Abstract
A major mechanism of hypercoagulability in the antiphospholipid syndrome (APS) is antiphospholipid antibody (aPL)-mediated up-regulation of tissue factor (TF) on monocytes via activation of toll-like receptors (TLR), p38 mitogen activated protein kinase (MAPK) and nuclear factor (NF) κB pathways. We examined whether monocyte signalling pathways are differentially activated by IgG from patients with vascular thrombosis (VT) alone compared with IgG from patients with pregnancy morbidity (PM) alone. We purified IgG from 49 subjects. A human monocyte cell line and ex vivo healthy monocytes were treated with 100μg/ml IgG for 6 hours and cell extracts examined by immunoblot using antibodies to p38MAPK and NFκB. To further investigate intracellular signalling pathways induced by these IgG, specific inhibitors of p38MAPK, NFκB, TLR4 and TLR2 were used to determine their effect on TF activity. Only IgG from patients with VT but no PM (VT+/PM−) caused phosphorylation of NFκB, p38MAPK and up-regulation of TF activity in monocytes. These effects were not seen with IgG from patients with PM alone (VT−/PM+), aPL-positive patients without APS or healthy controls. TF up-regulation caused by the VT+/PM− samples was reduced by inhibitors of p38MAPK, NFkB, and TLR4. The effects of VT+/PM− IgG on signalling and TF up-regulation were concentrated in the fraction that bound b-2-glycoprotein I. Our findings demonstrate that IgG from patients with diverse clinical manifestations of APS have differential effects upon phosphorylation of NFkB, p38MAPK and TF activity which may be mediated by differential activation of TLR4.
Introduction
The antiphospholipid syndrome (APS) is diagnosed in patients who suffer vascular thromboses (VT) and/or pregnancy morbidity (PM) in association with persistently positive blood tests for antiphospholipid antibodies (aPL) (1, 2). APS is the commonest cause of acquired venous and arterial thrombosis (3) and the most important treatable cause of recurrent miscarriage (4). Prospective clinical studies have shown a significant association between aPL and arterial and venous thrombosis (5) as well as PM (6). Patients with APS develop a wide range of clinical manifestations (7) and pathogenic aPL have been shown to exert their thrombotic effects through interactions with endothelial cells (EC) (8), platelets (9) and monocytes (10).
aPL are commonly identified by the anticardiolipin (aCL) enzyme-linked immunosorbent assay (ELISA), anti-beta-2-glycoprotein I (β2GPI) ELISA and lupus anticoagulant (LA) assay (2). Some patients who test positive in these assays will develop VT, others PM, some will have both and some will develop neither despite the persistent presence of serum aPL (7). Fewer than 4.2% of patients with PM due to APS go onto develop VT (11, 12). This study investigates the hypothesis that aPL present in these VT−/PM+ patients lack the ability to act on target cells to promote thrombosis. In particular, we compared the ability of polyclonal IgG from VT+/PM− or VT−/PM+ patients with APS to increase activity of tissue factor (TF), the major initiator of coagulation produced by monocytes.
TF expression is increased in monocytes from patients with APS (13, 14) and on healthy monocytes exposed to aPL in vitro (15, 16). This aPL-mediated up-regulation of TF in monocytes occurs via extracellular signal regulated kinase (ERK)-1, p38-mitogen activated protein kinase (MAPK) and nuclear factor (NF) κB signalling pathways (10, 17). Similarly, aPL-mediated activation of p38MAPK and NFκB pathways has been shown in cultured EC (18) and toll-like receptor (TLR) 4 has been implicated in this process by both in vitro (19) and in vivo (20) studies. aPL react with the β2GPI-TLR4-Annexin A2 complex in human monocyte plasma membranes (21). TLR2 is implicated in the inflammatory activation of mouse fibroblasts by human aPL, but there are no previous studies of the effects of aPL on TLR2 in monocytes (22).
Previous studies (10, 17) of the effects of aPL upon monocytes, have tested samples of purified polyclonal aPL from limited numbers of patients with mostly VT alone. Only one study however, has clearly examined large numbers of patients with different manifestations of the APS; VT alone and PM alone (23). Interestingly, by proteomic analysis of monocytes isolated from 51 patients with the APS this group identified the differential expression of several monocyte proteins between the different clinical sub-groups. Thus, reinforcing our hypothesis that aPL from patients with different clinical manifestations of the APS may have differential effects upon target cells. In this study we compared the effects of a large number of polyclonal IgG samples, derived from different APS patient subsets and control groups, on TLR, p38MAPK and NFκB signalling pathways as well as TF function in a human monocyte cell line and ex vivo healthy monocytes.
Materials and Methods
Patients
Serum samples from 49 individuals were obtained for this study from patients under our care at University College London Hospital (UCLH), London, UK and through Professor Silvia Pierangeli at University of Texas Medical Branch, Galveston, USA and Dr Ware Branch at University of Utah Health Services Center, Salt Lake City, Utah, USA. All subjects signed consent forms approved by the local ethics committees at each institution. Of 27 patients fulfilling the classification criteria for APS (2) ten had a history of VT alone (VT+/PM−), seven had both VT and PM (VT+/PM+) and ten had experienced only PM (VT−/PM+). Serum samples from patients were collected after the clinical event and stored at −80 °C. Twelve patients (nine with SLE) were aPL positive but lacked the APS (aPL+/APS−) and ten healthy individuals were aPL and APS negative (aPL-negative).
Purification and immunological characterisation of IgG
IgG was purified from all serum samples by protein G sepharose affinity chromatography (GE Healthcare Lifesciences, Sweden), passed through Detoxi-Gel™ Endotoxin removing columns (Thermo Scientific, UK) and confirmed to be endotoxin free (<0.06 endotoxin units/ml) by the Limulus Amoebocyte Lysate assay (Sigma, UK). The concentration of purified IgG was determined using the Nanodrop ND-1000 Spectrophotometer (LabTech International, UK). The aCL and anti-β2GPI activity of IgG was measured as previously described (24) using international calibrators in G phospholipid units (GPLU, from Louisville APL Diagnostics, USA) and the IgG Sapporo standard, HCAL (Center for Disease Control, USA) (2). LA activity was measured by clinical assays in the routine hospital laboratory using the dilute Russell's viper venom time (dRVVT) test.
