Abstract
Introduction
Animal studies show that atrial fibrillation (AF) may emanate from sites of high rate and regularity, with fibrillatory conduction to adjacent areas. We used simultaneous mapping to find evidence for potential drivers in human AF defined as sites with higher rate and regularity than surrounding tissue.
Materials and Methods
In 24 patients (age 61±10 years; 12 persistent) we recorded AF simultaneously from 32 left atrial bipolar basket electrodes in addition to pulmonary veins (PV), coronary sinus and right atrial electrodes. We measured AF cycle length by Fourier transform and electrogram regularity at each electrode, referenced to patient-specific atrial anatomy.
Results
We analyzed 10,298 electrode-periods. Evidence for potential AF drivers was found in 11 patients (5 persistent). In persistent AF, these sites lay at the coronary sinus and left atrial roof but not PVs, while in paroxysmal AF 6 of 9 sites lay at PVs (p<0.05). During ablation, a subset of patients experienced AF CL prolongation or termination with a focal lesion; in each case this lesion mapped to potential driver sites on blinded analysis. Conversely, sequential mapping failed to reveal these sites, possibly due to fluctuations in dominant frequency at driver locations in the context of migratory AF.
Conclusions
Simultaneous multi-site recordings in human AF reveal evidence for drivers that lie near PVs in paroxysmal but not persistent AF, and were sites where ablation slowed or terminated AF in a subset of patients. Future work should determine if real-time ablation of AF-maintaining regions defined in this fashion eliminates AF.
Keywords: Atrial Fibrillation, Substrate Mapping, Fourier Analysis, Fibrillatory Conduction
Introduction
Atrial fibrillation is the most common arrhythmia in the United States, yet its treatment is suboptimal. While ablation is effective in patients with paroxysmal AF, ablation is less successful in persistent AF due to an incomplete understanding of its pathophysiology. Elegant animal studies show that AF may emanate from rapid and regular sources with fibrillatory conduction causing less rapid and less organized activity in surrounding tissue (1; 2). Many sites of rapid atrial activity are seen in human AF (3–7), but it is unclear whether these sites ‘drive’ AF because recent work suggests that some may represent sites of wavefront collision or artifact (8).
We hypothesized that human AF may be maintained by a driver, evidenced as the fastest and most organized site (i.e. with repeatable electrograms over time) with radial decrement in rate and regularity to surrounding tissue. This physiologically-based definition is consistent with prior studies in canine and sheep models of AF (2). Human studies have recently proposed that a single driver may perpetuate AF (7). Indeed, Dibs et al. (9) identified localized sites of rapid and organized atrial activity in human AF, but radial activity from these sites was based on sequential point-by-point mapping (7), that cannot examine spatial relationships if drivers move over time (10).
We set out to use simultaneous multipole mapping to study if human AF exhibits drivers with fibrillatory conduction to surrounding tissue by studying spatial relationships in AF rate and regularity from instantaneous maps from 44 bipolar electrodes of the left atrium (LA), pulmonary veins (PV), coronary sinus (CS) and right atrium, compared to sequential mapping from these electrodes, in relation to ablation in patients with paroxysmal and persistent AF.
Methods
Patient Recruitment
We enrolled 24 consecutive patients (age 61 ± 10 years) with persistent (n=12) or paroxysmal AF, defined per guidelines (11), referred to the Arrhythmia Service of the Veterans Affairs San Diego Healthcare System and University of California San Diego Medical Centers (VASDHS/UCSD). The study was approved by our joint Institutional Review Board, and all patients provided written informed consent. All patients were anti-coagulated and/or showed no evidence of thrombus on transesophageal echocardiography (table 1).
Table 1.
Clinical Characteristics.
