies were custom made by Open Biosystems (Huntsville, AL) using the phosphopeptides CAPAEKpTPVKKKA, KKpTPKKAKKPC, CKKVAKpSPKKA, and AKpSPAKAKAC (pS phosphoserine, and pT is phosphothreonine), encompassing potential Cdk1 phosphorylation sites at Thr-18, Thr-154, Ser-172, and Ser-186 based on the histone H1 variant H1e. These antibodies were screened for cross-reactivity with mitotically phosphorylated human histone H1, and the phospho-specific histone H1 (Thr-154) antibody was used in this study. A rabbit anti-human phospho-Aurora A (Thr-288) antibody was raised with the peptide APSSRRTpTLCGTLD. Goat anti-mouse Alexa Fluor 568, goat anti-rabbit Alexa Fluor 647, Sytox Green, and propidium iodide were purchased from Molecular Probes (Eugene, OR). ECL donkey anti-rabbit IgG, horseradish peroxidase (HRP) linked whole antibody and ECL sheep anti-mouse IgG, and HRP linked whole antibody were purchased from Amersham Biosciences. Reconstituted recombinant active Cdk1/cyclin B1 was purchased from UBI, New England BioLabs (Beverly, MA), and Upstate. Purified histone H1 from calf thymus was purchased from Invitrogen. An Aurora A selective kinase inhibitor, MLN8054, discovered by Millennium Pharmaceutical (14), dual Aurora A and Aurora B kinase inhibitors ZM44739 from AstraZeneca (17), VX-680 from Vertex Pharmaceutical (18), a selective Plk1 kinase inhibitor BI2536 from Roche Applied Science (19), and a selective Cdk1 kinase inhibitor RO3306 from Roche Applied Science (20) were synthesized in-house by Eli Lilly chemists based on the publicly disclosed chemical structures. Enzymatic and cell-based activities of these kinase inhibitors are summarized from Lilly in-house assays and are consistent with those reported previously (supplemental Table 1).
Immunoprecipitation and Kinase Assay
For Cdk1 and Aurora A kinase assays after immunoprecipitation, 100 or 325 μg of protein lysates precleared by centrifugation at 10,000 rpm for 10 min at 4 °C were incubated with either 10 μg of cyclin B1 (Santa Cruz) or 3.25 μg of Aurora A (BD Transduction Laboratories) antibody, respectively, for 2 h at 4 °C. The lysates were then incubated with 30 μl of protein A beads for 1.5 h at 4 °C, washed 2 times with lysis buffer, and then washed with kinase assay buffer (4). Cdk1 and Aurora A kinase assays with the immunoprecipitation complex were performed using histone H1 and histone H3 as the substrates, respectively. The reaction was stopped by the addition of SDS sample buffer and heating at 95 °C for 5 min. Phosphorylated histone H1 and histone H3 proteins were separated by SDS-PAGE on 12 and 15% gels, respectively, and visualized by autoradiography.
Immunofluorescence and Confocal Microscopy
For immunofluorescence and confocal microscopy, cells were plated in Lab-Tek 4 chamber slides (Fisher) at 2.5 × 104 per chamber. Cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then permeabilized with 0.2% Triton X-100 for 5 min. After blocking with 1% bovine serum albumin, 1× phosphate-buffered saline for 1 h, cells were incubated with primary antibodies in blocking solution for 1 h. Cells were washed 3× with phosphate-buffered saline and incubated with secondary antibodies at 1:1000 and DNA stain in blocking solution for 1 h. After washing 3× with phosphate-buffered saline, cells were imaged with a Bio-Rad MRC600 confocal microscope at 60× magnification or Zeiss LSM510 confocal microscope at 40× magnification.
Time-lapsed Microscopy and Flow Cytometry
Cell culture and sample preparations for cell cycle analysis by flow cytometry were done as previously described (11, 13). For time-lapsed live cell imaging, cells (1 × 105) were plated in a 60-mm Petri dish containing a 40-mm glass coverslip and incubated overnight at 37 °C and 5% CO2. The Focht Closed System (FCS2) was assembled as described by the manufacturer. The FCS2 chamber with cells were placed on the microscope stage and allowed to equilibrate to 37 °C using the FCS2 temperature controller with a perfusion rate of 4 μl/min. Cells were treated with conditioned medium with or without inhibitor. Differential interference contrast images were taken every 15 min at 40× magnification on a Leica DMI6000 microscope equipped with a Leica digital camera. A U2OS H2B-green fluorescent protein stable cell line was generated similarly according to Kanda et al. (21). The cells were seeded in the live cell imaging slide chamber assembly overnight at 37 °C incubator and then mounted on the RTM-3.0 real time microscope (Richardson Technologies Inc., Toronto, Canada) with constant perfusion at 4 μl/min and 37 °C. The images were collected at 20× magnification for 24–72 h at 15 min/frame.
RESULTS
Inactivation of Aurora Kinases Uncouples the Nuclear Cycle from Cytokinesis
Recently we showed that inactivation of Aurora B or simultaneous inactivation of both Aurora A and B kinases resulted in polyploidy and large multinucleated cells (13). Polyploidy may be generated in two different ways; 1) DNA endoreduplication without intervening mitosis (22) or 2) nuclear division (endomitosis) without cytokinesis (23). To determine how inactivation of Aurora kinases actually leads to polyploidy, we followed the cell cycle by live cell imaging in the presence of a dual Aurora A and B kinase inhibitor.