To establish the effects of exposing monocytes to IgG, initially we used pooled samples from the four groups of subjects VT+/PM−, VT−/PM+, aPL+/APS− and aPL-negative. To ensure reproducibility of results we tested two different pooled samples, obtained by combining an equal concentration of IgG from several individual samples, for each of the four groups. In each group the first pooled sample was derived from five individuals and the second was derived from seven individuals (two subjects were common to both pools in each group). aCL and anti-β2GPI binding of each pooled IgG sample were tested at 100μg/ml. Pooled IgG from both VT+/PM− and VT−/PM+ groups had high aCL (>52 GPLU) and anti-β2GPI (>136% binding compared to a concentration of 100μg/ml HCAL) binding. Pooled IgG from the aPL+/APS− groups had moderate aCL (33 GPLU) but low anti-β2GPI (26% binding compared to a concentration of 100μg/ml HCAL) activity, whilst pooled IgG from the aPL-negative group did not bind either CL or β2GPI.
Affinity purification of APS-IgG that bind β2GPI
Human β2GPI (1.5mg) (Louisville APL Diagnostics, USA) was coupled to 1ml of CNBr-activated Sepaharose 4B (Pharmacia). Total IgG fractions from APS patients were applied to the β2GPI column and incubated overnight at 4°C, after which the column was washed. Eluted 1ml fractions containing IgG anti-β2GPI antibodies were obtained by applying 0.1M glycine, pH 2.7 and immediately neutralized with 100ml of 1M Tris, pH 9.0. Eluted fractions were concentrated (YM-30 centrifugal filter devices, Millipore) and resuspended into PBS, pH 7.4. In our hands this technique enables us to obtain IgG samples enriched for anti-β2GPI such that the binding of anti-β2GPI in the affinity-purified fraction is more than twice as high as in the residual sample that did not bind the column.
Isolation of healthy monocytes
Peripheral venous blood samples from a healthy donor were used to isolate mononuclear blood cells by Ficoll-Paque Plus (GE Healthcare) density gradient centrifugation. Monocytes were purified using the immunomagnetic Easysep human CD14+ve selection protocol (StemCell Technology,UK). Purified monocytes (1×105/ml) were cultured with serum-free RPMI 1640 medium and treated with 100μg/ml purified IgG or 3μg/ml LPS for six hours. The six-hour incubation period has been shown to be appropriate for experiments addressing the effects of aPL on monocytes by Lopez-Pedrera et al. (10, 23) and was also consistent with the results of our time-course experiments on the U937 cell line (see below).
In vitro exposure of monocytes to IgG and inhibitors
The human promonocytic (U937) cell line - (ECACC, UK), derived from a patient with generalised histiocytic lymphoma (25) - was maintained in RPMI 1640 medium containing 10% foetal calf serum (FCS), 100units/ml penicillin and 100μg/ml streptomycin. U937 cells (1×105/ml) were incubated with 100μg/ml purified IgG or 3μg/ml LPS or 100ng/ml TNFα for time periods between 5 minutes and 24 hours.
In some experiments, cells were pre-treated with specific inhibitors for 30 minutes prior to exposure to IgG. Bay 11-7082 (Alexis Biochemicals, UK) a specific inhibitor of NFκB activity, was used at 50μM. SB203580 (Calbiochem, UK), a specific p38MAPK inhibitor, was used at 1μM. All inhibitors were dissolved in <1% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS). Anti-human TLR2 antibody (eBioscience, USA) and E. coli K12 msbB LPS, a TLR4 antagonist (InvivoGen, USA) were used at 1μg/ml. To confirm specific effects of these inhibitors, we repeated experiments using 1% DMSO/PBS alone or SB202474 (Calbiochem), a non-functional analogue of SB203580 in DMSO.
Western blotting for the analysis of NFκB and p38MAPK signalling pathways
Cell extracts were prepared by addition of 100ml lysis buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 2mM EDTA, 1% NP-40, 0.1% SDS, 10mM NaF, 1mM Na3VO4, complete mini protease inhibitor cocktail tablets [Roche, UK]). Samples were resolved on a 10% SDS polyacrylamide gel electrophoresis under reducing conditions, transferred to nitrocellulose membranes, blocked and incubated overnight at 4°C with 1:1,000 dilution of rabbit monoclonal anti-human phosphorylated (Ser536) and total NFκB p65, phosphorylated (Thr180/Tyr182) and total p38MAPK, followed by 1 hour incubation in 1:2,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG. Equivalent protein loading was demonstrated using an anti-human actin polyclonal antibody (Santa Cruz Biotechnology, USA). Protein bands were visualised by chemiluminescence (GE Healthcare) and their intensity quantified by densitometric analysis (QuantityOne software, Biorad, USA) and results were expressed as a ratio of relative expression.
Assay for monocyte TF activity
Monocyte TF activity was determined using a chromogenic assay (Actichrome TF; American Diagnostica, USA) that measures factor Xa after activation by the TF-factor VII complex. U937 cells were treated for six hours with IgG (100μg/ml) and cell lysates then tested for TF activity.
Statistical analysis
Non-parametric statistical analyses were performed. Mean values are shown and Mann-Whitney-Wilcoxon scores carried out to compare two groups of unpaired data using GraphPad Prism software 4.0c (GraphPad Software, San Diego, USA).
Results
Characteristics of subjects and IgG samples
Table I gives the relevant clinical and laboratory features of the 49 subjects. 46 (94%) were women. aCL and anti-β2GPI levels and LA positivity were similar in the three APS groups (VT+/PM−, VT−/PM+ and VT+/PM+) but considerably lower in the aPL+/APS− group as expected since higher titres of IgG aCL and IgG anti-β2GPI are markers of increased risk of developing VT or PM (2). The comparable levels of aPL detected by all three assays in the VT+/PM− and VT−/PM+ groups indicate that differences in the functional effects of IgG from these two groups are not likely to be due to differences in levels of aPL.
