Abstract
Presynaptic GABAA receptors occur at hippocampal mossy fiber synapses. Whether and how they modulate orthodromic signaling to postsynaptic targets is poorly understood. We show that an endogenous neurosteroid selective for high-affinity δ-subunit-containing GABAA receptors depolarizes rat mossy fiber boutons, enhances action potential-dependent Ca2+ transients, and facilitates glutamatergic transmission to pyramidal neurons. Conversely, blocking GABAA receptors hyperpolarizes mossy fiber boutons, increases their input resistance, decreases spike width, and attenuates action potential-dependent presynaptic Ca2+ transients, indicating that a subset of presynaptic GABA receptors are tonically active. Blocking GABAA receptors also interferes with the induction of long-term potentiation at mossy fiber-CA3 synapses. Presynaptic GABAA receptors thus facilitate information flow to the hippocampus both directly and by enhancing LTP.
Presynaptic GABAA receptors exist in several areas of the CNS. In comparison with somatodendritic receptors, relatively little is known of their pharmacological properties and physiological roles. Although they mediate presynaptic inhibition of glutamate release from primary muscle afferents in the spinal cord, they have a facilitatory effect on neurotransmitter release in the brainstem and cerebellum1,2. Whether presynaptic receptors in the cerebral cortex enhance or depress neurotransmitter release is less clear. Mossy fibers in the hippocampal formation offer a well-defined and relatively accessible pathway where presynaptic GABAA receptors can be examined3. In immature rats activation of such receptors is accompanied by an increase in the amplitude of the fiber volley evoked by axon stimulation4. Furthermore, application of the GABAA receptor agonist muscimol enhances glutamate release from isolated boutons onto acutely dissociated pyramidal neurons5. Although these observations suggest that presynaptic GABAA receptors facilitate orthodromic transmission, this remains to be shown in a more intact preparation.
It is also unclear whether presynaptic GABAA receptors in mossy fibers are active in the absence of evoked GABA release. Examination of mossy fiber excitability in response to electrical stimulation revealed bi-directional effects of the GABAA receptor antagonist SR95531 (gabazine) depending on [Cl−]i in individual granule cells3, arguing that the receptors can be tonically active. However, whole-terminal recordings from mossy fiber boutons have not detected an effect of blocking GABAA receptors on input resistance6. Although direct recordings have confirmed that presynaptic GABAA receptors are sufficiently sensitive to detect GABA spillover from neighboring inhibitory terminals (see also refs. 3,4), their affinity estimated from pressure-applied GABA was relatively low (EC50 ≈ 60 μM). Furthermore, the pharmacological profile was consistent with presence of the α2 subunit, which has been detected immunohistochemically at mossy fibers3, and which is not thought to contribute to high-affinity receptors, implying that they may not be able to detect low ambient GABA concentrations.
Nevertheless, the identification of relatively low-affinity receptors (see also ref. 7) does not exclude the additional presence of high-affinity receptors. Dentate granule cells express tonically active δ-subunit-containing GABAA receptors in their somatodendritic compartment, which are sensitive to changes in ambient GABA 8-10. Such receptors, which commonly also contain α4 subunits, are generally thought to be extrasynaptic, have a high affinity for GABA, and exhibit relatively little desensitization 11,12,9,13. They are also sensitive to the endogenous neurosteroid tetrahydrodeoxycorticosterone (THDOC) at nanomolar concentrations that occur in the brain14, and to the hypnotic drug 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP, also known as gaboxadol, GBX). Although α4 and δ subunits are abundant in granule cell bodies and dendrites15,9, it is not known if either or both also occur in mossy fibers.
Here, we report that GABAA receptors, tonically active in quiescent acute brain slices in the absence of added GABA, directly modulate mossy fiber membrane potentials, with a pharmacological profile consistent with the presence of δ subunits, and affect spike properties and spike-driven Ca2+ influx in individual boutons. We also show that potentiating and blocking high-affinity GABAA receptors have opposite effects on excitatory synaptic transmission to CA3 pyramidal cells. Finally, presynaptic GABAA receptors paradoxically facilitate the induction of long-term potentiation at mossy fiber synapses. These results reveal an important mechanism by which information flow to the hippocampus can be bidirectionally modulated.
RESULTS
High-affinity GABAA receptors affect mossy fiber excitability
We looked for evidence for δ-subunit-containing GABAA receptors at mossy fibers by examining the effects of the endogenous neurosteroid allosteric modulator THDOC and of the agonist GBX on antidromic action current (AC) threshold in granule cells held in voltage clamp. Ionotropic glutamate and GABAB receptors were blocked throughout with 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoxaline (NBQX, 50 μM), D-2-amino-5-phosphonovalerate (APV, 50 μM) and CGP52432 (5 μM). We measured the success rate for evoking ACs in response to electrical stimuli applied at different intensities via a bipolar electrode in stratum lucidum. Both THDOC (10 nM) and GBX (1 μM) reversibly decreased the AC success rate (see Supplementary Fig. 1a,b online). For each condition, we estimated the stimulus intensity (in arbitrary units) yielding a 50% AC success rate (Stim50). THDOC reversibly increased the AC threshold (Stim50 Control: 3.7 ± 0.5; Stim50 THDOC: 7.4 ± 0.8; n = 8; mean ± S.E.M, paired t-test: p < 0.01; Supplementary Fig. 1c). Application of GBX had a similar effect (Stim50 Control: 5.3 ± 0.3; Stim50 GBX: 7.5 ± 0.3; n = 4; p < 0.02). These results indicate that ligands selective for δ-subunit-containing GABAA receptors reduce axonal excitability.
The whole-cell recording method can alter [Cl−]i in the axon3. We therefore tested the effect of THDOC on AC success rate in granule cells using gramicidin perforated-patch recordings. THDOC had a similar effect on AC threshold in perforated-patch as with whole-cell recordings (Stim50 Control: 1.6 ± 0.4; Stim50 THDOC: 3.6 ± 0.8; n = 3; p < 0.05; not shown). Taken together with previous evidence that either activating or blocking GABAA receptors alters mossy fiber excitability in opposite directions depending on [Cl−]i (ref. 3), these results imply that high-affinity neurosteroid-sensitive GABAA receptors have a profound effect on mossy fiber excitability in the absence of manipulations to increase ambient GABA.