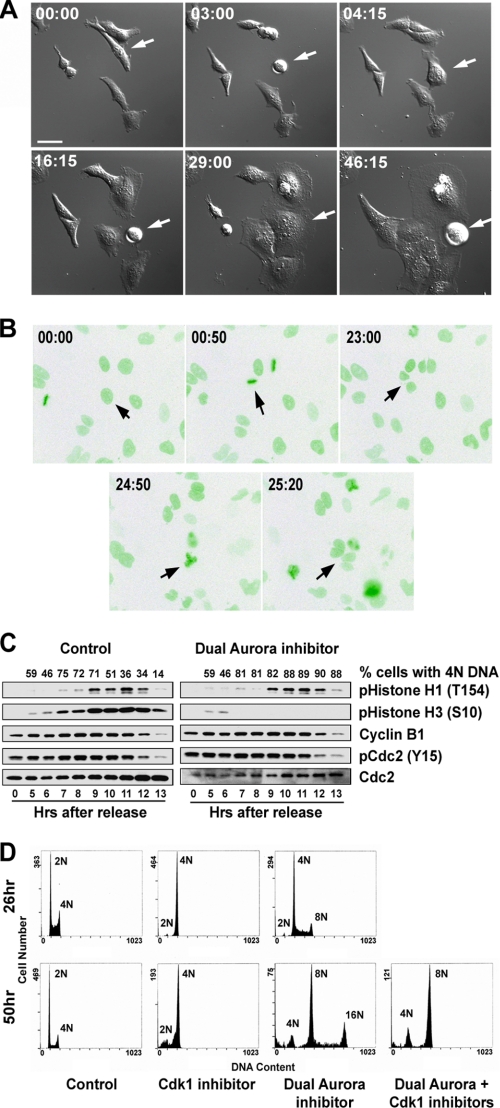
Live cell imaging showed that cells in the presence of the dual Aurora kinase inhibitor, ZM44739, underwent multiple rounds of mitosis, seen as repeated cell round-up, but failed to divide, resulting in increasingly larger cell size after each round of mitosis (Fig. 1A and supplemental Fig. S1). This suggests that the nuclear division cycle may have been uncoupled from cytokinesis and continued without cell division. To directly observe the nuclear division cycle, we tagged the H2B protein with green fluorescent protein, which allows direct visualization of chromosomes in living cells, and then observed the green fluorescent protein-tagged H2B cell growth in the presence of the dual Aurora kinase inhibitor under a fluorescent microscope. Indeed, multiple rounds of nuclear division cycles occurred in the presence of the dual Aurora kinase inhibitor (Fig. 1B and supplemental Fig. S2).
FIGURE 1.
Effects of inactivation of Aurora kinases on cell cycle progression. Repeated nuclear division without cytokinesis in the presence of a dual Aurora kinase inhibitor revealed by live cell imaging is shown. A, shown are snapshots of differential interference contrast images of HeLa cells undergoing repeated rounds of mitosis without the ensuing cytokinesis in the presence of a dual Aurora kinase inhibitor. The arrow points to a cell that underwent three rounds of mitosis with increasing cell size. B, shown are snapshots of fluorescent green fluorescent protein images of U2OS cells expressing green fluorescent protein-H2B in the presence of a dual Aurora kinase inhibitor. The same cell that underwent more than two rounds of mitosis is indicated by arrows in each image. The time after the addition of the dual Aurora kinase inhibitor when the image was acquired is indicated in each image. Increased size of the cells as a function of time after the dual Aurora kinase inhibitor addition (panel A) was noted. Bar = 40 μm. C, shown is a Western blot analysis of a synchronized mitosis in the presence or absence of Aurora kinase activities. Cells were synchronized by double thymidine block/release, and the dual Aurora kinase inhibitor, ZM44739, was added 5 h after thymidine release. The numbers on the top represent the % of cells with 4N DNA. D, nuclear division in the absence of Aurora kinase activities is Cdk1-dependent. DNA contents of synchronized HeLa S3 cells at the times indicated after treatment with a selective Cdk1 kinase inhibitor or a dual Aurora kinase inhibitor or a sequential treatment with a dual Aurora kinase inhibitor first for 24 h followed by a selective Cdk1 kinase inhibitor.
Consistent with live cell imaging, biochemical analysis shows that HeLa cells synchronized by double thymidine block were able to go through a synchronized mitosis in the presence of the dual Aurora kinase inhibitor with a similar kinetic as the DMSO-treated control cells, but they stayed in the 4N DNA content state (Fig. 1C). This includes similar patterns of phosphorylation/dephosphorylation of histone H1 at Thr-154, histone H3 at Ser-10, and Cdc2 at Tyr-15 and accumulation/degradation of cyclin B1 (Fig. 1C). Together, the results demonstrate that cells can undergo mitosis in the absence of Aurora A and Aurora B kinase activities, and consequently, the nuclear division cycle is uncoupled from cytokinesis and continues to oscillate without cytokinesis.
Normally DNA replication occurs once and once only per cell cycle. Cdk1 activity is shown to be essential for entry into mitosis and also for preventing DNA re-replication within a cell cycle (24, 25). To investigate whether the nuclear division cycle in the absence of Aurora A and B kinase activities requires Cdk1, we synchronized HeLa cells at G1 by centrifugal elutriation. We allowed cells to undergo a synchronized mitosis to first uncouple nuclear division from cytokinesis in the presence of the dual Aurora kinase inhibitor. We then added a selective Cdk1 kinase inhibitor, RO3306, to the cells. We hypothesized that if nuclear division in the absence of Aurora kinase activities is Cdk1-independent, then nuclear division should continue in the presence of Cdk1 kinase inhibition. On the other hand, if the addition of the Cdk1 inhibitor prevented further nuclear division, then nuclear division in the absence of Aurora kinase activities would still require Cdk1. As shown in Fig. 1D, the addition of the Cdk1 kinase inhibitor to the cells pretreated with the Aurora kinase inhibitor effectively prevented further increase in DNA content of the cells. These cells had a 4–8N DNA content at the time of Cdk1 inhibitor addition, and their DNA content remained at 4–8N 24 h later, whereas the cells treated with Aurora kinase inhibitor alone had up to 16N DNA content by this time of the experiment (Fig. 1D). Thus, these data show that the nuclear division cycle without Aurora kinase activities still requires Cdk1.