Table I. Clinical and laboratory features of the subjects used as a source of IgG.
| VT+/PM− (n=10) |
VT−/PM+ (n=10) |
VT+/PM+ (n=7) |
aPL+/APS− (n=12) |
aPL-negative (n=10) |
|
|---|---|---|---|---|---|
| Age (mean yrs±SEM) | 51.8±4.8 | 57.1±3.3 | 45.3±3.4 | 50.4±4.4 | 33.6±2.9 |
| Sex | 1Male/9Female | 10Female | 7Female | 1Male/11Female | 1Male/9Female |
| PAPS | 5(50%) | 7(70%) | 6(86%) | 0 | 0 |
| SAPS | 5(50%) | 3(30%) | 1(14%) | 0 | 0 |
| Other ARD | 5SLE | 3SLE | 1SLE | 9SLE | 0 |
| Live Births | 8 | 17 | 7 | 8 | 4 |
| Total APS− related PM | 0 | 20 (10FT, 10ST, 1 pre- eclampsia) |
13 (4FT, 6ST, 1 pre- eclampsia) |
0 | 0 |
| Arterial – VT | 5 (3stroke, 5TIA) |
0 | 6 (5stroke) | 0 | 0 |
| Venous – VT | 5 (4DVT, 1PE) | 0 | 6 (4DVT, 3PE) |
0 | 0 |
| LA positive | 8 (80%) | 9 (90%) | 6, 1NT | 3 (25%) | 0 |
| aCL (mean GPLU±SEM) |
62.5±8.8 | 52.2±9.5 | 77.3±12.2 | 33.4±4.9 | 0 |
| Anti-β2GPI* (mean OD±SEM) |
148 (0.90±0.12) |
136 (0.83±0.09) |
90 (0.69±0.15) |
26 (0.18±0.06) |
0 |
| Aspirin | 3 | 8 | 2 | 4 | 0 |
| Warfarin | 10 | 2§ | 6 | 0 | 0 |
| Corticosteroids** | 3 | 1 | 1 | 6 | 0 |
| Immunosuppressives** | 2 | 2 | 1 | 7 | 0 |
Abbreviations: aCL, anticardiolipin; GPLU, IgG phospholipid units; ARD, Autoimmune rheumatic disease; DVT, Deep Vein Thrombosis; FT, First trimester; ST, Second trimester; PL, Pregnancy Loss; TIA, Transient Ischaemic Attack; PE, pulmonary embolus; SLE, Systemic Lupus Erythematosus; LA, Lupus Anticoagulant; NT, Not Tested.
Anti-β2GPI activity was calculated as mean % binding to a concentration of 100μg/ml HCAL.
In the VT+/PM− group one patient was taking 7mg prednisolone, a second was taking 100mg azathioprine and a third patient 10mg prednisolone and 100mg azathioprine. In the VT−/PM+ group one patient was taking 4mg prednisolone and 50mg azathioprine whilst another patient was taking 400mg hydroxychloroquine. Two of the VT−/PM+ were given warfarin on clinicians judgement for primary prevention and/or warfarin responsive headache with normal brain scan.
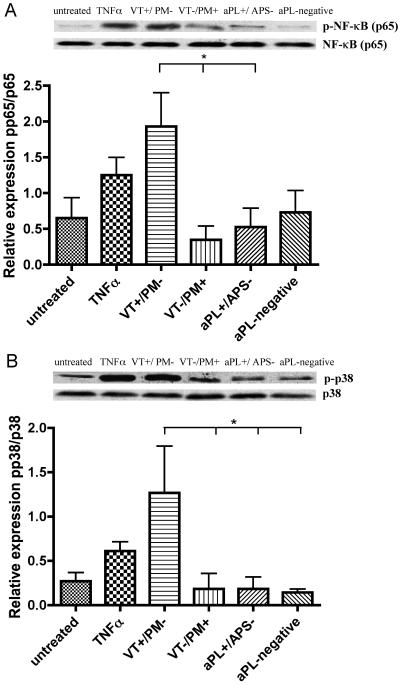
Pooled IgG from VT+/PM− patients but not VT−/PM+ promoted phosphorylation of NFκB and p38MAPK in U937 cells
To establish the effects of exposing monocytes to IgG, initially we used pooled IgG samples from the four clinical groups VT+/PM−, VT−/PM+, aPL+/APS− and aPL-negative. U937 cells were treated with pooled IgG for 0, 5, 10 and 15 minutes, 1, 6, and 24 hours. Maximal differences in phosphorylation of NFκB and p38MAPK were observed between the different groups after six hours exposure to IgG (as shown in Figure 1). U937 cells exposed to these IgG or to TNFa between 0 minutes and 1 hour had very little phosphorylation of NFκB and p38MAPK (too little to see differences between untreated cells and positive control), whereas cells cultured for 24 hours under any conditions (i.e exposure to IgG from all groups, TNFα or even in medium alone) showed equal levels of non-specific phosphorylation of both NFκB p65 and p38MAPK. After six hours incubation, VT+/PM− IgG caused a ~4-fold increase in phosphorylation of NFκB compared with IgG from the other three groups (p<0.05) (as shown in Figure 1A). Similarly, a ~6-fold increase in phosphorylation of p38MAPK was seen with the VT+/PM− sample compared to the other groups (p<0.05) (as shown in Figure 1B). In contrast, IgG from VT−/PM+ patients had no greater effect on NFkB and p38MAPK phosphorylation than IgG from the control (aPL+/APS− and aPL-negative) groups or medium alone.
Figure 1. IgG from patients with different clinical manifestations of the APS preferentially activate different signalling pathways.
