Abstract
Psoriasis is an inflammatory skin disorder with aberrant regulation of keratinocytes and immunocytes. While it is well known that uncontrolled keratinocyte proliferation is largely driven by pro-inflammatory cytokines from the immunocytes, the functional role of keratinocytes in the regulation of immunocytes is poorly understood. Recently, we found that Trim32, an E3-ubiquitin ligase, is elevated in the epidermal lesions of human psoriasis. We previously showed that Trim32 binds to Piasy and mediates its degradation through ubiquitination. Interestingly, the Piasy gene is localized in the PSORS6 susceptibility locus on chromosome 19p13 and Piasy negatively regulates the activities of several transcription factors including NF-κB, STAT, and SMADs that are implicated in the pathogenesis of psoriasis. In this study, we show that Trim32 activates, and Piasy inhibits, keratinocyte production of CCL20, a psoriatic chemokine essential for the recruitment of dendritic cells and Th17 cells to the skin. Further, Trim32/Piasy regulation of CCL20 is mediated through Piasy interaction with the RelA/p65 subunit of NF-κB. As CCL20 is activated by Th17 cytokines, the up-regulation of CCL20 production by Trim32 provides a novel positive feedback loop of CCL20 and Th17 activation in the self-perpetuating cycle of psoriasis.
Introduction
CCL20 is an inflammatory chemokine responsible for the recruitment of leukocytes to sites of injury and inflammation. It is mainly expressed in surface epithelial cells such as keratinocytes (Homey et al., 2000), bronchial epithelium (Reibman et al., 2003), and intestinal epithelium (Fujiie et al., 2001). CCL20 expression from keratinocytes is induced by pro-inflammatory cytokines such as TNFα, IL-1, and IL-17 (Harper et al., 2009; Homey et al., 2000; Nograles et al., 2008). In contrast to the promiscuous nature of many other chemokines and their receptors, CCR6 is the only known receptor for CCL20. CCR6 is expressed in a variety of immunocytes including neutrophils, memory B cells, Treg, immature DCs, and Th17 cells. CCL20/CCR6 plays important roles in chemostasis of immunocytes, particularly for those in the Th17 pathway. CCR6 is induced by nuclear receptor RORgammat (Manel et al., 2008), and found in virtually all Th17 cells (Singh et al., 2008). Moreover, CCR6 is essential for the pathogenesis of encephalomyelitis and psoriasis in animal models (Hedrick et al., 2009; Reboldi et al., 2009).
Th17 activation is a defense mechanism against extracellular infection in tissue (Ghilardi and Ouyang, 2007). The receptors for Th17 cytokines are expressed in a variety of epithelial cells including keratinocytes. Th17 cytokines contribute to tissue immunity through 1) production of antimicrobial peptides, 2) recruitment of immunocytes via induction of chemokines such as CCL20, and 3) tissue repair by enhancing epithelial proliferation (Zheng et al., 2007). The Th17 signaling pathway is tightly controlled to ensure tissue homeostasis, and is terminated after infection is ablated and tissue repair complete. However, Th17 signaling is persistently activated in psoriasis (Blauvelt, 2008). The recruitment of Th17 cells by CCL20 and induction of CCL20 from keratinocytes by Th17 cytokines in psoriasis highlights the critical role of CCL20 in Th17 activation and psoriasis pathogenesis. Yet, the regulation of CCL20 induction in keratinocytes is largely unknown.
Trim32 is a scaffold protein with E3 ubiquitin ligase activity (Ozato et al., 2008). We found that Trim32 contributes to the survival of keratinocytes under conditions that induce terminal differentiation and apoptosis (Horn et al., 2004). Furthermore, we identified Piasy as a Trim32 physiological substrate (Albor et al., 2006), where Trim32 mediates Piasy ubiquitylation and degradation in keratinocytes. Piasy is a transcriptional repressor for a number of transcription factors with roles in inflammation and keratinocyte proliferation, such as STATs, SMADs, and NF-κB (Shuai and Liu, 2005). The PIASY gene is localized in a psoriasis susceptibility locus (PSORS6) on chromosome 19p13 (Lee et al., 2000); however the genetic alterations responsible for the pathogenesis of psoriasis in this locus remain to be identified. The fact that Piasy regulates a number of factors whose activation is associated with psoriasis-like phenotypes in mouse models, including constitutive NF-κB activation by IκBα deletion (Klement et al., 1996), epidermal overexpression of STAT3 (Sano et al., 2005), and TGFβ which activates Smads (Li et al., 2004), highlights a central role for Trim32 and Piasy in psoriasis pathogenesis.