Cdk1-dependent Phosphorylation of Histone H1 at Thr-154 during Mitosis
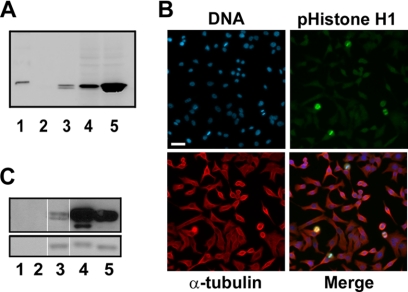
Histone H1 has been used widely as a substrate for in vitro Cdk1 kinase assays. However, whether histone H1 is actually a physiological substrate of Cdk1 has not been well established. We identified several potential Cdk1 phosphorylation sites, Thr-18, Thr-154, Ser-172, and Ser-186, largely conserved in human histone H1 variants based on sequence analysis and generated antibodies using peptides encompassing these phospho-residues as antigens. An antibody against phosphorylated Thr-154 that specifically recognized the mitotically phosphorylated histone H1 by Western blotting was identified (Fig. 2A). This antibody also showed specific staining of the mitotic chromatin (Fig. 2B). A selective Cdk1 kinase inhibitor inhibited histone H1 phosphorylation at Thr-154 in a concentration-dependent manner (data not shown and see Fig. 3). Furthermore, the antibody strongly reacted with histone H1 phosphorylated by Cdk1 in vitro (Fig. 2C). Taken together, our data show that Cdk1 phosphorylates histone H1 at Thr-154 during mitosis. While our study was ongoing, Sarg et al. (26) independently discovered that Cdk1 phosphorylates histone H1 at Thr-154 at mitosis.
FIGURE 2.
Characterization of a Cdk1-dependent phospho-specific histone H1 (Thr(P)-154) antibody. A, shown is Western analysis of phospho-histone H1 antibody (Thr(P)-154) showing specific cross-reactivity with mitotically phosphorylated histone H1. The loading of the gel was as follows: lane 1, molecular weight marker; lane 2, 1 μg of control unphosphorylated histone H1; lane 3, 1 μg of histone H1 isolated from colcemid-treated HeLa cells; lane 4, 28 μg of protein lysates from CaLu-6 cells; lane 5, 28 μg of protein lysates from CaLu-6 cells treated with nocodazole. B, shown is specific staining of mitotic cells by the phospho-specific histone H1 antibody (Thr(P)-154). Blue = DNA; green = phospho-histone H1; red = α-tubulin. Bar = 60 μm. C, shown is a Western blot analysis of histone H1 in vitro phosphorylated by Cdk1 with the phospho-specific histone H1 antibody (Thr(P)-154). Lanes 1 and 2, Cdk1 kinase alone, from UBI and New England BioLabs, respectively; lane 2, unphosphorylated histone H1 protein control; lanes 4 and 5, histone H1 protein phosphorylated in vitro by Cdk1 kinase from UBI and New England BioLabs, respectively. The upper panel is the Western blot, and the lower panel is Coomassie staining of the gel showing equal loading of histone H1 for Western blot analysis.
FIGURE 3.
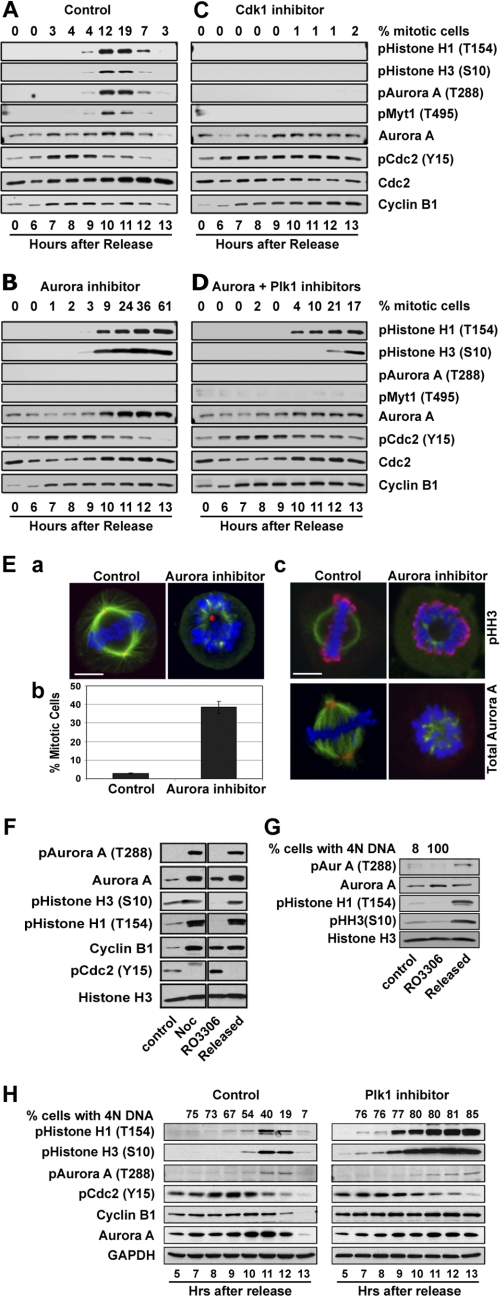
Aurora kinases are not required for entry into mitosis and activation of Cdk1. A, HeLa cells were synchronized by double thymidine block/release. Synchronized cells were treated with DMSO as control (A) or a selective Aurora A kinase inhibitor (B) or a selective Cdk1 kinase inhibitor (C) or a combination of Aurora A and Plk1 kinase inhibitors (D) 5 h after release from thymidine block. Mitotic index was analyzed by flow cytometry and mitotic progression was also followed biochemically by Western blotting for phospho-histone H1 and H3, autophosphorylation of Aurora A (Thr(P)-288), Myt1 phosphorylation at Thr-495, Cdc2 tyrosine 15 phosphorylation (Y15) and cyclin B1 and Aurora A protein levels. Total Cdc2 protein was used as loading control. E, shown is mitotic arrest with monopolar spindles of cells treated with a selective Aurora A kinase inhibitor. a, shown is a representative control and a treated HeLa cell showing normal bipolar mitotic spindle in the control and monopolar spindle in the treated cell. Blue = DNA; red = pericentrin staining of centrosome; green = α-tubulin staining. b, shown is the mitotic index of the control and cells treated with a selective Aurora kinase inhibitor, which was used at a concentration shown to selectively inhibit Aurora A kinase activity. Cells were fixed and stained as shown in panel A. At least 500 cells were counted, and mitotic index is expressed as percentage of cells at mitosis. c, shown is a mitotic control and a treated cell showing phospho-histone H3 and Aurora A staining. Blue = DNA; green = α-tubulin; red = phospho-histone H3 (upper) and Aurora A (lower). Note that the Aurora A kinase inhibitor prevented centrosome localization of the Aurora A protein. Bar = 7 μm. F, Cdk1 inhibitor, RO3306 (9 μm), block/release of HeLa cells is shown. Cells were treated with RO3306 for 20 h and then were released from the block by washing 3× with fresh medium without RO3306. Total cell lysates were analyzed by Western blot analysis for mitotic markers and activation of Aurora A kinase. Mitotic extract of cells treated with nocodazole for 20 h was used as a positive control. G, NIH-3T3 cells were similarly treated with RO3306 as described for panel F for Cdk1-dependent activation of Aurora A kinase during G2/M transition. H, shown is Western blot analysis of a synchronized cell cycle progression in the presence or absence of a selective Plk1 kinase inhibitor, BI2536. HeLa cells were synchronized by double thymidine block, and the Plk1 inhibitor was added 5 h after release from thymidine block. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Activation of Cdk1 Is Upstream of Aurora A Kinase during G2/M Transition