Western blot analysis of cell lysates from U937 cells treated with pooled IgG (100μg/ml) from four clinical groups (VT+/PM−, VT−/PM+, aPL+/APS− and aPL-negative) for 6 hours. Representative blots from a single experiment are illustrated and quantitative analysis (from three independent experiments, showing mean and standard error) with antibodies specific for human phosphorylated and total proteins are shown against: - (A) NFκB p65 and (B) p38MAPK. Statistically significant differences are shown (*p<0.05). For abbreviations see text.
These experiments with pooled samples enabled us to establish the ideal incubation time for further experiments as six hours exposure of monocytes to IgG. This finding was consistent with the previous work of Lopez-Pedrera et al (10, 23). To investigate the biological relevance of our initial findings using pooled samples and U937 cells, we addressed the following questions: are similar differences between VT+/PM− and VT−/PM+ samples seen in monocyte signalling when IgG from individual subjects is tested? ; are similar effects seen when ex vivo monocytes rather than U937 cells are used? ; what is the functional consequence of the six hour exposure to IgG?
Similar profiles of NFκB and p38MAPK phosphorylation occurred in U937 cells and ex vivo monocytes exposed to IgG from individual subjects
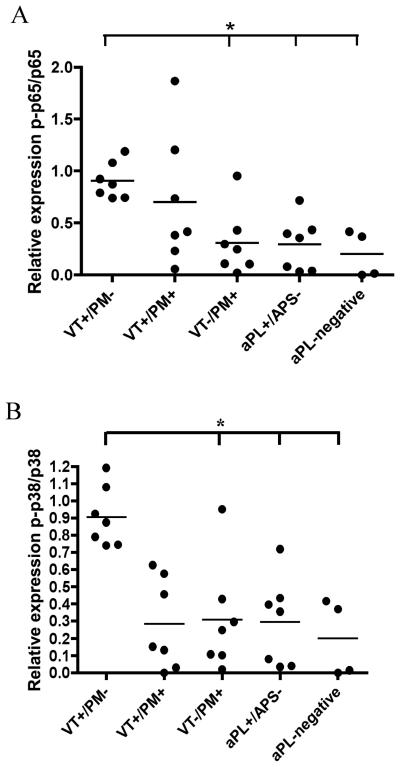
We examined the effects of IgG from individual patients chosen at random from the four groups previously tested (VT+/PM−, VT−/PM+, aPL+/APS− and aPL-negative). In general, the same pattern of phosphorylation found with pooled IgG was observed with individual IgG samples. NFκB p65 (Figure 2A) and p38MAPK (Figure 2B) phosphorylation in U937 cells exposed to IgG from VT+/PM− patients were ~3-fold higher (p<0.05) than from the other groups.
Figure 2. IgG from individual VT+/PM− patients but not VT−/PM+ promotes phosphorylation of NFκB and p38MAPK in U937 cells.
U937 cells were treated with purified IgG (100μg/ml) from seven individual patients chosen at random from four clinical groups (VT+/PM−, VT+/PM+, VT−/PM+, aPL+/APS−) or purified IgG (100μg/ml) from four healthy controls (aPL-negative) or TNFα (100ng/ml) for 6 hours. Quantitative analysis with antibodies specific for human phosphorylated and total proteins are shown against: - (A) NFκB p65 and (B) p38MAPK. Each value in the scattered plot of the quantitative analysis represents the mean of three experiments performed independently of each other. Statistically significant differences are shown (*p<0.05).
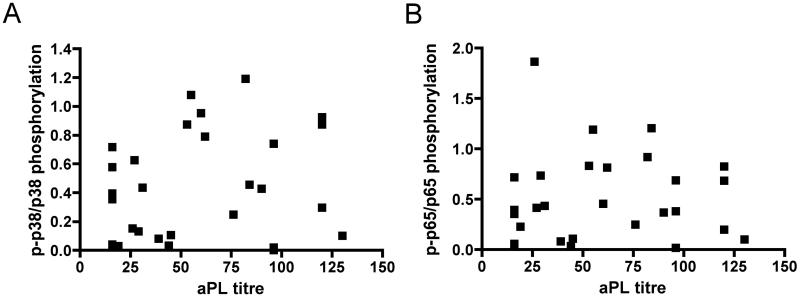
To investigate the possibility that differences in NFκB and p38MAPK phosphorylation between groups were due to differences in titre of aPL, we plotted aPL level against each of these outcome measures for all 28 samples in the VT+/PM−, VT−/PM+, VT+/PM+ and aPL+/APS− groups (Figure 3A and 3B). There was no correlation between aPL titre and either phosphorylation of NFκB (r2=0.0003117) or p38MAPK (r2=0.03016).
Figure 3. The effects of IgG on monocyte activation are not related to the aPL titres.
aPL titres of all 28 patients in the VT+/PM−, VT−/PM+, VT+/PM+ and aPL+/APS− groups (not healthy controls, who have no aPL) against both (A) p38MAPK (r2=0.03016) and (B) NFκB p65 (r2=0.0003117) phosphorylation show no correlation between aPL titre and either outcome.
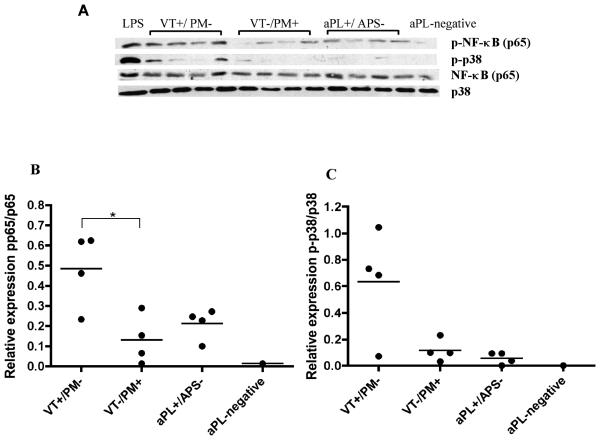
We then studied the effects of IgG from four individual patients chosen at random upon ex vivo monocytes isolated from a healthy donor (Figure 4A). We found a similar profile of IgG mediated NFκB p65 and p38MAPK phosphorylation in ex vivo monocytes compared to what we previously found in U937 cells (Figure 1&2). Therefore, IgG from patients with VT+/PM− increased NFκB p65 and p38MAPK (Figure 4B and C) phosphorylation compared with IgG from patients with VT−/PM+, although it only reached statistical significance (p<0.05) for NFκB p65.