Presynaptic GABAA receptors at mossy fibers
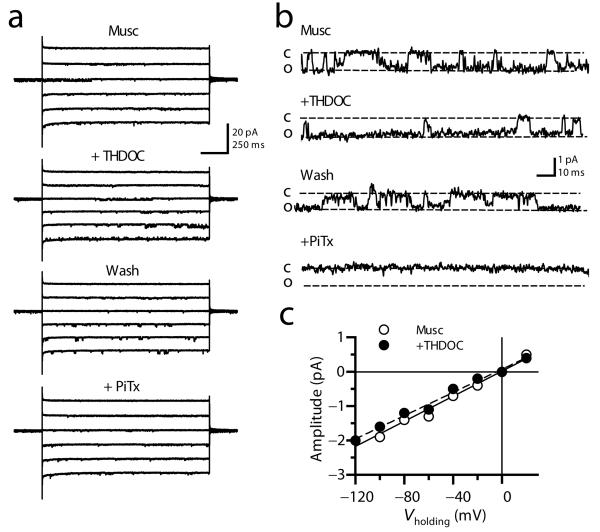
Measurements of mossy fiber excitability do not give a quantitative indication of the effects of GABAA receptors on the presynaptic membrane potential. Furthermore, such experiments do not distinguish between receptors in presynaptic boutons and in the rest of the axon. We therefore obtained patch-clamp recordings from mossy fiber boutons16,17 (Supplementary Fig. 2). Consistent with previous results6, the selective GABAA receptor agonist muscimol (10 μM) evoked channel currents in outside-out patches from mossy fiber boutons in the presence of a cocktail of blockers of ionotropic glutamate and GABAB receptors and voltage-dependent Ca2+ (CdCl2, 100-200 μM) and Na+ channels (TTX, 1 μM) (Fig. 1a,b). The modal single-channel conductance of GABAA receptors in mossy fiber boutons (γMFB) estimated from the linear fit of the current-voltage relationship was 23.7 pS (n = 5; Supplementary Fig. 3), not significantly different from that measured in patches excised from granule cell bodies (γGC = 27.1 pS; n = 7; p = 0.25). Application of picrotoxin (100 μM) blocked these currents, confirming that they were mediated by GABAA receptors (n = 3). THDOC (10 nM) increased the apparent open probability (Baseline Popen: 0.18 ± 0.02; THDOC Popen: 0.33 ± 0.03; n = 4, p < 0.05), with no effect on the single-channel conductance (Fig. 1c).
Fig. 1. THDOC modulates GABAA receptors in outside-out patches from mossy fiber boutons.
(a) Traces obtained in one patch at different holding potentials (−120 to −20 mV) showing single-channel currents evoked by muscimol (10 μM, Musc) and their reversible modulation by 10 nM THDOC (+ THDOC, Wash) and block by 100 μM picrotoxin (+ PiTx). (b) High magnification of a portion of the traces shown in (A), showing an increased open channel probability when THDOC was co-applied with muscimol. (c) The single-channel conductance calculated from the slope of the open channel current-voltage relationship was unaltered by THDOC (example from one patch).
At least some presynaptic GABAA receptors are thus modulated by neurosteroids. Because we selected patches where single channel openings could be resolved, and because the agonist was applied continuously, we cannot determine to what extent desensitizing THDOC-insensitive channels co-exist.
Presynaptic GABAA receptors depolarize boutons
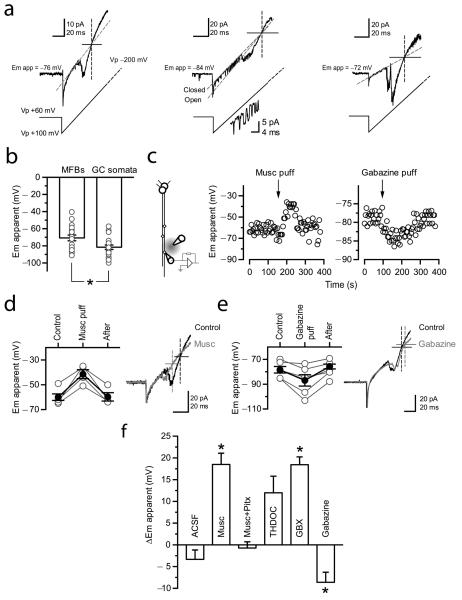
Previous studies have suggested that GABAA receptors depolarize mossy fiber boutons6,4, consistent with the actions of presynaptic GABAA receptors elsewhere in the CNS1,2. However, the methods used have not allowed a direct estimate of the actions of GABAA receptors on the presynaptic membrane potential: whole-terminal recordings perturb both [K+]i and [Cl−]i, which interact to determine the driving force for GABAA receptor-mediated ion fluxes. We therefore applied ramp voltage commands in the cell-attached patch-clamp recording mode, with a pipette containing 155 mM [K+], close to [K+]i (refs. 18,19). Because the K+ flux reverses when the pipette and intracellular potential are equal, this allows a minimally invasive estimate of the presynaptic membrane potential. Ramp voltage commands evoked an inward current that reversed polarity at −70.8 ± 3.3 mV (n = 19; Fig. 2a). We verified that this current was carried by K+ ions: the inward component was profoundly attenuated when recording with a low [K+] pipette solution (Supplementary Fig. 4). When the same method was used to estimate the membrane potential in dentate granule cell bodies, it yielded more negative values (−81.6 ± 2.6 mV; n = 21; p < 0.05; Fig. 2b).
Fig. 2. Non-invasive measurement of mossy fiber membrane potential and modulation via GABAA receptors.