To further elucidate the relationship between Cdk1 and Aurora A kinase during G2/M transition, we took advantage of rapid action by small molecule kinase inhibitors as compared with siRNA and added the kinase inhibitors to synchronized cells as they began to enter into the G2 phase. Furthermore, we followed the cellular activities of Cdk1 and Aurora A by directly analyzing the phosphorylation levels of their cellular substrates using phospho-specific antibodies, including autophosphorylation of Aurora A at Thr-288 (27, 28), Aurora A-dependent phosphorylation of Myt1 at Thr-495 by Plk1 (29), Aurora B phosphorylation of histone H3 at Ser-10 (30, 31), and Cdk1 phosphorylation of histone H1 at Thr-154 as described in Fig. 2. Cell cycle progression after release from thymidine block was monitored using multiple parameters by flow cytometry, immunofluorescence cell staining, and Western blotting. We reasoned that if Aurora A kinase indeed acts upstream of Cdk1, as suggested by Hirota et al. (10), then inactivation of Aurora A kinase during G2/M transition would prevent Cdk1 activation and arrest cells at G2, whereas inactivation of Cdk1 would have no effect on Aurora A kinase activation. Conversely, if Aurora A acts downstream of Cdk1 as some other previous studies suggested (15, 32) or parallel to Cdk1, activation of Cdk1 during G2/M progression would be independent of Aurora A kinase activity.
Upon release from double thymidine block, HeLa cells underwent a synchronous G2/M progression between 9 and 12 h (Fig. 3A). As shown in Fig. 3B, the Aurora kinase inhibitor, MLN8054, which was used at a concentration that selectively inhibits Aurora A activity (14), completely inhibited Aurora A autophosphorylation at Thr-288 and phosphorylation of Myt1 at Thr-495, demonstrating effective inhibition of Aurora A kinase activity by the inhibitor. However, the Aurora kinase inhibitor showed no significant effect on Cdk1 activation as compared with DMSO control (Fig. 3B). In fact, Cdk1 of cells treated with the Aurora kinase inhibitor became dephosphorylated at Tyr-15, and its substrate histone H1 became phosphorylated at Thr-154 to a similar level and at similar kinetics as in the control cells (Fig. 3, A and B). Moreover, the Aurora inhibitor caused sustained histone H1 and H3 phosphorylation and sustained accumulation of Aurora A and cyclin B1, indicating a mitotic block. Indeed, using histone H3 phosphorylation as a mitotic marker, flow cytometry and immunofluorescent microscopy showed that the treated cells had 4N DNA content with markedly elevated mitotic index (Fig. 3B). Immunofluorescence microscopy showed that cells treated with the Aurora A kinase inhibitor had monopolar spindles (Fig. 3E), in agreement with previous reports (14) and similarly as we observed previously with Aurora A specific siRNA (12). During mitosis, Aurora A is normally associated with centrosomes and poles of the mitotic spindle (15). Interestingly, we observed that the Aurora kinase inhibitor completely blocked Aurora A kinase localization to centrosomes or spindle poles (Fig. 3E). A recent report also showed that the kinase activity of Aurora A is important for its localization to the mitotic spindles in Xenopus egg extracts (33).
Mitotic Activation of Aurora A Kinase Requires Cdk1 Activity
In contrast, cells treated with the Cdk1 inhibitor were arrested in G2 with interphase microtubules, 4N DNA content and accumulation of cytoplasmic cyclin B1 (data not shown; Ref. 20). Inactivation of Cdk1 inhibited Aurora A autophosphorylation at Thr-288, Aurora A-dependent phosphorylation of Myt1, and histone H3 phosphorylation by Aurora B kinase (Fig. 3C). Although Aurora A protein accumulated in Cdk1 inhibitor-treated cells, its autophosphorylation at Thr-288 was not detectable by Western blot analysis, together indicating that mitotic activation of Aurora A requires Cdk1 activity. Interestingly, inactivation of Cdk1 also prevented dephosphorylation of itself at Tyr-15, suggesting that normally Tyr-15 dephosphorylation of Cdk1 by Cdc25 requires Cdk1 kinase activity, consistent with a positive feedback activation mechanism (15).