Figure 4. IgG from individual patients display similar pattern of activation of p38MAPK and NFκB on ex vivo monocytes.
Western blot analysis of cell lysates from healthy monocytes treated with IgG (100μg/ml) or LPS (3μg/ml) for 6 hours. (A) Representative blot with antibodies specific for human phosphorylated and total proteins against NFκB p65 and p38MAPK. The positive control (LPS) in lane 1 shows stimulation of p38MAPK and NFκB p65. Four individual patients from the VT+/PM− group are shown in lanes 2-5, four individual patients from the VT−/PM+ group are shown in lanes 6-9, four individual patients from the aPL+/APS− group are shown in lanes 10-13 and a pooled IgG sample from four healthy controls (aPL-negative) is shown in lane 14. (B) Quantitative densitometric analysis of the blots shown in (A) displaying ratio of phosphorylated to total protein against NFκB and (C) Quantitative densitometric analysis of the blots shown in (A) displaying ratio of phosphorylated to total p38MAPK. Statistically significant differences are shown (*p<0.05).
TF activity in U937 cells was stimulated by VT+/PM− IgG but not by VT−/PM+ IgG and this stimulation is blocked by inhibiting NFκB, p38MAPK or TLR4 signalling pathways.
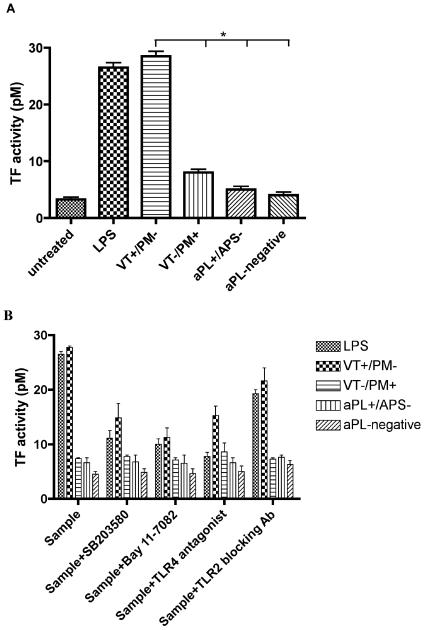
Exposure to pooled VT+/PM− IgG caused a ~4-fold increase in TF activity (p<0.05) compared with VT−/PM+, aPL+/APS− or aPL-negative IgG (Figure 5A). In contrast, VT−/PM+ IgG did not significantly increase TF activity compared with aPL+/APS− or aPL-negative IgG.
Figure 5. APS-IgG activates monocyte TF activity.
(A) Only pooled VT+/PM− IgG activates monocyte TF activity. U937 cells were treated with 100μg/ml pooled IgG from four clinical groups (VT+/PM−, VT−/PM+, aPL+/APS− and aPL-negative) or 3μg/ml LPS for 6 hours. Cells were lysed and TF activity (pM) was determined using the Actichrome TF assay. Values represent the mean and standard error of three independent experiments. Statistically significant differences are shown (*p<0.05). (B) APS-IgG activates monocyte TF activity via the NFκB, p38MAPK and TLR4 pathways. U937 cells were pre-treated for 1 hour with 1μM SB203580, 50μM Bay 11-7082, 1μg/ml anti-TLR2 blocking antibody or 1μg/ml TLR4 antagonist (E. coli K12 msbB LPS), then treated with 100μg/ml pooled IgG or 3μg/ml LPS for 6 hours. Cells were lysed and TF activity was measured. Values represent the mean and standard error of two different experiments. The positive control (LPS) confirms that NFκB, p38MAPK and TLR4 pathways were inhibited by addition of the inhibitors.
Figure 5B shows the effects of inhibitors of p38MAPK (SB203580), NFκB (Bay 11-7082) and TLR4 (E. coli K12 msbB LPS) and of anti-TLR 2 antibody on TF activity induced by the IgG samples. The low levels of TF activity in cells exposed to IgG from the VT−/PM+, aPL+/APS− and aPL-negative groups were not affected by any of the inhibitors. The increase in TF activity stimulated by VT+/PM− IgG was significantly reduced by inhibitors of p38MAPK (p=0.01), NFκB (p=0.01) and TLR4 (p=0.01) but not anti-TLR2 antibody (p=0.06). Addition of either DMSO alone or a non-functional analogue of SB203580 did not have any appreciable effects on TF activity (data not shown).
The stimulatory effects of VT+/PM− IgG on NFκB phosphorylation are concentrated in the IgG fraction that binds β2GPI
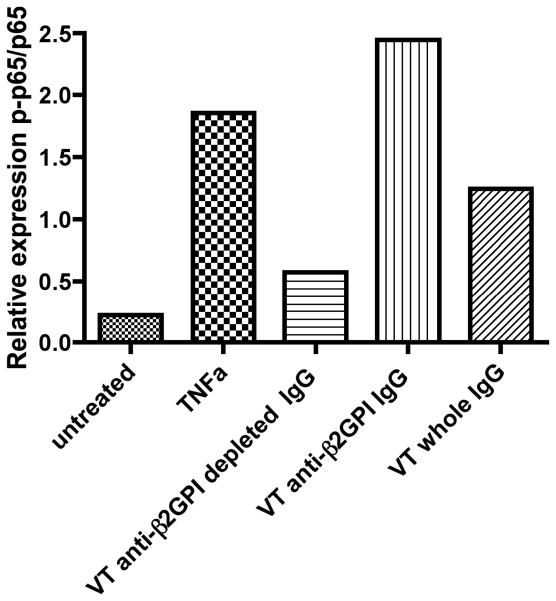
Affinity-purification of anti-β2GPI antibodies from a total IgG pooled sample of four VT+/PM− patients confirmed that the anti-β2GPI sub-fraction is responsible for increased phosphorylation of NFκB p65 (Figure 6). In contrast, the anti-β2GPI-depleted IgG fraction showed an appreciable reduction in ability to promote NFκB p65 phosphorylation compared with both the anti-β2GPI-enriched and whole IgG fractions. Anti-β2GPI activity was calculated as mean % binding to a concentration of 100μg/ml HCAL and was found to be 131 for the anti-β2GPI affinity purified IgG sample and 65 for the anti-β2GPI depleted IgG.