In this study, we found that Trim32 is elevated in psoriatic epidermis. Furthermore, we identified CCL20 as a downstream target of Trim32. Trim32 activates, and Piasy inhibits, keratinocyte production of CCL20 in mouse and in human keratinocytes. Further molecular dissection of this pathway revealed Trim32/Piasy regulation of CCL20 through NF-κB, mediated by Piasy interaction with the RelA/p65 subunit of NF-κB. As CCL20 is activated by Th17 cytokines, the up-regulation of CCL20 production by Trim32 provides a novel positive feedback loop and direct keratinocyte role of CCL20 and Th17 activation in the self-perpetuating cycle of psoriasis.
Results
Trim32 is up-regulated in psoriatic epidermis
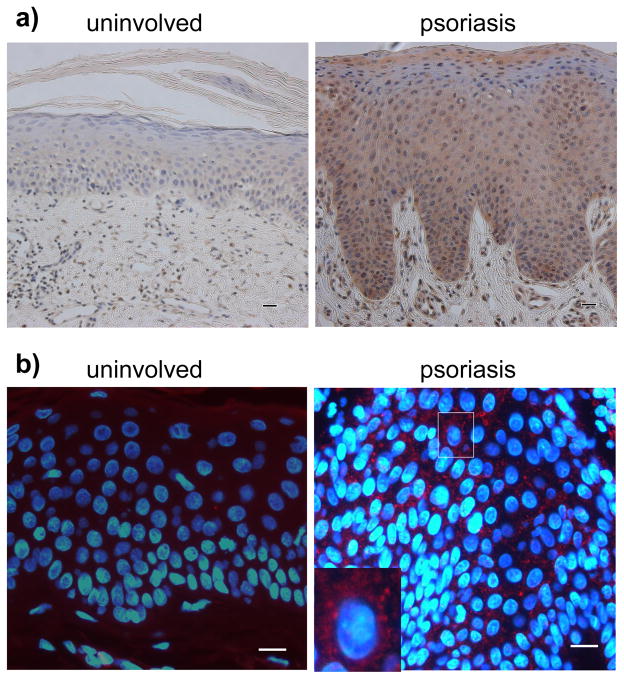
Trim32 is an E3 ubiquitin ligase that inhibits apoptosis in normal keratinocytes in response to UV-light and TNFα (Horn et al., 2004). Based upon reports of resistance to apoptosis as one of the common features of psoriatic keratinocytes (Wrone-Smith et al., 1997), we tested Trim32 expression in lesional specimens from human psoriasis patients, compared to uninvolved control epidermis (Fig. 1a). We found Trim32 overexpression in 20 out of 33 psoriasis specimens by immunohistochemical staining, with elevated Trim32 in the basal and spinous epidermal cell layers. Indirect immunofluorescence analysis revealed that Trim32 expression is predominantly cytoplasmic in the psoriatic keratinocytes (Fig. 1b), as expected on the basis of previous studies (Albor et al., 2006; Reymond et al., 2001).
Figure 1. Elevated Trim32 expression in human psoriasis.
a) Immunohistochemical analysis of Trim32 expression in psoriasis specimens. Trim32 was visualized in paraffin sections using 0.2 μg/ml chicken anti-Trim32 antibody and stained using the ABC technique. Trim32 was elevated in the epidermis of psoriatic lesions relative to uninvolved skin (200× magnification). b) Indirect immunofluorescence analysis of Trim32 expression in psoriasis specimens. Trim32 was visualized with chicken anti-Trim32 antibody followed by Texas red secondary antibody (600× magnification). The nucleus was stained with Hoechst 33342. The micrographs are representative of 20 Trim32 positive from 33 psoriatic skin specimens. The bar equals 50 micrometers.
Regulation of psoriatic chemokine CCL20 by Trim32/Piasy
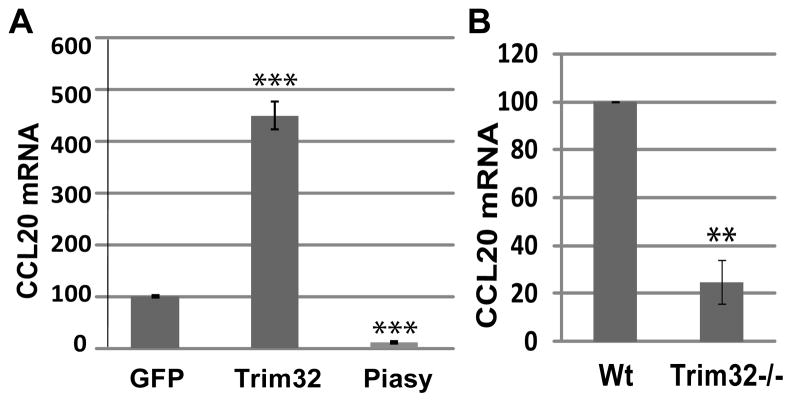
To explore how Trim32 might potentially contribute to the pathogenesis of psoriasis, we analyzed the expression of a panel of psoriatic cytokines and chemokines in keratinocytes infected with Trim32 or Piasy adenoviruses. We found that CCL20 expression was significantly increased in keratinocytes with Trim32 adenovirus and decreased in keratinocytes with Piasy adenovirus as compared with those infected with GFP adenovirus (Fig. 2a). In addition, CCL20 expression is reduced in primary keratinocytes isolated from Trim32 null mice as compared with those from their wild type littermates (Fig. 2b).