To further investigate Cdk1-dependent mitotic activation of Aurora A, we performed the Cdk1 inhibitor, RO3306, block/release experiment in HeLa cells (Fig. 3F). Cells were arrested in G2 with inactivated Cdk1 and then were released from inhibitor block to allow Cdk1 activation and entry into mitosis in the presence of nocodazole. Cells treated with nocodazole alone without prior treatment with RO3306 were used as a positive mitotic control. In the presence of RO3306, cells were arrested in G2 and had accumulation of inactive Aurora A kinase. Upon release from Cdk1 inhibitor treatment, cells entered into mitosis, and concurrently, the accumulated Aurora A became activated by autophosphorylation at Thr-288. To determine whether mitotic activation of Aurora A kinase in normal non-transformed cells is also similarly dependent on Cdk1, a Cdk1 inhibitor block/release experiment was repeated using NIH-3T3 cells (Fig. 3G). RO3306 effectively blocked NIH-3T3 cells in G2. At G2 arrest by inactivation of Cdk1, accumulated Aurora A kinase was not phosphorylated at Thr-288 and, thus, was inactive (Fig. 3G). Upon release from Cdk1 inhibitor block, cells entered mitosis rapidly, and Aurora A kinase became rapidly activated by autophosphorylation at Thr-288 similarly as observed in HeLa cells. Taken together, the data thus demonstrate that mitotic activation of Aurora A kinase is indeed downstream of Cdk1 in human cells.
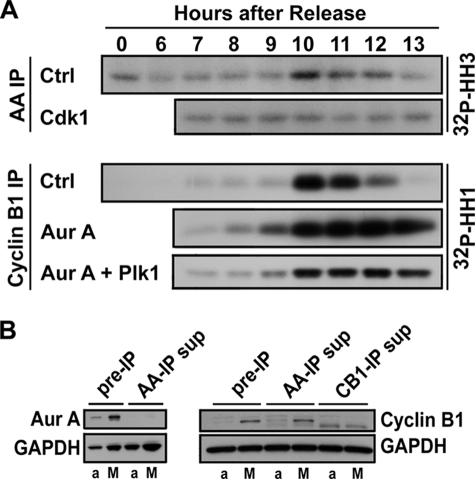
We next assayed Aurora A and Cdk1 kinase activities in vitro after immunoprecipitation during a synchronized cell cycle in the presence or absence of Cdk1 and Aurora kinase inhibitors. Consistent with cellular histone H1 phosphorylation by Western blotting, Cdk1 kinase was transiently activated 10 h after release from thymidine block as cells underwent synchronized G2/M transition (Fig. 4A). Then, as cells progressed through mitosis, Cdk1 activity was down-regulated. Activation of Aurora A kinase followed a similar pattern. Aurora A was also activated 10 h after release from thymidine block and was down-regulated as cells progressed through mitosis from 11 to 13 h after release. In agreement with cellular biochemical data shown in Fig. 3B, the addition of Aurora kinase inhibitor had no effect Cdk1 activity but instead led to sustained activation of Cdk1, as would be expected for a role of Aurora A kinase in mitotic progression downstream from Cdk1. In contrast, the addition of Cdk1 kinase inhibitor abolished mitotic activation of Aurora A kinase normally associated with G2/M progression after release from thymidine block, thus providing further support to the notion that activation of Aurora A kinase during mitosis is downstream of Cdk1 and, furthermore, requires Cdk1 activity. The complete depletion of cyclin B1 and Aurora A by immunoprecipitation from assay protein lysates indicates that Cdk1 and Aurora A kinase activities, as shown in Fig. 4A, should represent their cellular activity levels (Fig. 4B).
FIGURE 4.
In vitro kinase assays. HeLa cells were synchronized by double thymidine block/release and were treated with the kinase inhibitors as described for Fig. 3. A, Cdk1 and Aurora A kinases were immunoprecipitated (IP) using cyclin B1 and Aurora A antibodies, and their kinase activities were assed in vitro with histone H1 (HH1) and histone H3 (HH3) as substrates, respectively. Ctrl, control. B, effective depletion of Aurora A (Aur A) and cyclin B1 by immunoprecipitation is shown. An asynchronous growing cell sample (a) and a mitotic cell sample (M) were incubated with Aurora A (AA-IP)- and cyclin B1 (CB1-IP)-specific antibodies, respectively, under the same conditions for kinase assays by IP as shown in panel A. After removal of the IP complex, the supernatants (sup) were analyzed by Western blotting for completeness of target protein depletion by immunoprecipitation. Please note that immunoprecipitation completely depleted Aurora A and cyclin B1 from both asynchronous and mitotic cells samples. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Aurora A and Plk1 Have a Redundant Role in Feedback Activation of Cdk1 during G2/M Transition
We suspected that Aurora A and Plk1 kinases may have a redundant role in Cdk1 activation during G2/M transition. To explore this possibility, we first investigated whether Plk1 plays a role in Cdk1 activation with a selective Plk1 kinase inhibitor in a similar manner as described above for Aurora kinase inhibitor treatment in a synchronized mitosis. Similar to inactivation of Aurora A kinase, inactivation of Plk1 also had no significant effect on Cdk1 activation and entry into mitosis, as evidenced by a concurrent decrease in Cdc2 Tyr-15 phosphorylation and increase in histone H1 phosphorylation in similar kinetics as those in the DMSO control cells (Fig. 3H). Moreover, sustained high levels of phosphorylated histone H1 and H3 and accumulation of Aurora A and cyclin B1 and 4N DNA content together indicate that cells with inactive Plk1 were arrested at mitosis, in agreement with previous publications (9). We then simultaneously inactivated Aurora A and Plk1 kinases with selective kinase inhibitors during a synchronized cell cycle and followed cell cycle progression into mitosis (Fig. 3D). Cells treated with both Aurora and Plk1 kinase inhibitors had a marked decrease in mitotic index as compared with cells treated with Aurora kinase inhibitor alone. This drop in mitotic index in the presence of both kinase inhibitors was associated with a decrease in Cdk1 activation. A substantial fraction of Cdk1 remained to be phosphorylated at Tyr-15 13 h after release from thymidine block, and the level of phosphorylated histone H1 was markedly decreased (Fig. 3D). Indeed, the in vitro Cdk1 kinase assay further showed that cells treated with both Aurora and Plk1 kinase inhibitors had significantly lower Cdk1 kinase activity (Fig. 4A). The data, thus, suggest that Aurora A and Plk1 likely have redundant roles in the feedback activation of Cdk1 during G2/M transition in human cells.