Figure 6. The effects of pooled VT+/PM− IgG on monocyte signalling pathways are mediated by anti-β2GPI antibodies.
Pooled IgG (100μg/ml) from patients with VT alone (VT+/PM−) was passed through a β2GPI affinity purification column. Eluted fractions were used at 100μg/ml to treat U937 cells for 6 hours. Quantitative analysis displaying ratio of phosphorylated and total protein against NFκB.
What are the effects on monocytes of IgG samples from APS patients who have both VT and PM (VT+/PM+)?
The results of testing IgG from seven VT+/PM+ individuals are included in Figure 2. Three of these seven VT+/PM+ IgG samples showed both the greatest ability to cause p38MAPK phosphorylation and the greatest NFκB phosphorylation. These samples resemble VT+/PM− samples. The other four VT+/PM+ samples had far less effect on either signalling pathway and resemble VT−/PM+ samples. There was no correlation with the most recent clinical event. In four cases the most recent event was PM, in one it was VT and in two VT and PM had occurred during the same clinical episode. We found a similar spread of results when we tested the effect of seven individual VT+/PM+ samples on TF activity in monocytes. The results for these seven samples were 1, 3.3, 6.8, 11.8, 18.5, 32.5 and 54 (TF activity in pM, results are mean of two separate experiments).
Discussion
We have shown that IgG from VT+/PM− patients promotes up-regulation of monocyte TF activity that is prevented by specific inhibitors of p38MAPK and NFκB. In contrast, IgG from VT−/PM+ patients could not promote phosphorylation of these signalling molecules or stimulate up-regulation of TF activity. These functional differences were seen despite the fact that the VT−/PM+ and VT+/PM− samples had similarly high levels of CL-binding and β2GPI-binding.
Previous studies on monocytes (10, 13, 17, 21) tested polyclonal aPL samples from limited numbers of patients with VT alone. Two groups (10, 26) purified total IgG from seven patients with VT+/PM−, whilst another study (21) used polyclonal IgG anti-β2GPI antibodies from three patients with VT+/PM−. We investigated IgG from larger numbers of subjects and are the first to compare effects of purified IgG from VT+/PM− and VT−/PM+ patients with APS. The only previous studies to compare VT+/PM− and VT−/PM+ groups looked at ex vivo monocytes (10, 23) and showed that monocytes extracted from APS patients with VT (including both VT+/PM− and VT+/PM+) had higher TF mRNA, higher surface expression of TF and altered signalling compared to monocytes from VT−/PM+ APS patients, aPL-negative patients with VT and healthy controls (10). Furthermore, monocytes from patients with APS and thrombosis have higher levels of vascular endothelial growth factor (VEGF) and tyrosine kinase Flt-1 than those from patients with APS and no thrombosis or healthy controls (26). Proteomic analysis and mass spectrometry identified six proteins whose expression was significantly different in monocytes from the APS/VT group than the three other groups. They showed the same results of TF expression, signalling and proteomics in monocytes from healthy people exposed to purified IgG from patients with APS, but these IgG samples were derived only from patients with VT and were pooled before addition to the monocytes. Our results agree with and complement those of Lopez-Pedrera et al (10). We also found increased TF and increased p38MAPK and NFκB signalling in monocytes exposed to VT+/PM− IgG but have further demonstrated that purified VT−/PM+ IgG does not cause similar effects. This finding is consistent with the fact that those effects have not been seen on monocytes derived from VT−/PM+ patients [9]. Lopez-Pedrera et al studied TF expression while we studied activity. Expression and activity of TF are not linked directly because cell surface TF may be inactive due to encryption or complex formation with inhibitors. In future studies we will measure both activity and expression because it is now recognised that TF may have pathogenic pro-inflammatory effects in APS separate from the effects on thrombosis that are measured in the activity assay (27, 28).
The distinct differences in IgG mediated phosphorylation of monocyte signalling molecules we observed at six hours supports the findings of Lopez-Pedrera et al. and other groups who have examined the time course of aPL mediated signalling activation in platelets and HUVECs (18). We then confirmed the biological significance of this six hour exposure to IgG by correlating these differences in signalling with our findings from the TF activity assay.
A possible confounder of our results would be misallocation of samples between clinical groups. A patient who had only ever suffered PM might nevertheless possess aPL capable of causing VT and might subsequently develop VT. Therefore, they would be allocated to the VT−/PM+ group whereas their true allocation should be VT+/PM+. Such misallocations would reduce our ability to detect true differences between the VT+/PM− and VT−/PM+ groups rather than creating false-positive differences. We feel that allocation of patients to VT+/PM− or VT−/PM+ groups was secure due to the long duration of follow-up (mean = 13 years) without clinical events that would alter that allocation. Of 9 female VT+/PM− subjects, 5 had live births after their VT events and the other 4 did not become pregnant for reasons other than APS (e.g. partner's infertility). Of these 9 patients, three are already post-menopausal and six are over 40 so the chance that they will develop APS-related PM in the future is low.