(a) Three examples of bouton-attached recordings. Depolarizing voltage ramps (−100 to +200 mV; 3mV/ms) induced a current that reversed polarity, superimposed on a leak current (fitted with the red dashed line). The corresponding voltage ramp protocol is indicated below (not to scale). The vertical dashed line indicates the intersection between the current trace and the linear fit to the leak current, corrresponding to the reversal potential for the K+ current (the apparent resting membrane potential, Emapp.). Single K+ channels were seen in some traces (middle). A straight line fitted through the open state current also crosses the intersection. Single channel currents are shown at higher magnification below. Action potentials were occasionally detected (right). (b) Summary plot of estimated membrane potential (Em apparent) measured in bouton- and soma-attached recordings (error bars: s.e.m.). The estimated membrane potential in mossy fiber boutons was more depolarized than that measured at granule cell somata. *, p = 0.03, Mann-Whitney U test. (c) Left, Experimental design used to measure the effect of muscimol or gabazine on bouton membrane potential. Right, Apparent membrane potential in two mossy fiber boutons showing the effects of pressure application of muscimol (Musc puff) or gabazine (Gabazine puff), as indicated. (d – e) Effects of muscimol (D) and gabazine (E) on Em apparent, with sample traces before (Control, black) and immediately after (red) drug application, as in (A). (f) Summary data for the effect of pressure application of ACSF (n = 3), muscimol (n = 6), muscimol in a background of picrotoxin (n = 6), THDOC (n = 3), GBX (n = 8) and gabazine (n = 4), respectively. Traces show averages of 5 consecutive trials before (black) and immediately after (red) pressure application of muscimol or gabazine. *, p = 0.01, paired t-test.
We applied repeated ramp commands to investigate the effect of either activating or blocking GABAA receptors on the apparent membrane potential in mossy fiber boutons. Local pressure application of muscimol (10 μM, <150 μm from the bouton) depolarized boutons by 18.5 ± 3.7 mV (n = 4; p < 0.05; Fig. 2c,d). Conversely, pressure application of gabazine hyperpolarized boutons by 8.5 ± 4.5 mV (n = 6; p < 0.05; Fig. 2c—f). We verified that muscimol had no effect when GABAA receptors were pre-blocked with picrotoxin (−0.7 ± 1.4 mV; n = 3; Fig. 2f). Similarly, local pressure-application of artificial CSF (ACSF) also had no significant effect (3.3 mV ± 2.1 mV; n = 3; Fig. 2f), arguing against movement artifact as the cause of the change in apparent bouton potential.
Prompted by the evidence that δ-containing receptors are present in boutons, we tested the effect of GBX. Local pressure-application of GBX depolarized mossy fiber boutons by 18.4 ± 1.8 mV (n = 8; p < 0.05; Fig. 2f). (A similar trend was observed with THDOC application: 12 ± 4 mV; n = 3; p = 0.08; Fig. 2f.) Taken together, these results directly demonstrate that presynaptic GABAA receptors sensitive to δ-selective agents have a depolarizing action when [K+]i and [Cl−]i are relatively unperturbed.
Tonically active GABAA receptors alter spike shape
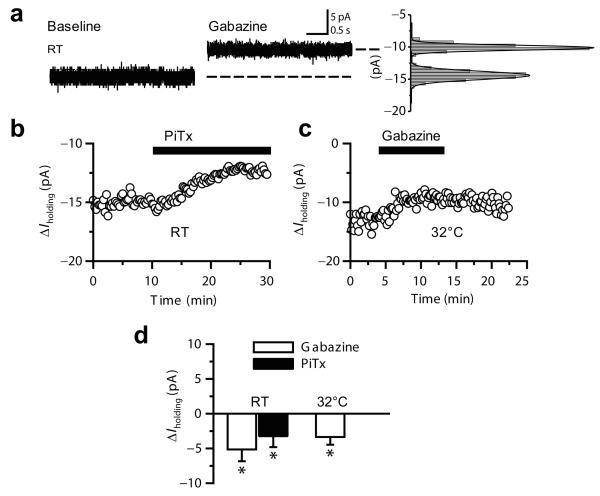
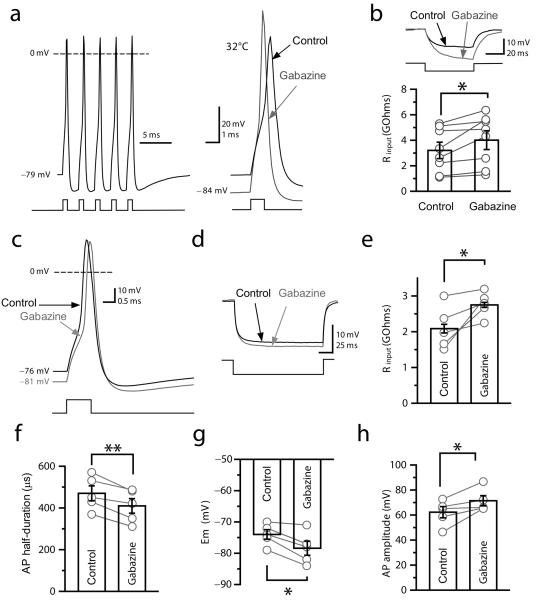
Although bouton-attached recordings provide a minimally invasive measurement of membrane potential, the high access resistance makes it difficult to record the action potential shape. We therefore used whole-bouton recordings to understand how tonic GABAA receptor-mediated depolarization might affect neurotransmitter release. Ionotropic glutamate and metabotropic GABA receptors were blocked as before with NBQX (50 μM), APV (50 μM) and CGP-52432 (5 μM). Initially, we voltage-clamped boutons at −70 mV with a Cs-based pipette solution containing 155 mM [Cl−]. Application of picrotoxin (100 μM) or gabazine (5 or 10 μM) decreased the holding current both at room temperature (picrotoxin: 3.2 ± 1.5 pA, n = 7; gabazine: 5.1 ± 1.7 pA, n = 2, Fig. 3a, b, d) and at 32°C (gabazine: 3.3 ± 1.1 pA, n = 3; Fig. 3c,d). These results confirm that GABAA receptors in mossy fibers are tonically active in a quiescent slice. We then examined the role of the tonic GABAA conductance in action potentials by recording from mossy fiber boutons in current-clamp mode with a pipette containing 155 mM KCl at 32°C (Fig. 4a,b). Blocking tonically active GABAA receptors with gabazine reversibly hyperpolarized all boutons recorded (Control: −73.3 ± 2 mV; gabazine: −78.5 ± 2.6 mV, n = 8, p < 0.004; Fig. 4a, see also Supplementary Fig. 5). This effect was accompanied by an increase in input resistance (Control: 3.2 ± 0.6 GΩ; gabazine: 4.0 ± 0.7 GΩ; p < 0.03; Fig. 4b), an increase in the amplitude of the action potential (Control: 85.9 ± 3.9 mV; gabazine: 95.1 ± 3.4 mV; p < 0.02) and an acceleration of its wave-form (Half-duration Control: 504 ± 34 μs; gabazine: 423 ± 24 μs; p < 0.02) (Fig. 4a, see also Supplementary Fig. 5).