Cdk1-independent Activation of Aurora A Kinase at Centrosomes in G2
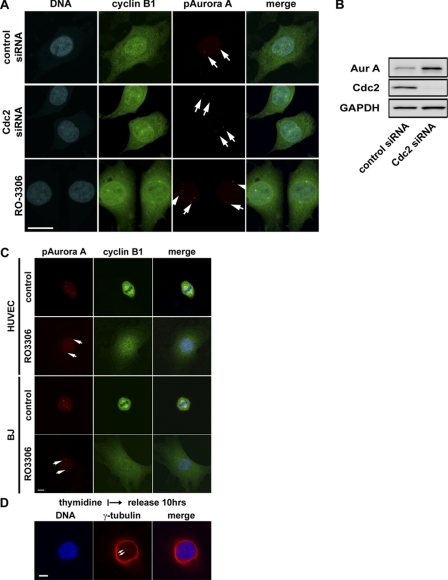
Active Aurora A kinase first appears at the centrosomes in late G2 or early prophase (Fig. 5A). To our surprise, inactivation of Cdk1 in HeLa cells either by a selective small kinase inhibitor or siRNA, which effectively inhibited Cdc2 expression (Fig. 5B), had no effect on the activation of Aurora A kinase at centrosomes (Fig. 5A). Furthermore, centrosomes were separated apparently normally at G2 in the absence of Cdk1 kinase activity. Similarly, inactivation of Cdk1 in non-transformed human umbilical vein endothelial and BJ human cells also arrested cell cycle at G2 with activated Aurora A localized to separated centrosomes (Fig. 5C).
FIGURE 5.
Cdk1-independent activation of Aurora A at centrosomes and centrosome separation at G2. A, representative images of HeLa cells treated with the small molecule Cdk1 inhibitor, RO3306 (9 μm), or Cdk1-specific siRNA and stained for cyclin B1, phospho-specific Aurora A (Thr-288), and DNA are shown. Bar = 20 μm. B, Western blots show effective knockdown of Cdc2 proteins by the Cdc2-specific siRNA oligo. Accumulation of Aurora A (Aur A) kinase in the absence of Cdk1 activity is consistent with G2 cell cycle arrest. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C, shown are representative images of human umbilical vein endothelial (HUVEC) and BJ cells, non-transformed human cells treated with the Cdk1 kinase inhibitor RO3306 (9 μm) and stained for cyclin B1, and phospho-specific Aurora A (pAurora A; Thr-288) and DNA. Bar = 10 μm. D, shown are representative images of HeLa cells treated with Cdk1 and Aurora kinase inhibitors as indicated and stained for DNA and γ-tubulin. Bar = 10 μm. Cells with inactivated Cdk1 were cleanly arrested at G2. Several hundred cells were examined under the microscope, and all had cytoplasmic cyclin B1 accumulation and activated Aurora A kinase localized to centrosomes which are well separated.
As shown in Fig. 3E, Aurora A kinase activity is required for its association with centrosomes and for centrosome separation for the formation of bipolar mitotic spindles. To determine whether Aurora A kinase activity associated with centrosomes is required for centrosome separation at late G2, we inactivated both Aurora A and Cdk1 kinase activities by treatment of HeLa cells with both Aurora and Cdk1 kinase inhibitors. Concurrent inactivation of both Cdk1 and Aurora A kinase arrested cells in G2 and, as suspected, prevented centrosome separation (Fig. 5D). No activated Aurora A kinase was detectable in centrosomes of cells treated, as would be expected, with both Cdk1 and Aurora A kinase inhibitors (data not shown). Together, the data indicate that activation of Aurora A kinase at the centrosomes is independent of Cdk1 kinase activity and is required for centrosome separation in late G2.
DISCUSSION
This study focuses on elucidating the relationship between Cdk1 and Aurora A kinase during G2/M transition of human cells. We employed a chemical biology approach using selective small molecule kinase inhibitors to inactivate the mitotic kinases and then analyzed the effect of their inactivation on entry into mitosis and on activation of the other mitotic kinases during synchronized cell cycle progression. We also followed the kinase activities directly through analysis of in vivo phosphorylation of their physiological substrates using phospho-specific antibodies. We find that Aurora A kinase is first activated at centrosomes and is required for centrosome separation in late G2 independently of Cdk1 activity. Upon entry into mitosis, Aurora A becomes mitotically activated in a Cdk1-dependent manner. We show that inactivation of Cdk1 with a small molecule kinase inhibitor arrests cells in G2 and prevents mitotic activation of Aurora A kinase, suggesting that Aurora A kinase acts downstream of Cdk1 during G2/M transition. Indeed, chemical inactivation of Aurora A kinase has no significant effect on Cdk1 activation and entry into mitosis. Instead, cells are arrested at mitosis with activated Cdk1, similarly as previously described for inactivation of Aurora A kinase by RNAi-mediated knockdown (13). Together, our data show that Cdk1 kinase plays a major and essential role in promoting entry into mitosis upstream of mitotic activation of Aurora A kinase activity.