Given the clear differences in properties between the VT+/PM− and VT−/PM+ samples, an obvious question was which properties would be dominant in VT+/PM+ patients. One possible answer was that VT+/PM+ would all resemble VT+/PM− on the grounds that the presence of aPL with the ability to stimulate the p38MAPK and NFκB pathways is essential in all APS patients who suffer VT. Alternatively, IgG aPL present in VT+/PM+ subjects might be a mixture of antibodies capable of causing VT (with properties similar to VT+/PM− IgG) and those not capable of causing VT (with properties similar to VT−/PM+ IgG). Therefore, the relative amounts of these two types of antibody in each individual VT+/PM+ sample would determine whether it stimulated the p38MAPK and NFκB pathways in our assays. Lastly, the character of the aPL present in an individual might change with time such that the properties of a VT+/PM+ IgG sample would depend on the most recent clinical event in that person. For instance, if they had suffered VT long ago but PM recently then their antibodies would behave like VT−/PM+ IgG. We believe that our findings of a mixed effect upon these signalling pathways with no correlation with the most recent clinical event support the second of the hypotheses suggested above – that the population of aPL in these patients is a mixture of aPL resembling the VT+/PM− group and aPL resembling VT−/PM+.
Use of pooled samples was necessary to carry out the multiple experiments to establish the optimal time of incubation with IgG. To reduce the chance of erroneous findings arising from atypical individual samples within pools, we tested two different pools for each clinical group and repeated the experiments using individual samples drawn at random from those used to create each pool. These experiments confirmed the differences between effects of VT+/PM− and VT−/PM+ samples. It is unlikely that these effects are due to non-aPL autoantibodies since affinity-purified anti-β2GPI from our VT+/PM− group had a particularly strong effect. IgG from the aPL+/APS− group had minimal effect on monocytes in any assay, despite the fact that 9/12 patients from this group had SLE and a range of other serum autoantibodies (e.g. anti-dsDNA, anti-Ro).
What is the mechanism of the effects exerted on monocytes by our VT+/PM− IgG samples? We believe that IgG anti-β2GPI antibodies within those samples bind to β2GPI (derived from FCS used in cell culture of U937 cells or attached to the monocytes extracted from human serum) and that these complexes interact with TLR4 on the monocyte surface. This hypothesis would be consistent with our results showing that the effect of APS-IgG upon monocytes is concentrated within the anti-β2GPI fraction of VT+/PM− IgG and inhibited by the TLR4 antagonist E. coli K12 msbB LPS. This antagonist is known to be specific for the TLR4 pathway (from experiments in EC) and acts upstream of the MyD88 adaptor (29-31). However, aPL can also act by other mechanisms involving other receptors (32). Our results did show some effect of TLR2 blocking antibody though this was not clear enough to convince us that TLR2 is involved in the effect of our VT+/PM− samples on monocytes. For example, aPL could be acting by a complement dependent mechanism, as suggested in other studies (33, 34) because complement is present in the FCS used in our U937 culture medium. Furthermore, we have previously detected β2GPI in IgG purified monoclonal aPL (24) and have confirmed (data not shown) that co-purified β2GPI is also present in our purified polyclonal IgG aPL.
It would be of interest to know whether these differences in effects of IgG samples from patients with different clinical manifestations of APS are mirrored in their effects when tested in in-vivo models. Though several such models have been described for both thrombosis (35, 36) and pregnancy loss (37), careful analysis of the methods sections of these papers (both ours and others) shows that very few samples from patients with PM alone have ever been tested in in-vivo models. Thus it is currently not possible to reach a conclusion as to whether VT+/PM− and VT−/PM+ IgG aPL samples have different effects in those models.
In conclusion, our data support our hypothesis that aPL from VT+/PM− and VT−/PM+ patients with APS differ in their effects on p38MAPK and NFκB signalling pathways and TF activity. These differences may be mediated by preferential activation of TLR4 by IgG aPL from VT+/PM− patients.
Footnotes
This work was supported by The Wellcome Trust. IG is funded by the Rosetrees Trust. PT is funded by BBSRC. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
Disclosures
S.P. is a co-owner and founder of Louisville APL Diagnostics, Inc. The authors have no other conflicting financial interests.
References
- 1.Hughes GR, Harris NN, Gharavi AE. The anticardiolipin syndrome. J Rheumatol. 1986;13:486–489. [PubMed] [Google Scholar]
- 2.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PG DEG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 3.Petri M. Classification and epidemiology of the Antiphospholipid Syndrome. In: Asherson RA, Cervera R, Piette J-C, Shoenfeld Y, editors. The Antiphospholipid Syndrome II: Autoimmune Thrombosis. Second ed. Elsevier Science B.V.; Amsterdam: 2002. pp. 11–20. [Google Scholar]
- 4.Rai R, Regan L. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:1354. doi: 10.1016/s0029-7844(02)02574-7. [DOI] [PubMed] [Google Scholar]
- 5.Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332–338. doi: 10.1016/s0002-9343(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 6.Lynch A, Marlar R, Murphy J, Davila G, Santos M, Rutledge J, Emlen W. Antiphospholipid antibodies in predicting adverse pregnancy outcome. A prospective study. Ann Intern Med. 1994;120:470–475. doi: 10.7326/0003-4819-120-6-199403150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Boffa MC, Hughes GR, Ingelmo M. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 8.Pierangeli SS, Colden-Stanfield M, Liu X, Barker JH, Anderson GL, Harris EN. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation. 1999;99:1997–2002. doi: 10.1161/01.cir.99.15.1997. [DOI] [PubMed] [Google Scholar]