Fig. 3. Blocking GABAA receptors reveals a tonic current in mossy fiber boutons.
(a) Whole-bouton voltage-clamp recording (−70 mV) showing a 4.5 pA tonic current in the presence of gabazine (5 μM). Right, all-point histograms and Gaussian fits derived from the traces. RT: room temperature. (b – c) Time course of holding current in two mossy fiber boutons recorded at RT (22 - 24°C) (B) and at 32°C (C), showing the effect of blocking GABAA receptors with picrotoxin (100 μM) or gabazine (5 μM). (d) Summary of changes in holding current measured in mossy fiber boutons in experiments performed at RT and 32°C (± s.e.m; *, p < 0.05; paired t-test).
Fig. 4. Tonic GABAA receptor-mediated currents modulate the electrical properties of mossy fiber boutons.
(a) Current-clamp recording from a mossy fiber bouton at 32°C (155 mM KCl). Left, the bouton fired with high fidelity in response to 5 consecutive depolarizing pulses at 200 Hz (average of 10 traces). Right, sample traces (averages of 10 consecutive trials) showing the action potential before (black) and after (gray) superfusion of gabazine (5 μM). (b) Top, response to hyperpolarizing current injection, showing an increase in input resistance in gabazine. Bottom, summary of changes in input resistance in mossy fiber boutons recorded with 155 mM [Cl−]i. (c) Current-clamp recording from a mossy fiber bouton with 20 mM [Cl−]i. Sample traces show the action potential (averages of 10 consecutive trials) before (black) and after (gray) superfusion of gabazine (5 μM). Inset, same waveforms superimposed showing the increase in action potential half-duration after superfusion of gabazine. (d - e) Left, Response to hyperpolarizing current injection, showing the increase in input resistance in gabazine. Right, summary plot of the effect of gabazine on input resistance. (f – h) Summary plots showing the effects of gabazine on action potential parameters measured in mossy fiber boutons with 20 mM [Cl−]i. Bar charts: mean ± s.e.m; gray symbols illustrate individual experiments; *, p < 0.05; ** p, < 0.01; paired t-test.
We repeated the measurement of action potential wave-forms with a lower internal chloride concentration (20 mM) to match estimates obtained at a brainstem synapse (ref. 20). Again, gabazine applied at 32°C hyperpolarized mossy fiber boutons (Control: −74 ± 1.5 mV; gabazine: −78.4 ± 2.2 mV; p < 0.05; Fig. 4c,g), and increased their input resistance (Control: 3.2 ± 0.6 MΩ; gabazine: 4.0 ± 0.7 MΩ; p < 0.05; Fig. 4d,e). This effect was accompanied by a reduction in the half-duration of action potentials (Control: 470 ± 36 μs; gabazine: 410 ± 35 μs; p < 0.01; n = 5; Fig. 4c,f), and an increase in their amplitude (Control: 62.3 ± 4.5; gabazine: 71.5 ± 4.1 mV; p < 0.05; Fig. 4h). These effects were partly reversible upon gabazine wash-out (membrane potential: −76 ± 2 mV; input resistance: 2.5 ± 0.4 MΩ; action potential half-duration: 430 ± 33 μs; action potential amplitude: 67.1 ± 3.7 mV). Thus, tonic activation of GABAA receptors can modulate both passive and active properties of mossy fiber boutons under relatively physiological conditions, with intact GABA uptake mechanisms.
Tonically active GABAA receptors modulate Ca2+ transients
How do tonically active GABAA receptors affect presynaptic Ca2+ kinetics in mossy fiber boutons? We applied two-photon excitation microscopy to identify individual giant boutons up to 1000 μm from their parent granule cell body21-23. The whole-cell pipette contained both a fluorescent Ca2+ indicator (Fluo-4) and a morphological tracer (Alexa Fluor 594). Giant mossy fiber boutons were unambiguously identified by their characteristic size and shape, including thin filopodial protrusions (Fig. 5a,c). Gabazine at near-physiological temperature (34 °C) had no significant effect on the resting Ca2+-dependent fluorescence, but decreased the size of the action-potential-dependent fluorescence transient (ΔF/R: 83 ± 5 % of baseline; n = 7; p = 0.032; Fig. 5d; see Methods). In contrast, THDOC (10 nM) increased the action-potential-dependent fluorescence signal (ΔF/R: 114 ± 5 % of baseline; n = 8; p = 0.021), again with no significant effect on the resting fluorescence.
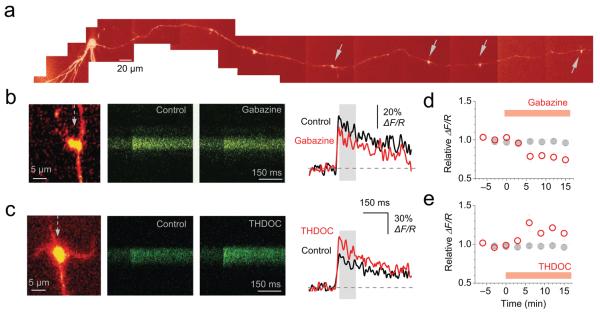
Fig. 5. Tonically active neurosteroid-sensitive GABAA receptors enhance presynaptic action potential-dependent Ca2+ transients in giant mossy fiber boutons.