To address the relationship between Cdk1 and Aurora A in human cells, Hirota et al. (10) used Aurora A-specific siRNA to inactivate Aurora A in HeLa cells between two thymidine block/release that was used to synchronize the cell cycle. They found that RNAi inactivation of Aurora A prevented activation of Cdk1 and entry into mitosis of HeLa cells. They, thus, proposed that Aurora A kinase is required for the initial activation of Cdk1 and entry into mitosis. However, we and others showed that RNAi inactivation of Aurora A in non-synchronized HeLa cells does not block entry into mitosis but, instead, arrests cells in mitosis with monopolar spindles (11, 12, 34), suggesting that to the contrary, Aurora A kinase is not essential for Cdk1 activation and entry into mitosis. To further resolve the relationship between Cdk1 and Aurora A kinases during G2/M transition of human cells, in this study we employed a chemical biology approach using selective small molecule kinase inhibitors. Here we provide multiple lines of evidence to show that Aurora A kinase is not essential for activation of Cdk1 and entry into mitosis. First, selective inactivation of Aurora A with a small molecule kinase inhibitor arrested cells at mitosis with monopolar spindles, the same phenotype also induced by Aurora A-specific siRNA as we and others described previously (11–13). Second, we repeated the cell synchronization experiment as described by Hirota et al. (10) with selective small molecule kinase inhibitors and took advantage of their rapid actions as compared with siRNA to inactivate Aurora A and Cdk1 activity specifically in G2. As in non-synchronized cells, inactivation of Aurora A kinase during a synchronized G2/M progression did not inhibit entry into mitosis but instead arrested cells at mitosis with activated Cdk1. Furthermore, the time course showed that mitotic progression and Cdk1 activation in the presence or absence of Aurora A kinase activity occurred with similar kinetics. Third, inactivation of Cdk1 blocked entry into mitosis and mitotic activation of Aurora A. Therefore, our data convincingly show that Cdk1 is an upstream kinase and is required for both entry into mitosis and mitotic activation of Aurora A kinase. Our current findings in human cells are consistent with Aurora A functions in Drosophila where Aurora A was originally identified and characterized. In Drosophila, Aurora A kinase plays an important and essential role in centrosome maturation and separation for bipolar mitotic spindle formation (35). Additionally, Cdk1-dependent activation of Aurora A kinase was also previously observed in Xenopus oocyte (36) and human cells (32).
We also show that like Aurora A kinase, Plk1 kinase does not play an essential role in entry into mitosis and activation of Cdk1 in human cells, in agreement with several recent studies (8, 34). However, simultaneous inactivation of both Aurora A and Plk1 kinases markedly delays Cdk1 activation and entry into mitosis, suggesting that Aurora A and Plk1 play a redundant role in Cdk1 feedback activation during G2/M transition. Consistent with this, Cdk1 remains largely phosphorylated at Tyr-15 in the absence of Aurora A and Plk1 activities. In further support of feedback activation of Cdk1, Cdk1 remains phosphorylated at Tyr-15 in G2 when its activity was inhibited. Therefore, Cdk1, Aurora A, and Plk1 are likely to act in a positive feedback activation loop for Cdk1 activation through Cdc25-mediated dephosphorylation during G2/M transition. Indeed, human Plk1 and Aurora A are shown to be able to phosphorylate and activate Cdc25 phosphatase activity toward Cdk1 in vitro (37). Feedback activation of Cdk1 through multiple mitotic kinases would provide a platform for checkpoint regulation in response to different types of cell cycle perturbations and also provides a molecular explanation how Cdk1-independent expression of Plk1 or Aurora A overrides the G2/M DNA damage checkpoint arrest (15, 38, 39). Recent studies show that Plk1 plays an essential role in the recovery from G2/M DNA damage-induced arrest in human cells (40).
In summary, our results suggest that Cdk1, Aurora A, and Plk1 form a positive feedback activation loop in human cells. Activation of Cdk1 initiates the entry into mitosis and mitotic activation of Aurora A and Plk1 kinases. Activated Aurora A and Plk1 then further feedback-activate Cdk1 to promote rapid and timely entry into mitosis and coordinately regulate various aspects of mitosis, such as bipolar mitotic spindle formation and checkpoint response. In addition, inactivation of both Aurora A and Aurora B kinases uncouples the nuclear division cycle from cytokinesis leading to polyploidy and multinucleated cells.
Supplementary Material
Acknowledgments
We thank Shufen Cai, Teresa Burke, and Jack Dempsey for the analysis of custom-made antibodies. We thank Dr. Dan Li for the analysis of human histone H1 sequence and identification of potential Cdk1 phosphorylation sites. We also thank Dr. Jake Starling and Dr. Richard Gaynor for interest and support throughout this research effort.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2 and Table 1.
- siRNA
- small interfering RNA
- RNAi
- RNA interference.