Figure 2. Regulation of CCL20 expression in keratinocytes by Trim32 and Piasy.
a) CCL20 mRNA expression in mouse keratinocyte line 291 infected with adenovirus expressing GFP, Trim32, or Piasy. b) Analysis of CCL20 mRNA in primary mouse keratinocytes isolated from Trim32 null mice (Trim32−/−) and wild type littermates (Wt). CCL20 mRNA was normalized to GAPDH. Data are representative of three independent experiments for 1a and two independent experiments for 1b (**, P< 0.01; ***, P< 0.001).
Trim32 correlates with CCL20 expression in psoriasis
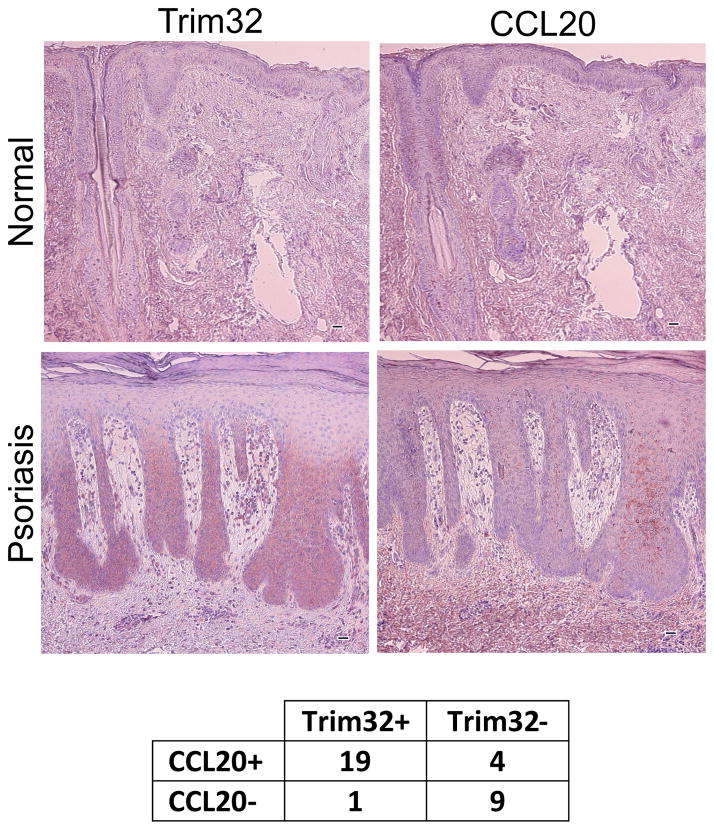
CCL20 is a psoriatic chemokine overexpressed in psoriatic epidermis (Homey et al., 2000). To test whether Trim32 expression correlated with CCL20 expression, we carried out immunostaining of tissue sections for each of these antigens in human psoriatic skin specimens versus uninvolved skin controls (Fig. 3). CCL20 was not detectable in the uninvolved skin. However, CCL20 was detected along with Trim32 overexpression in a majority of psoriatic skin specimens. CCL20 was detected in 23 out of 33 psoriasis specimens from 33 different patients, correlating with Trim32 positivity in 19 cases. Lack of Trim32 correlated with lack of CCL20 expression in 9 additional cases. Thus CCL20 expression in psoriatic lesions correlated with Trim32 status in 87% of the psoriatic specimens (Fig. 3).
Figure 3. Trim32 and CCL20 expression in human psoriasis.
Paraffin embedded psoriasis lesional tissues from 33 patients were sectioned serially and stained with chicken anti-Trim32 antibody and goat anti-CCL20 antibody (100× magnification). The results are summarized in the table. Correlation of overexpression of Trim32 with CCL20 expression was statistically significant (*Two-tailed Fisher exact test, p-value = 0.00015). The bar equals 50 micrometers.