(a) Reconstruction of a dentate granule cell and its intact axon (Alexa Fluor 594 channel, λx = 800 nm). The somatic patch pipette is seen at left. The collage was obtained from Kalman-filtered averages of 10-15 μm stacks. Arrows indicate giant boutons equipped with characteristic thin filopodia. (b) Blocking GABAA receptors with gabazine reduced spike-dependent presynaptic Ca2+ entry. Left panel: representative giant bouton (‘red’ Alexa channel; dotted arrow shows position of line-scan). Line-scans and traces: Ca2+ responses in the mossy fiber bouton shown in the left panel (‘green’ Fluo-4 channel, 10-trace average; averaging window for ΔF/R indicated by gray area) following a single action potential induced at the soma, in control conditions and in 1 μM gabazine. (c) THDOC (10 nM) enhanced spike-dependent presynaptic Ca2+ entry; notation as in (b). (d-e) Summary of the effects of gabazine (d) (n = 7) and THDOC (e) (n = 8) on the average spike-evoked Ca2+ response. Each point is the average of two trials. Gray symbols show the average ΔF/R time course in control conditions (n = 7); error bars, s.e.m.
These results imply that tonically active GABAA receptors normally enhance the Ca2+ influx that accompanies action potentials, and furthermore that this phenomenon can be potentiated by endogenous neurosteroids acting on δ-subunit-containing GABAA receptors. Taken together with the results of direct mossy fiber bouton recordings (Fig. 4) these effects are consistent with the observation that Ca2+ influx is positively correlated with action potential width24,17.
In contrast to mossy fiber boutons, action potential-dependent fluorescence transients in boutons of CA3 axons, recorded under identical conditions, were unaffected by gabazine (n = 8) or THDOC (n = 6; see Supplementary Fig. 6), implying that tonically active GABAA receptors are not ubiquitously expressed in hippocampal glutamatergic boutons.
Presynaptic GABAA receptors enhance synaptic transmission
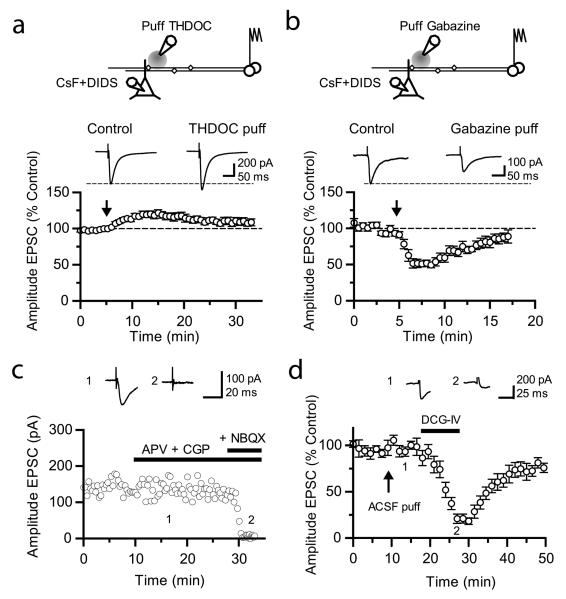
How do tonically active GABAA receptors affect excitatory synaptic transmission to CA3 pyramidal neurons? We recorded from pyramidal neurons with a pipette solution containing CsF and 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) in order to block postsynaptic GABAA receptors intracellularly25, while leaving presynaptic receptors intact. Mossy fibers were stimulated in stratum granulosum and a pressure-application pipette containing THDOC or gabazine was positioned in stratum lucidum near the apical dendrite of the recorded CA3 pyramidal neuron. We thus avoided potential effects of manipulating GABAA receptors at the site of axon recruitment by the electrical stimulus. Local pressure-application of THDOC (50 nM, 10 – 30 psi, 150 – 300 ms) reversibly increased the EPSC amplitude by 22 ± 4 % (n = 8; p < 0.03; Fig. 6a). Conversely, pressure-ejection of gabazine reversibly decreased the EPSC amplitude by 44 ± 6 % (n = 6; p < 0.01; Fig. 6b). We verified that superfusion of NBQX (20 μM) completely abolished residual EPSCs, thus confirming that they were mediated by AMPA/kainate receptors (n = 3, Fig. 6c). We did not detect any effect of puffing ACSF (n = 5, Fig. 6d), arguing against involvement of movement artifacts. We also verified that superfusion of the group II-selective metabotropic glutamate receptor agonist DCG-IV ((2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine, 1 μM) depressed evoked EPSCs by 82 ± 4 % (n = 4, Fig. 6d) confirming that they originated from mossy fibers. Finally, neither THDOC nor gabazine affected the input resistance of CA3 pyramidal neurons.
Fig. 6. Bi-directional modulation of synaptic transmission via tonically active GABAA receptors at mossy fibers.
(a) (Top) Schematic illustrating the experimental design to study presynaptic GABAA receptors at mossy fiber – CA3 synapses. Postsynaptic GABAA receptors in CA3 pyramidal cells were blocked using an intracellular pipette solution containing CsF and DIDS. A pressure-application pipette containing THDOC (50 nM, left) was positioned ~50 – 200 μm from the apical dendrite of the recorded neurons. (Bottom) Time-course of effects of pressure application of THDOC (n = 8). Traces are averages of 10 consecutive EPSCs before and after drug application. NMDA and GABAB receptors were blocked throughout. (b) Effect of gabazine (10 μM) applied in the same way (n = 6). (c) Plot of evoked postsynaptic current amplitude against time in control experiments, showing the complete blockade of synaptic transmission upon NBQX (20 μM) application. Sample traces (averages of 10 consecutive EPSCs) were taken as indicated on the graph. (d) Time course of the effects of pressure-application of ACSF (arrow) followed by superfusion of DCG-IV (1 μM) on evoked EPSCs recorded with a pipette solution containing Cs-F and DIDS (n = 5). Sample traces show the average of 10 consecutive EPSCs before and after superfusion of DCG-IV. Error bars: s.e.m.