REFERENCES
- 1.Nigg E. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 21–32 [DOI] [PubMed] [Google Scholar]
- 2.Nurse P. (1994) Cell 79, 547–550 [DOI] [PubMed] [Google Scholar]
- 3.Osmani S. A., Ye X. S. (1996) Biochem. J. 317, 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X. S., Xu G., Pu R. T., Fincher R. R., McGuire S. L., Osmani A. H., Osmani S. A. (1995) EMBO J. 14, 986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Y. W., Erikson E., Taieb F. E., Maller J. L. (2001) Mol. Biol. Cell 12, 1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abrieu A., Brassac T., Galas S., Fisher D., Labbé J. C., Dorée M. (1998) J. Cell Sci. 111, 1751–1757 [DOI] [PubMed] [Google Scholar]
- 7.Liu Q., Ruderman J. V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5811–5816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Erikson R. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8672–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., Peters J. M. (2007) Curr. Biol. 17, 304–315 [DOI] [PubMed] [Google Scholar]
- 10.Hirota T., Kunitoku N., Sasayama T., Marumoto T., Zhang D., Nitta M., Hatakeyama K., Saya H. (2003) Cell 114, 585–598 [DOI] [PubMed] [Google Scholar]
- 11.Du J., Hannon G. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8975–8980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kufer T. A., Silljé H. H., Körner R., Gruss O. J., Meraldi P., Nigg E. A. (2002) J. Cell Biol. 158, 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H., Burke T., Dempsey J., Diaz B., Collins E., Toth J., Beckmann R., Ye X. (2005) FEBS Lett. 579, 3385–3391 [DOI] [PubMed] [Google Scholar]
- 14.Manfredi M. G., Ecsedy J. A., Meetze K. A., Balani S. K., Burenkova O., Chen W., Galvin K. M., Hoar K. M., Huck J. J., LeRoy P. J., Ray E. T., Sells T. B., Stringer B., Stroud S. G., Vos T. J., Weatherhead G. S., Wysong D. R., Zhang M., Bolen J. B., Claiborne C. F. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4106–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindqvist A., Rodríguez-Bravo V., Medema R. H. (2009) J. Cell Biol. 185, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl A. F., Donaldson K. L. (2003) in Current Protocols in Cell Biology (Bonifacino J. S., Dasso M., Harford J. B., Lippincott-Schwartz J., Yamada K. M. eds) pp. 8.5.1–8.5.9, John Wiley & Sons, Inc., New York [Google Scholar]
- 17.Ditchfield C., Johnson V. L., Tighe A., Ellston R., Haworth C., Johnson T., Mortlock A., Keen N., Taylor S. S. (2003) J. Cell Biol. 161, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gizatullin F., Yao Y., Kung V., Harding M. W., Loda M., Shapiro G. I. (2006) Cancer Res. 66, 7668–7677 [DOI] [PubMed] [Google Scholar]
- 19.Steegmaier M., Hoffmann M., Baum A., Lénárt P., Petronczki M., Krssák M., Gürtler U., Garin-Chesa P., Lieb S., Quant J., Grauert M., Adolf G. R., Kraut N., Peters J. M., Rettig W. J. (2007) Curr. Biol. 17, 316–322 [DOI] [PubMed] [Google Scholar]
- 20.Vassilev L. T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D. C., Chen L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10660–10665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda T., Sullivan K. F., Wahl G. M. (1998) Curr. Biol. 8, 377–385 [DOI] [PubMed] [Google Scholar]
- 22.Fisher D. L., Nurse P. (1996) EMBO J. 15, 850–860 [PMC free article] [PubMed] [Google Scholar]
- 23.Geddis A. E., Fox N. E., Tkachenko E., Kaushansky K. (2007) Cell Cycle 6, 455–460 [DOI] [PubMed] [Google Scholar]
- 24.Blow J. J., Dutta A. (2005) Nat. Rev. Mol. Cell Biol. 6, 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuarin J., Buck V., Nurse P., Millar J. B. (2002) Cell 111, 419–431 [DOI] [PubMed] [Google Scholar]
- 26.Sarg B., Helliger W., Talasz H., Förg B., Lindner H. H. (2006) J. Biol. Chem. 281, 6573–6580 [DOI] [PubMed] [Google Scholar]
- 27.Bayliss R., Sardon T., Vernos I., Conti E. (2003) Mol. Cell 12, 851–862 [DOI] [PubMed] [Google Scholar]
- 28.Eyers P. A., Maller J. L. (2004) J. Biol. Chem. 279, 9008–9015 [DOI] [PubMed] [Google Scholar]
- 29.Macùrek L., Lindqvist A., Lim D., Lampson M. A., Klompmaker R., Freire R., Clouin C., Taylor S. S., Yaffe M. B., Medema R. H. (2008) Nature 455, 119–123 [DOI] [PubMed] [Google Scholar]
- 30.Adams R. R., Maiato H., Earnshaw W. C., Carmena M. (2001) J. Cell Biol. 153, 865–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giet R., Glover D. M. (2001) J. Cell Biol. 152, 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marumoto T., Hirota T., Morisaki T., Kunitoku N., Zhang D., Ichikawa Y., Sasayama T., Kuninaka S., Mimori T., Tamaki N., Kimura M., Okano Y., Saya H. (2002) Genes Cells 7, 1173–1182 [DOI] [PubMed] [Google Scholar]
- 33.Sardon T., Peset I., Petrova B., Vernos I. (2008) EMBO J. 27, 2567–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane H. A., Nigg E. A. (1996) J. Cell Biol. 135, 1701–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glover D. M., Leibowitz M. H., McLean D. A., Parry H. (1995) Cell 81, 95–105 [DOI] [PubMed] [Google Scholar]
- 36.Maton G., Thibier C., Castro A., Lorca T., Prigent C., Jessus C. (2003) J. Biol. Chem. 278, 21439–21449 [DOI] [PubMed] [Google Scholar]
- 37.Roshak A. K., Capper E. A., Imburgia C., Fornwald J., Scott G., Marshall L. A. (2000) Cell. Signal. 12, 405–411 [DOI] [PubMed] [Google Scholar]
- 38.Cazales M., Schmitt E., Montembault E., Dozier C., Prigent C., Ducommun B. (2005) Cell Cycle 4, 1233–1238 [DOI] [PubMed] [Google Scholar]
- 39.Krystyniak A., Garcia-Echeverria C., Prigent C., Ferrari S. (2006) Oncogene 25, 338–348 [DOI] [PubMed] [Google Scholar]
- 40.van Vugt M. A., Brás A., Medema R. H. (2004) Mol. Cell 15, 799–811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.