Trim32 sensitizes keratinocytes to TNFα and IL-17A induced CCL20 expression
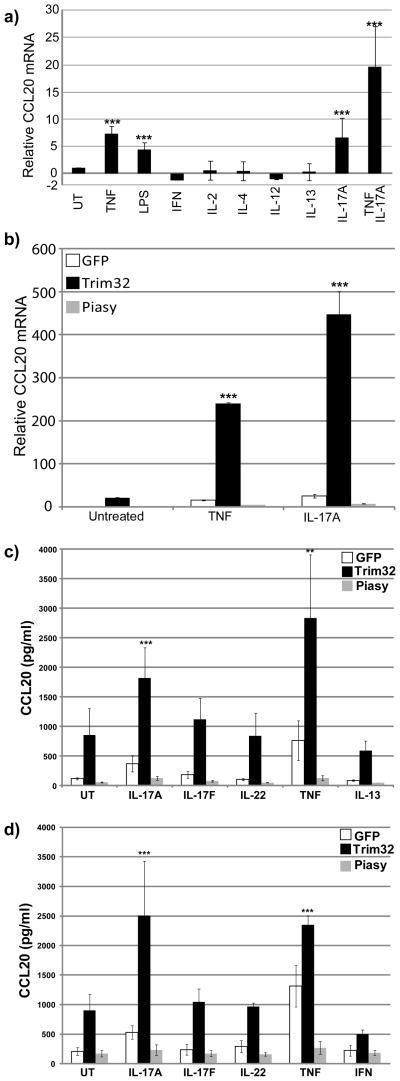
To define the role of CCL20 in the T cell response, we evaluated CCL20 expression in keratinocytes treated with Th1 (interferon-γ, IL-2, IL-12), Th2 (IL-4, IL-13), and Th17 (IL-17A, TNFα) cytokines. TNFα and IL-17A showed potent effects on CCL20 expression in keratinocytes (Fig. 4a), while neither Th1 nor Th2 cytokines showed any significant effect on CCL20 expression. As activated Th17 cells produce both IL-17A and TNFα, we evaluated their combined effects on CCL20 induction. Although TNFα is also produced from other T helper cells, the enhanced induction of CCL20 by the combined treatment of IL-17A and TNFα is consistent with CCL20 as a Th17 chemokine regulated by Th17 cytokines.
Figure 4. Regulation of Th17 induced CCL20 by Trim32 and Piasy.
a) CCL20 mRNA was measured by qRT-PCR in mouse 291 keratinocytes treated with indicated cytokines and LPS. b) CCL20 mRNA was measured by qRT-PCR in mouse 291 keratinocyte infected overnight with adenovirus expressing GFP, Trim32, or Piasy, then treated with indicated cytokines for 4 hours. CCL20 mRNA was normalized to GAPDH. c) CCL20 protein was measured by ELISA in mouse 291 keratinocyte infected overnight with adenovirus expressing GFP, Trim32, or Piasy, then treated with indicated cytokines for 6 hours. d) Human keratinocyte line HEKnV was infected with adenovirus expressing GFP, Trim32, or Piasy, then treated with indicated cytokines for 6 hours. CCL20 protein was measured from the culture supernatant by ELISA. Data are representative of three independent experiments. The statistical analysis was conducted by one-way ANOVA followed by Bonferroni post-test (**, P< 0.01; ***, P< 0.001).
To test whether Trim32/Piasy regulate the expression of CCL20 in response to TNFα and IL-17A, we measured CCL20 expression in keratinocytes infected with Trim32- or Piasy-expressing adenoviruses followed by treatment with TNFα or IL-17A. Trim32 significantly increased, whereas Piasy repressed, TNFα and IL-17A induction of CCL20 expression (Fig. 4b). These effects of Trim32 and Piasy on induction of CCL20 by TNFα or IL-17A were confirmed by ELISA analysis of CCL20 protein expression in both mouse (Fig. 4c), and human (Fig. 4d) keratinocytes. Trim32 showed enhanced induction of CCL20 by TNFα or IL-17A but not by IL-17F, IL-13 and interferon-γ. The cooperation of Trim32 expression with TNFα or IL-17 signaling to induce CCL20 expression in keratinocytes suggests that Trim32 is a positive regulator that targets rate-limiting steps in TNFα and IL-17 signaling pathways.