Neurosteroid-sensitive GABAA receptors thus exert a bi-directional modulatory influence on mossy fiber transmission to CA3 pyramidal neurons. We asked whether tonically active GABAA receptors have a different effect on mossy fiber transmission to interneurons in stratum lucidum (with DCG-IV applied at the end of the experiment to verify that EPSCs were sensitive). Although gabazine had a qualitatively similar effect, the depression of mossy fiber EPSCs in interneurons was smaller on average than in CA3 pyramidal neurons (25 ± 9 %; n = 9, p < 0.05, not shown), and also more variable, ranging from 0 to 81%. We did not attempt to identify interneurons to determine whether this variability correlated with morphological or other parameters. Finally, in a separate set of experiments we recorded EPSCs in CA3 pyramidal neurons under identical conditions, but evoked via an electrode positioned in stratum radiatum to elicit action potentials in associational-commissural axons, which include recurrent collaterals of CA3 pyramidal cells. In contrast to the robust effect on mossy fiber-evoked EPSCs, gabazine did not affect the EPSC amplitude (95 ± 14% of baseline, p = 0.73, n = 4; see Supplementary Fig. 7).
Presynaptic GABAA receptors facilitate LTP induction
Mossy fibers exhibit NMDA receptor-independent LTP, the induction of which is sensitive to the presynaptic membrane potential26. The depolarizing action of GABAA receptors in mossy fibers suggests that they may facilitate LTP induction by enhancing presynaptic Ca2+ influx. Although presynaptic Ca2+ transients in individual boutons evoked by single action potentials are attenuated by blocking GABAA receptors (Fig. 5d), this finding cannot necessarily be extrapolated to high-frequency trains of action potentials in multiple axons, which are required to induce LTP at mossy fibers. We therefore asked whether local pressure-application of gabazine immediately before high-frequency stimulation (HFS, 100 Hz, 200 ms) in the dentate gyrus could interfere with Ca2+-dependent fluorescence signals detected with the high-affinity ratiometric indicator Fura-2 bulk-loaded to multiple axons in stratum lucidum. The indicator was delivered by injection of the AM-ester close to the site of stimulation27. We then waited for it to be taken up by mossy fibers and to diffuse to stratum lucidum in CA3 before delivering HFS trains at 32°C, with glutamate receptors blocked with NBQX and D-APV. Because HFS-evoked Ca2+ signals (estimated from the fluorescence ratio excited at 340 nm and 380 nm) decreased in peak amplitude with repeated trials (as a result of photobleaching and continued redistribution of Fura-2), we alternately applied gabazine by local pressure either on the first trial or on the second trial in interleaved paired-trial experiments (Fig. 7a). The HFS-evoked Ca2+ signal decreased significantly more when gabazine was applied on the second trial (Δratio340/380, 1st trial minus 2nd trial = 1.13 ± 0.31 %, n = 5) than when applied on the first trial (0.16 ± 0.12 %, n = 5; p < 0.02; Fig. 7b) in the pair. Although this result is consistent with a facilitatory role of GABAA receptors in presynaptic Ca2+ signaling, it does not necessarily indicate that high-affinity receptors mediate the effect, because GABA released by the stimulus could have activated low-affinity receptors4,28. Indeed, when the benzodiazepine agonist zolpidem was applied immediately before the HFS, it had the opposite effect to gabazine (zolpidem on first trial: Δratio340/380, 1st trial minus 2nd trial = 0.77 ± 0.17 %, n = 6; zolpidem on second trial: −0.66 ± 0.26 %, n = 5; p = 0.011; Fig. 7c).
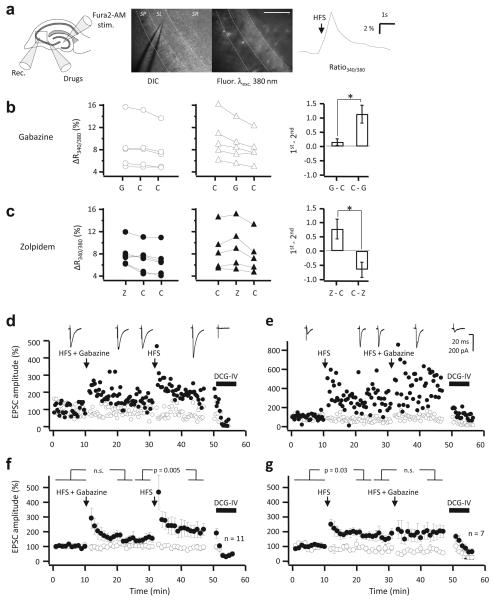
Fig. 7. GABAA receptors facilitate mossy fiber LTP.
(a) Schematic (left) and infrared DIC and fluorescence images (middle), illustrating the Fura2 imaging. A recording electrode (Rec.) was used to monitor mossy fiber EPSPs prior to glutamate receptor blockade and imaging. Mossy fibers were loaded (Fura2-AM) and stimulated (Stim.) in the dentate gyrus. Gabazine, zolpidem or vehicle were pressure-applied just outside the imaged field (Drugs). Scalebar: 100 μm. Right: sample Ca2+ fluorescence trace showing a transient increase in the fluorescence ratio excited at 340 and 380 nm evoked by high-frequency stimulation (HFS). (b) Gabazine was pressure-applied immediately before the first (left) or second (middle) of three trains delivered with 4 minute intervals. The HFS-evoked fluorescence ratio transient (ΔRatio340/380) was significantly attenuated by gabazine (G), as revealed by comparing with control conditions (C) (right). (c) Pressure-applied zolpidem (Z) increased the HFS-evoked fluorescence ratio transient (ΔRatio340/380). (d) EPSC amplitudes recorded in a CA3 pyramidal neuron in response to stimulation of two pathways. HFS was delivered to one pathway (filled symbols) at the times indicated, either together with local pressure application of gabazine in stratum lucidum (HFS + Gabazine) or alone (HFS). DCG-IV was applied at the end of the experiment to confirm that the responses were profoundly depressed, typical of mossy fibers. The sample traces were obtained in the test pathway, aligned with the times at which they were recorded. (e) Example of a cell where the LTP induction protocols were applied in reverse order. (f) Average time course of 11 experiments as in (d). (g) Average time course of 7 experiments as in (e). Error bars: s.e.m.