- 9.Vega-Ostertag M, Harris EN, Pierangeli SS. Phosphorylation of p38MAPK is involved in antiphospholipid antibody mediated platelet activation. Arthritis Rheum. 2003;48:S161. [Google Scholar]
- 10.Lopez-Pedrera C, Buendia P, Cuadrado MJ, Siendones E, Aguirre MA, Barbarroja N, Montiel-Duarte C, Torres A, Khamashta M, Velasco F. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheum. 2006;54:301–311. doi: 10.1002/art.21549. [DOI] [PubMed] [Google Scholar]
- 11.Cervera R, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Kiss E, Zeher MM, Tincani A, Kontopoulou-Griva I, Galeazzi M, Bellisai F, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Piette JC, Espinosa G, Bucciarelli S, Pisoni CN, Bertolaccini ML, Boffa MC, Hughes GR. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicenter prospective study of 1,000 patients. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.093179. [DOI] [PubMed] [Google Scholar]
- 12.Quenby S, Farquharson RG, Dawood F, Hughes AM, Topping J. Recurrent miscarriage and long-term thrombosis risk: a case-control study. Hum Reprod. 2005;20:1729–1732. doi: 10.1093/humrep/deh844. [DOI] [PubMed] [Google Scholar]
- 13.Cuadrado MJ, Lopez-Pedrera C, Khamashta MA, Camps MT, Tinahones F, Torres A, Hughes GR, Velasco F. Thrombosis in primary antiphospholipid syndrome: a pivotal role for monocyte tissue factor expression. Arthritis Rheum. 1997;40:834–841. doi: 10.1002/art.1780400509. [DOI] [PubMed] [Google Scholar]
- 14.Dobado-Berrios PM, Lopez-Pedrera C, Velasco F, Aguirre MA, Torres A, Cuadrado MJ. Increased levels of tissue factor mRNA in mononuclear blood cells of patients with primary antiphospholipid syndrome. Thromb Haemost. 1999;82:1578–1582. [PubMed] [Google Scholar]
- 15.Reverter JC, Tassies D, Font J, Khamashta MA, Ichikawa K, Cervera R, Escolar G, Hughes GR, Ingelmo M, Ordinas A. Effects of human monoclonal anticardiolipin antibodies on platelet function and on tissue factor expression on monocytes. Arthritis Rheum. 1998;41:1420–1427. doi: 10.1002/1529-0131(199808)41:8<1420::AID-ART11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Kornberg A, Blank M, Kaufman S, Shoenfeld Y. Induction of tissue factor-like activity in monocytes by anti-cardiolipin antibodies. J Immunol. 1994;153:1328–1332. [PubMed] [Google Scholar]
- 17.Bohgaki M, Atsumi T, Yamashita Y, Yasuda S, Sakai Y, Furusaki A, Bohgaki T, Amengual O, Amasaki Y, Koike T. The p38 mitogen-activated protein kinase (MAPK) pathway mediates induction of the tissue factor gene in monocytes stimulated with human monoclonal anti-beta2Glycoprotein I antibodies. Int Immunol. 2004;16:1633–1641. doi: 10.1093/intimm/dxh166. [DOI] [PubMed] [Google Scholar]
- 18.Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS. Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum. 2005;52:1545–1554. doi: 10.1002/art.21009. [DOI] [PubMed] [Google Scholar]
- 19.Raschi E, Testoni C, Bosisio D, Borghi MO, Koike T, Mantovani A, Meroni PL. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 20.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, Nguyen-Oghalai T, Meroni PL. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Rigano R, Ortona E, Valesini G. Anti-beta(2)-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 22.Satta N, Dunoyer-Geindre S, Reber G, Fish RJ, Boehlen F, Kruithof EK, de Moerloose P. The role of TLR2 in the inflammatory activation of mouse fibroblasts by human antiphospholipid antibodies. Blood. 2007;109:1507–1514. doi: 10.1182/blood-2005-03-024463. [DOI] [PubMed] [Google Scholar]
- 23.López-Pedrera C, Cuadrado M, Herández V, Buendïa P, Aguirre M, Barbarroja N, Torres L, Villalba J, Velasco F, Khamashta M. Proteomic analysis in monocytes of antiphospholipid syndrome patients: Deregulation of proteins related to the development of thrombosis. Arthritis Rheum. 2008;58:2835–2844. doi: 10.1002/art.23756. [DOI] [PubMed] [Google Scholar]
- 24.Giles I, Lambrianides N, Pattni N, Faulkes D, Latchman D, Chen P, Pierangeli S, Isenberg D, Rahman A. Arginine residues are important in determining the binding of human monoclonal antiphospholipid antibodies to clinically relevant antigens. J Immunol. 2006;177:1729–1736. doi: 10.4049/jimmunol.177.3.1729. [DOI] [PubMed] [Google Scholar]
- 25.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 26.Cuadrado MJ, Buendia P, Velasco F, Aguirre MA, Barbarroja N, Torres LA, Khamashta M, Lopez-Pedrera C. Vascular endothelial growth factor expression in monocytes from patients with primary antiphospholipid syndrome. J Thromb Haemost. 2006;4:2461–2469. doi: 10.1111/j.1538-7836.2006.02193.x. [DOI] [PubMed] [Google Scholar]
- 27.Redecha P, Franzke CW, Ruf W, Mackman N, Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J Clin Invest. 2008;118:3276–3278. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coats SR, Reife RA, Bainbridge BW, Pham TT, Darveau RP. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect Immun. 2003;71:6799–6807. doi: 10.1128/IAI.71.12.6799-6807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somerville JEJ, Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Invest. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Re F, Strominger JL. Separate functional domains of human MD-2 mediate Toll-like receptor 4-binding and lipopolysaccharide responsiveness. J immunol. 2003;171:5272–5276. doi: 10.4049/jimmunol.171.10.5272. [DOI] [PubMed] [Google Scholar]
- 32.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–430. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 33.Oku K, Atsumi T, Bohgaki M, Amengual O, Kataoka H, Horita T, Yasuda S, Koike T. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–5. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 34.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vega-Ostertag ME, Pierangeli SS. Mechanisms of aPL-mediated thrombosis: effects of aPL on endothelium and platelets. Current rheumatology reports. 2007;9:190–197. doi: 10.1007/s11926-007-0031-0. [DOI] [PubMed] [Google Scholar]
- 36.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, Bossi F, Ziller F, Sblattero D, Meroni P, Tedesco F. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 37.Salmon JE, Girardi G, Holers VM. Activation of complement mediates antiphospholipid antibody-induced pregnancy loss. Lupus. 2003;12:535–538. doi: 10.1191/0961203303lu397oa. [DOI] [PubMed] [Google Scholar]