CCL20 regulation by Piasy is mediated through the p65/RelA subunit of NF-κB
The regulation of TNFα and IL-17 induced CCL20 by Trim32/Piasy suggests Trim32/Piasy acts at a common pathway between TNFα and IL-17. In fact, NF-κB is a common downstream effector of TNFα, IL-17, IL-1, and LPS. Because of our previous results showing that NF-κB is regulated by Trim32/Piasy (Albor et al., 2006), we evaluated the effect of Trim32 and Piasy on NF-κB dependent CCL20 expression. We found that CCL20 is induced upon transfection of a constitutively active p65/RelA subunit of NF-κB (Fig. 5a), but that p65 mediated CCL20 expression was only marginally increased by co-transfection of Trim32. This suggests that p65 acts downstream of Trim32, and therefore bypasses the regulation of CCL20 expression by Trim32. Note that the overall induction of CCL20 by Trim32 plasmid transfection (9.6 fold) was less than CCL20 induction by Trim32 adenovirus (20 fold in Fig. 4), consistent with plasmid transfection being less efficient than adenoviral infection. p65-dependent CCL20 expression was significantly repressed by co-transfection of Piasy, suggesting that p65 is a target of Piasy. This is supported by Piasy association with p65, as detected by co-immunoprecipitation of endogenous p65 with Flag-tagged Piasy in 291 keratinocytes (Fig. 5b), suggesting that CCL20 regulation by Trim32/Piasy is mediated through NF-κB.
Figure 5. Piasy regulates CCL20 through p65/RelA subunit of NF-κB.
a) CCL20 mRNA was measured in 291 keratinocytes transfected with plasmids expressing GFP, p65, Trim32 plus p65, or Piasy plus p65. Data are representative of two independent experiments. Statistical analysis of Trim32 and Piasy effect was conducted relative to p65 alone control (*, P< 0.05). b) Piasy interacts with endogenous p65 in keratinocytes. Flag-tagged Piasy and Flag-tagged Erk (negative control) were transfected into 291 keratinocytes. The bound p65 was immunoprecipitated with an anti-Flag antibody and visualized by immunoblotting with anti-p65 antibody.
Discussion
Psoriasis is a chronic inflammatory skin disorder characterized by epidermal hyperproliferation and immunocyte infiltration (Lowes et al., 2007; Nickoloff et al., 2007). T cell-derived cytokines have been prevalently viewed as the driving force for aberrant keratinocyte proliferation and differentiation, whereas keratinocytes are generally considered as the downstream effector of T cell activation. Yet, unlike many other autoimmune diseases such as systemic lupus erythematosus and pemphigus vulgaris, psoriasis is triggered and exacerbated by external insults such as microbial infections and physical injury (Koebner phenomenon), suggesting that defects in the immune system alone are not sufficient for psoriasis pathogenesis. The perturbation of keratinocyte response to external insults may contribute to the pathogenesis of psoriasis through chemokine production to recruit immunocytes. CCL20 is a chemokine abundant in psoriatic plaques (Harper et al., 2009; Homey et al., 2000; Kryczek et al., 2008), and is induced by proinflammatory cytokines. In addition, CCL20 can be induced by physical stimuli, such as tape stripping of skin (Schmuth et al., 2002) and dynamic compression of chondrocytes (Haudenschild et al., 2008). The induction of CCL20 can also be achieved by bacterial infection such as Staphylococcus aureus (Fahy et al., 2004) and Streptococcus mutans (Takahashi et al., 2008) with different toll-like receptors (TLR) in keratinocytes specific to different types of microbes. As keratinocytes constitute the first line of defense to protect the body from external insults, CCL20 production from keratinocytes could be an initial trigger for Th17 activation, and aberrant CCL20 induction in keratinocytes may contribute to the pathogenesis of psoriasis.
In this study, we showed up-regulation of Trim32, an E3 ubiquitin ligase, in psoriatic epidermis and identified Trim32 and its substrate Piasy as regulators of CCL20. Trim32 increases CCL20 induction by TNFα and IL-17 in keratinocytes. Systematic analysis of CCL20 induction in response to cytokines revealed that CCL20 is induced by Th17, but not Th1 or Th2 cytokines. This indicates that CCL20 is a chemokine specific to Th17 activation. These results are consistent with the results of Harper et al. demonstrating that CCL20 is induced by TNFα and IL-17A (Harper et al., 2009). In conjunction with CCL20 induction by Th17 cytokines, the involvement of CCL20 in Th17 activation is also supported by: 1) CCL20 recruitment of CCR6 positive CD11c+ DCs that produce IL-23 for Th17 cell activation (Lee et al., 2004); 2) high CCR6 expression in virtually all Th17 cells but not in Th1 and Th2 cells (Singh et al., 2008; Yamazaki et al., 2008), and 3) CCL20 overexpression in psoriatic epidermis (Homey et al., 2000). Therefore, CCL20 expression in keratinocytes could be a key component in maintaining the self-perpetuating cycle of Th17 activation in psoriasis.