Notwithstanding the confounding effect of different subtypes of GABAA receptors present in mossy fibers (see also refs. 6,7), the small but significant effect of gabazine on HFS-evoked Ca2+ signaling suggests that they may modulate the induction of LTP. To test this hypothesis we attempted to elicit LTP at mossy fiber synapses by recording EPSCs in CA3 pyramidal neurons at 32°C. A second pathway acted as a control for non-specific changes in recording condition. APV was included in the perfusion solution, and 5 mM EGTA in the pipette solution. We delivered HFS (100 Hz, 1 s) to the mossy fiber pathway, immediately following local pressure application of gabazine in stratum lucidum in the vicinity of the recorded CA3 pyramidal neuron. This yielded a non-significant increase in EPSC amplitude (p = 0.12, n = 11; Fig. 7d,f). When the HFS was subsequently delivered to the same pathway without gabazine, robust LTP was elicited (p = 0.005), reaching 205 ± 39 % of the baseline amplitude.
In a separate set of experiments, we inverted the order of conditioning stimuli, initially delivering HFS without gabazine, and then repeating it, after an identical delay, with pressure-applied gabazine. In this case, LTP was readily elicited by HFS alone (170 ± 25 %, n = 6; p = 0.03). HFS repeated with gabazine elicited a small additional potentiation that did not reach significance (Fig. 7e,g).
Although this experiment does not distinguish between tonically active receptors and lower-affinity receptors activated upon tetanization, the effect of gabazine argues that mossy fiber LTP is indeed facilitated by GABAA receptors. Because postsynaptic depolarization plays little or no role in mossy fiber LTP induction29, the results imply a role for presynaptic GABAA receptors in glutamatergic synaptic plasticity.
DISCUSSION
We have shown that tonically active and neurosteroid-sensitive GABAA receptors enhance neurotransmitter release in the brain by affecting action potential shape and Ca2+ signaling. We have also shown that GABAA receptors facilitate the induction of a presynaptic form of LTP.
The evidence reported here for tonically active presynaptic GABAA receptors is highly unlikely to be explained by a remote effect of GABAA receptors in the somato-dendritic compartments or axon initial segment. First, although sub-threshold depolarization can propagate a long distance in mossy fibers30,22, only a minority of axons supplying giant boutons are preserved during slice preparation. Mossy fibers were connected to their parent granule cells in experiments on action current threshold and Ca2+ imaging in individual boutons, but these were carried out under somatic voltage clamp. Second, the bi-directional modulation of mossy fiber membrane potential and synaptic transmission to CA3 was achieved by locally applied gabazine or THDOC. And third, we have directly demonstrated THDOC-sensitive channels in outside-out patches excised from giant boutons. These results indicate that tonically active neurosteroid-sensitive receptors are present in the synaptic varicosities that synapse on CA3 pyramidal neurons. Although Alle and Geiger (ref. 6) reported zolpidem-sensitive receptors with intermediate GABA sensitivity, as expected for α2 and γ-subunit-containing receptors (see also ref. 3), they saw no effect of blocking GABAA receptors on the input resistance of mossy fiber boutons unless GABA uptake was inhibited. A possible explanation for the discrepancy is that the input resistance is relatively insensitive to GABAA receptor manipulation, compared to the holding current and membrane potential. In any case, the presence of zolpidem-sensitive receptors does not exclude the additional presence of high-affinity receptors. A further possibility is that the same receptors might be sensitive to both zolpidem and THDOC. Although such GABAA receptors have not been reported, novel stoichiometries with unusual pharmacological profiles have been identified in extrasynaptic membranes of hippocampal neurons31,32, and diazepam has been reported to modulate GABAA receptor openings in the absence of GABA (ref. 33).
The bouton-attached recordings obtained here minimize perturbation of [K+]i and [Cl−]i. The resting apparent membrane potential in boutons was estimated around −70 mV, about 10 mV above that of granule cell somata. Although a leak conductance through the patch can bias the estimate of the membrane potential34, this potential artifact has little impact on the qualitative effects of either blocking tonically active GABAA receptors with gabazine or potentiating them with THDOC. These manipulations altered the mossy fiber bouton membrane potential in opposite directions, indicating that the GABAA reversal potential is depolarized relative to the resting membrane potential. This is consistent with presynaptic GABAA receptors elsewhere in the CNS, and implies that Cl− extrusion mechanisms are unable to drive ECl down to EK (ref. 20).
In the spinal cord presynaptic GABAA receptor-mediated depolarization of primary afferents is accompanied by a depression of neurotransmitter release, secondary to Na+ and/or Ca2+ channel inactivation and action potential propagation failure1. In contrast, presynaptic depolarization at most sites studied in the brain, caused by GABAA, glycine or kainate receptor activation35-38, or as a result of electrotonic propagation of sub-threshold somatic depolarization30,22,39, is accompanied by enhanced evoked neurotransmitter release (although see ref. 40). In the present study, blocking tonically active GABAA receptors decreased spike half-width and action potential-dependent presynaptic Ca2+ transients monitored with multi-photon excitation fluorescence microscopy. The change in action potential shape is consistent with de-inactivation of K+ channels secondary to hyperpolarization. Because a linear dependence of Ca2+ influx on action potential half-width has been reported in mossy fiber boutons24,17, this provides a ready explanation for the effect on glutamate release and orthodromic transmission to CA3 pyramidal neurons. However, the relationship between presynaptic membrane potential and exocytosis is affected by several other processes41,22,42,43, the relative contributions of which are beyond the scope of this study.
The effects of gabazine on mossy fiber bouton membrane potential, action potential shape and orthodromic transmission, imply that the low ambient GABA level in a quiescent slice is sufficient for baseline activity of high-affinity receptors. The extracellular GABA concentration in the intact brain is difficult to measure, but estimates as high as 2 - 4 μM have been obtained with microdialysis44. It may also fluctuate with behavioral state45. Taken together with evidence that neurosteroid levels fluctuate extensively in normal and pathological situations14,46, the present results reveal a powerful mechanism for bi-directional modulation of mossy fiber transmission to CA3.