As a tissue immune defensive mechanism, the Th17 signaling pathway is activated upon exposure to pathogens or tissue injury, and is tightly controlled to ensure tissue homeostasis (Ghilardi and Ouyang, 2007). Th17 signaling is terminated after infection is ablated and tissue repair complete. The persistent Th17 activation in psoriasis suggests that defects exist in Th17 signal regulation in psoriasis. Persistent Th17 activation in psoriatic skin is characterized by 1) the infiltration of IL-23 producing DCs and Th17 cells, and 2) epidermal overexpression of Th17 chemokines. As the recruitment of Th17 cells and IL-23 producing DCs is an essential step for Th17 activation, uncontrolled CCL20 production by keratinocytes could be central to persistent Th17 activation in psoriasis. In this study, we have demonstrated that CCL20 induction by TNFα and IL-17A is enhanced by Trim32 and attenuated by Piasy (Fig. 4). Thus, Trim32 overexpression in human psoriatic lesions observed in this study provides a plausible mechanism for how keratinocytes in epidermis could contribute to excessive CCL20 production in psoriasis. As Piasy is a substrate of Trim32, Piasy alterations could be primary causes of aberrant CCL20 induction in psoriasis cases without elevated Trim32. In this regard it will be interesting to determine whether the PIASY gene is functionally involved in the psoriasis susceptibility associated with the PSORS6 locus on chromosome 19p13 (Lee et al., 2000). Because Piasy is also a negative regulator for NF-κB, Smad, and Stat transcription factors that have been implicated in the development of psoriasis-like phenotypes in animal models, taking advantage of animal models and genetic approaches will help to further define the role of the Trim32/Piasy axis in Th17 activation and psoriasis pathogenesis.
So far, the mechanism of CCL20 regulation by the Trim32/Piasy axis is largely unknown. The magnitude of CCL20 induction enhanced by Trim32 suggests that Trim32 acts at rate-limiting step in CCL20 regulation. Because NF-κB is the common downstream effector of TNFα, IL-1, IL-17, and LPS, we focused our study in the NF-κB pathway. Although CCL20 induction by TNFα and IL-17 was significantly sensitized by Trim32 (Fig. 4), CCL20 induction by the p65/RelA subunit of NF-κB was only marginally enhanced by Trim32 (Fig. 5a). Bypassing the action of Trim32 by the constitutively active p65/RelA suggests that p65/RelA is the downstream effector of Trim32. Our evidence that p65/RelA is a Piasy interacting protein, and that p65/RelA-mediated CCL20 induction was repressed by Piasy (Fig. 5a), suggest that p65/RelA is a target regulated by the Trim32/Piasy axis in CCL20 expression.
Taken together, our results show that Trim32 and Piasy can regulate CCL20 in mouse and human keratinocytes, that Trim32 and CCL20 expression positively correlate in psoriasis lesional samples, and that Trim32 antigen activity is elevated in psoriatic epidermis. These results provide evidence for a novel pathway by which CCL20 is regulated in keratinocytes, and by which keratinocytes may contribute to the Th17 role in psoriasis. Further study to define the mechanism of CCL20 regulation by the Trim32/Piasy axis in keratinocytes will be essential to developing novel therapeutic approaches that target these E3 ligases to disrupt the self-perpetuating cycle of Th17 activation in psoriasis.
Materials and Methods
Keratinocyte Cell cultures
The primary mouse keratinocyte cell strain 291 was derived from neonatal mouse skin, and displays normal regulation of proliferation and differentiation by extracellular Ca2+ characteristic of primary epidermal cultures (Kulesz-Martin et al., 1985). Primary mouse keratinocytes were isolated from neonatal Trim32 knock-out mice epidermis and their wild type littermates. These cells were maintained in “low calcium” medium (final concentration of 0.03–0.05 mM Ca2+) as described (Dlugosz et al., 1995; Kulesz-Martin et al., 1988). Human keratinocytes HEKnV were cultured in EpiLife keratinocyte medium supplemented with a semi-defined human keratinocyte growth supplement (Cascade Biologics, Portland, OR) as described (Iordanov et al., 2002).
Adenoviral production and infection
The recombinant Trim32 and Piasy adenoviruses were generated as described using Adeno-X (Clontech, Mountain View, CA) (Liu et al., 2004). The recombinant Trim32 adenovirus was characterized using anti-Trim32 antibody to detect Trim32 expression in 291 keratinocytes infected with the virus. The viral particles were titered by immunostaining of Trim32 in 291 keratinocytes infected with limiting dilutions of virus. The minimum number of viral particles required for 100% infection of 291 keratinocytes was calibrated for experiments.
Cytokine treatment
Once the keratinocyte culture reached 100% confluence, the medium was replaced with LCEM without the fibroblast conditioned media. The mouse and human keratinocyte cultures were treated with species specific cytokines, respectively: TNFα (20 ng/ml), 1 ug/ml LPS (Sigma, St. Louis, MO), IFNγ (20 ng/ml), IL-2 (20 ng/ml), IL-4 (50 ng/ml), IL-13 (50 ng/ml), IL-17A (100ng/ml), IL-17F (100ng/ml), or IL-22 (100ng/ml), purchased from Peprotech (Rocky Hill, NJ).
Quantitative real time polymerase chain reaction (RT-PCR) for CCL20
Triplicate qPCR reactions (15 μl) for each sample and primer set were performed in a 384 well plate (Applied Biosystems, Inc. Foster City, CA) using Ccl20 gene-specific primers (6 μMeach, validated for linearity and target specificity) Fwd: TGCTCTTCCTTGCTTTGGCATGGGTA, Rev: TCTGTGCAGTGATGTGCAGGTGAAGC, in the presence of UDP-N-glycosylase (Invitrogen, Carlsbad, CA) and SYBR-Green I dye (Applied Biosystems Inc., Foster City, CA). Gene expression data was collected using a 7900HT thermocycler and SYBR-Green I fluorescence was analyzed using the ΔΔCT method (Applied Biosystems, Inc., Foster City, CA). The relative Ccl20 mRNA was normalized with GAPDH.
Keratinocyte transfection and immunoprecipitation
291 keratinocytes were transfected with Flag-tagged Piasy vector (Albor et al., 2006) using Mirus transfection reagent (Mirus Bio LLC, Madison, WI) when the culture reached 70% confluence. The cell lysates were collected in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% SDS, 10 mM EDTA, 50 mM Tris-HCl pH 8.0) and subjected to immunoprecipitation with agarose beads conjugated with anti-Flag antibody, clone M2 (Sigma, St. Louis, MO). The bound proteins were eluted for immunoblotting with anti-p65/RelA antibody, clone C20(Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Indirect immunofluorescence and immunohistochemistry
Archival human psoriasis lesional skin specimens biopsied and diagnosed in the OHSU Department of Dermatology were accessed with approved OHSU IRB protocol. Uninvolved human skin controls were obtained from non-psoriasis donors undergoing surgery in the Departments of Dermatology or Otolaryngology. The non-specific binding of antibody was blocked with PAB (PBS, 0.1% sodium azide, 0.5% BSA) containing 10% normal goat serum for CCL20 or 10% blokhen (AVES Lab, Portland, OR) for Trim32. The sections were then incubated with affinity-purified chicken anti-Trim32 antibody generated in our laboratory (Albor et al., 2006) or goat anti-CCL20 antibody, clone AF360 (R&D Systems Inc., Minneapolis, MN) overnight at 4°C. The specificity of chicken anti-Trim32 antibody was verified by immunoblotting and immunostaining as described (Albor et al., 2006) and in the Supplemental data herein (Figure S1). The staining signal was visualized by the ABC approach (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin. The immunofluorescent staining of Trim32 was carried out with Texas red secondary antibody. The nucleus was stained with 1 μg/ml Hoechst 33342.
Statistics
The data were expressed as mean ± SD. Statistical significance of CCL20 regulation by Trim32 and Piasy was determined using a two-tailed Student’s t test (Fig. 2 and 5) and one-way ANOVA followed by Bonferroni post-test (Fig. 4). Statistical significance of correlated expression of Trim32 and CCL20 (Fig. 3) was determined by two-tailed Fisher exact test.
Supplementary Material
Acknowledgments
We thank Hao Ding for providing Trim32 null mice, Mihail Iordanov for providing human keratinocyte HEKnV, Susan Lillie for providing clinical specimens, Carolyn Gendron and the Knight Pathology Core for histology assistance, Knight DNA Sequencing & Synthesis Core Lab for oligo synthesis, Erin Harper for CCL20 qPCR assistance, and Andrew Blauvelt for helpful discussion. This study was supported by National Psoriasis Foundation research grant to Yuangang Liu, 2007–08 OHSU Presidential Bridge Funding, Knight Cancer Institute Core grant CA69533 and NIH AR55651.
Abbreviations
- Trim32
tripartite motif-containing 32
- Piasy
protein inhibitor of activated STAT Y
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- DC
dendritic cell
- IL
interleukin
- Th
T helper
- TNF
tumor necrosis factor
- IFN
interferon
- LPS
lipopolysaccharides
- IHC
immunohistochemistry
Footnotes
Conflict of interest:
The authors have no conflict of interest.