Remarkably, although tonically active GABAA receptors in the somatodendritic compartment reduce granule cell excitability47,48, they enhance glutamate release from mossy fiber terminals, arguing against the use of ‘tonic inhibition’ as synonymous with the action of these receptors.
We have further shown that blocking GABAA receptors impairs the induction of mossy fiber LTP. Although we cannot rule out a more complex cascade, whereby GABAA receptors affect the release of a neuromodulator which, in turn, alters the induction of LTP, the results are most simply interpreted as an effect on presynaptic receptors on mossy fibers themselves. Indeed, they are consistent with the finding that manipulations that depolarize presynaptic boutons facilitate mossy fiber LTP induction26. Indeed, the dual role of presynaptic GABA receptors in modulating transmission and LTP at this synapse parallels the effects reported for presynaptic kainate receptors. Although we have not determined whether this effect is mediated by tonically active receptors, or by lower affinity receptors recruited by tetanic stimulation, it further underlines that presynaptic GABAA receptors have a paradoxical pro-excitatory role.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureneuroscience/.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to P. Jonas and to D. Engel for help in optimizing mossy fiber bouton recordings, and to C. Henneberger, M.C. Walker and K. Volynski for comments on the manuscript. This work was supported by the Medical Research Council (UK), the Wellcome Trust, the European Research Council, and the Fondation pour la Recherche Médicale (France).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
References
- 1.Kullmann DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trigo FF, Marty A, Stell BM. Axonal GABAA receptors. Eur. J. Neurosci. 2008;28:841–848. doi: 10.1111/j.1460-9568.2008.06404.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz A, et al. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–73. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura M, Sekino Y, Manabe T. GABAergic interneurons facilitate mossy fiber excitability in the developing hippocampus. J. Neurosci. 2007;27:1365–1373. doi: 10.1523/JNEUROSCI.4672-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang I, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Alle H, Geiger JRP. GABAergic spill-over transmission onto hippocampal mossy fiber boutons. J. Neurosci. 2007;27:942–950. doi: 10.1523/JNEUROSCI.4996-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han J, et al. Differential pharmacological properties of GABA(A) receptors in axon terminals and soma of dentate gyrus granule cells. J. Neurochem. 2009;109:995–1007. doi: 10.1111/j.1471-4159.2009.06018.x. [DOI] [PubMed] [Google Scholar]
- 8.Chandra D, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangan PS, et al. Cultured Hippocampal Pyramidal Neurons Express Two Kinds of GABAA Receptors. Mol. Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- 12.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 15.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 16.Bischofberger J, Engel D, Li L, Geiger JRP, Jonas P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- 17.Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fastinactivating K(+) channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–39. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 18.Fricker D, Verheugen JA, Miles R. Cell-attached measurements of the firing threshold of rat hippocampal neurones. J. Physiol. (Lond.) 1999;517(Pt 3):791–804. doi: 10.1111/j.1469-7793.1999.0791s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verheugen JA, Fricker D, Miles R. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J. Neurosci. 1999;19:2546–2555. doi: 10.1523/JNEUROSCI.19-07-02546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price GD, Trussell LO. Estimate of the chloride concentration in a central glutamatergic terminal: a gramicidin perforated-patch study on the calyx of Held. J. Neurosci. 2006;26:11432–11436. doi: 10.1523/JNEUROSCI.1660-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott R, Rusakov DA. Main determinants of presynaptic Ca2+ dynamics at individual mossy fiber-CA3 pyramidal cell synapses. J. Neurosci. 2006;26:7071–7081. doi: 10.1523/JNEUROSCI.0946-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott R, Ruiz A, Henneberger C, Kullmann DM, Rusakov DA. Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+ J Neurosci. 2008;28:7765–73. doi: 10.1523/JNEUROSCI.1296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott R, Lalic T, Kullmann DM, Capogna M, Rusakov DA. Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses. J Neurosci. 2008;28:13139–49. doi: 10.1523/JNEUROSCI.2932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bischofberger J, Geiger JRP, Jonas P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 2002;22:10593–602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yakushiji T, Tokutomi N, Akaike N, Carpenter DO. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987;22:1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- 27.Regehr WG, Tank DW. The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron. 1991;7:451–459. doi: 10.1016/0896-6273(91)90297-d. [DOI] [PubMed] [Google Scholar]
- 28.Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–15. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 29.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 30.Alle H, Geiger JRP. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- 31.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. (Lond.) 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birnir B, Eghbali M, Everitt AB, Gage PW. Bicuculline, pentobarbital and diazepam modulate spontaneous GABA(A) channels in rat hippocampal neurons. Br. J. Pharmacol. 2000;131:695–704. doi: 10.1038/sj.bjp.0703621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavas J, Marty A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J. Neurosci. 2003;23:2019–2031. doi: 10.1523/JNEUROSCI.23-06-02019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- 36.Stell BM, Rostaing P, Triller A, Marty A. Activation of presynaptic GABA(A) receptors induces glutamate release from parallel fiber synapses. J. Neurosci. 2007;27:9022–9031. doi: 10.1523/JNEUROSCI.1954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trigo FF, Chat M, Marty A. Enhancement of GABA release through endogenous activation of axonal GABA(A) receptors in juvenile cerebellum. J. Neurosci. 2007;27:12452–12463. doi: 10.1523/JNEUROSCI.3413-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 39.Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- 40.Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science. 1993;259:531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]
- 41.Huang H, Trussell LO. Control of presynaptic function by a persistent Na(+) current. Neuron. 2008;60:975–979. doi: 10.1016/j.neuron.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hori T, Takahashi T. Mechanisms underlying short-term modulation of transmitter release by presynaptic depolarization. J. Physiol. (Lond.) 2009 doi: 10.1113/jphysiol.2009.168765. doi:10.1113/jphysiol.2009.168765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 44.Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi L, et al. Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochem. Res. 2003;28:565–573. doi: 10.1023/a:1022881625378. [DOI] [PubMed] [Google Scholar]
- 46.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 47.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog. Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 48.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.