References
- Albor A, El Hizawi S, Horn EJ, Laederich M, Frosk P, Wrogemann K, et al. The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in limb-girdle muscular dystrophy type 2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkappaB. J Biol Chem. 2006;281:25850. doi: 10.1074/jbc.M601655200. [DOI] [PubMed] [Google Scholar]
- Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. The Journal of investigative dermatology. 2008;128:1064–1067. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz AA, Glick AB, Tennenbaum T, Weinberg WC, Yuspa SH. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- Fahy OL, Townley SL, Coates NJ, Clark-Lewis I, McColl SR. Control of Salmonella dissemination in vivo by macrophage inflammatory protein (MIP)-3alpha/CCL20. Laboratory investigation; a journal of technical methods and pathology. 2004;84:1501–1511. doi: 10.1038/labinvest.3700176. [DOI] [PubMed] [Google Scholar]
- Fujiie S, Hieshima K, Izawa D, Nakayama T, Fujisawa R, Ohyanagi H, et al. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3alpha/CCL20 in mucosal epithelial cells through NF-kappaB [correction of NK-kappaB] International immunology. 2001;13:1255–1263. doi: 10.1093/intimm/13.10.1255. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ouyang W. Targeting the development and effector functions of TH17 cells. Seminars in immunology. 2007;19:383–393. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. The Journal of investigative dermatology. 2009 doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Nguyen B, Chen J, D’Lima DD, Lotz MK. Rho kinase-dependent CCL20 induced by dynamic compression of human chondrocytes. Arthritis and rheumatism. 2008;58:2735–2742. doi: 10.1002/art.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. The Journal of clinical investigation. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- Horn EJ, Albor A, Liu Y, El Hizawi S, Vanderbeek GE, Babcock M, et al. RING protein Trim32 associated with skin carcinogenesis has anti-apoptotic and E3-ubiquitin ligase properties. Carcinogenesis. 2004;25:157. doi: 10.1093/carcin/bgh003. [DOI] [PubMed] [Google Scholar]
- Iordanov MS, Choi RJ, Ryabinina OP, Dinh TH, Bright RK, Magun BE. The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signal-regulated kinase signaling cascade from the activated epidermal growth factor receptor. Molecular and cellular biology. 2002;22:5380–5394. doi: 10.1128/MCB.22.15.5380-5394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, et al. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Molecular and cellular biology. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesz-Martin M, Yoshida MA, Prestine L, Yuspa SH, Bertram JS. Mouse cell clones for improved quantitation of carcinogen-induced altered differentiation. Carcinogenesis. 1985;6:1245. doi: 10.1093/carcin/6.9.1245. [DOI] [PubMed] [Google Scholar]
- Kulesz-Martin MF, Penetrante R, East CJ. Benign and malignant tumor stages in a mouse keratinocyte cell line treated with 7, 12-dimethylbenz[à]anthracene in vitro. Carcinogenesis. 1988;9:171. doi: 10.1093/carcin/9.1.171. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Ruschendorf F, Windemuth C, Schmitt-Egenolf M, Stadelmann A, Nurnberg G, et al. Genomewide scan in german families reveals evidence for a novel psoriasis-susceptibility locus on chromosome 19p13. American journal of human genetics. 2000;67:1020–1024. doi: 10.1086/303075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. Embo J. 2004;23:1770–1781. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lagowski JP, Vanderbeek GE, Kulesz-Martin MF. Facilitated search for specific genomic targets by p53 C-terminal basic DNA binding domain. Cancer BiolTher. 2004;3:1102. doi: 10.4161/cbt.3.11.1189. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nature immunology. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clinical reviews in allergy & immunology. 2007;33:45–56. doi: 10.1007/s12016-007-0039-2. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. The British journal of dermatology. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nature reviews. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature immunology. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. American journal of respiratory cell and molecular biology. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- Reymond A, Meroni G, Fantozzi A, Merla G, Cairo S, Luzi L, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140. doi: 10.1093/emboj/20.9.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- Schmuth M, Neyer S, Rainer C, Grassegger A, Fritsch P, Romani N, et al. Expression of the C-C chemokine MIP-3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Experimental dermatology. 2002;11:135–142. doi: 10.1034/j.1600-0625.2002.110205.x. [DOI] [PubMed] [Google Scholar]
- Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nature reviews. 2005;5:593–605. doi: 10.1038/nri1667. [DOI] [PubMed] [Google Scholar]
- Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi T, Yumoto H, Adachi T, Matsuo T. CCL20 production is induced in human dental pulp upon stimulation by Streptococcus mutans and proinflammatory cytokines. Oral microbiology and immunology. 2008;23:320–327. doi: 10.1111/j.1399-302X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- Wrone-Smith T, Mitra RS, Thompson CB, Jasty R, Castle VP, Nickoloff BJ. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–1329. [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.