| Characteristic | Paroxysmal AF | Persistent AF | P |
|---|---|---|---|
| Age/years | 61 ± 10.2 | 60 ± 10.3 | NS |
| Gender/M, F | 12,0 | 11,1 | NS |
| Duration of AF/months | 30 ± 33 | 91 ± 110 | NS |
| Ventricular CL at time of ablation/ms | 738 ± 181 | 837 ± 172 | NS |
| Left atrial diameter/mm | 42 ± 3.7 | 48 ± 4.4 | <0.001 |
| Left ventricular ejection fraction/% | 59 ± 7 | 52 ± 11 | NS |
| Right Atrial Enlargement/% | 5 (42) | 7 (58) | NS |
| Heart Failure/% | 0 | 4 (33) | NS |
| Hypertension/% | 8 (67) | 11 (92) | NS |
| Coronary Disease/n (%) | 2 (17) | 5 (42) | NS |
| Diabetes Mellitus/% | 6 (50) | 2 (17) | NS |
| Prior Cardiac Surgery/PCI/% | 1 (8) | 4 (33) | NS |
| Medications | |||
| ACEI/ARB/% | 6 (50) | 9 (75) | NS |
| Statins/% | 8 (67) | 8 (67) | NS |
| Beta-blockers/% | 9 (75) | 10 (83) | NS |
| Class I anti-arrhythmics/% | 2 (17) | 3 (25) | NS |
| Amiodarone/% | 1 (8) | 1 (8) | NS |
| Sotalol/% | 0 | 1 (8) | NS |
| Dofetilide/% | 2 (17) | 0 | NS |
Electrophysiologic Recordings
Electrophysiologic study was performed in the fasted state, at least 4 weeks after discontinuing amiodarone (n=2 patients; table 1) and 5 half-lives after discontinuing other anti-arrhythmic medications. In each patient, a duodecapolar catheter (St. Jude, 2 mm interelectrode/10 mm recording pair spacing) was used to record from the CS, inferior, and lateral right atrium. After trans-septal puncture, we advanced a 64-electrode basket (Constellation, Boston Scientific; 48 mm baskets: 4 mm interelectrode/4 mm recording pair spacing; 60 mm baskets: 5 mm interelectrode/5 mm recording pair spacing) sized according to LA echocardiographic anteroposterior diameter, and a steerable quadripolar mapping and ablation catheter (Boston Scientific, 2.5 mm interelectrode/2.5 mm recording pair spacing) into the LA (figure 1). Baskets were manipulated to ensure contact with the posterior LA, PV antra and orifice of the LA appendage using 3 criteria. First, we verified deformation of all splines with fluoroscopic imaging as they conformed to the atrial walls. Second, we ensured that all splines showed consistent movement artifact (even in atrial fibrillation) once good contact had been achieved. Third, electrogram signals were evaluated to ensure an adequate signal-to-noise ratio. The steerable mapping and ablation catheter was used for PV sampling and clinical ablation.
Figure 1.
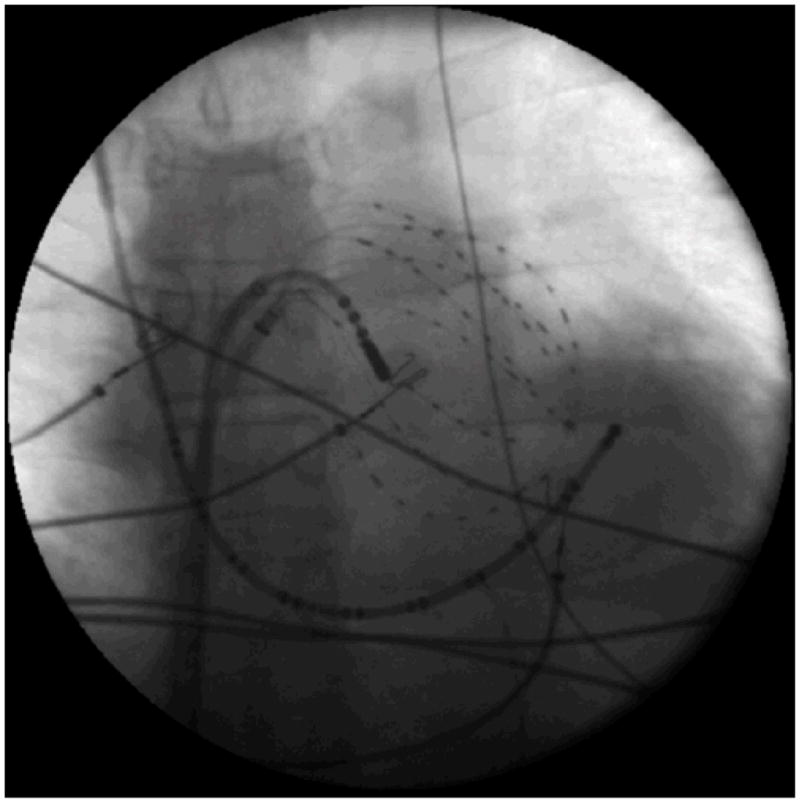
Left Atrial Fluoroscopy in the anteroposterior projection demonstrating a 64-electrode basket catheter in the left atrium, a deflectable 8-mm tip ablation catheter, and a duodecapolar catheter in the right atrium and coronary sinus.
We recorded AF for 5 minutes. Spontaneous AF was recorded unless the patient presented in sinus rhythm (n=10), when AF was induced by burst pacing from the CS, and analyzed after 2 minutes. During AF, we sampled each PV in turn. After completing the protocol, we performed ablation by circumferential PV isolation and additional linear lesions as clinically indicated. Ablation was performed during ongoing AF in 13 patients.
Spatial Distribution of AF Rate and Regularity Indices
Individualized pre-procedural CT images and NavX reconstructions (St. Jude Medical, Sylmar, CA) were used to individually relate each electrode to anatomic landmarks. We analyzed AF rate and regularity for electrodes grouped regionally as: (1) Pulmonary veins; (2) Posterior LA (16 electrodes); (3) LA roof (16 electrodes); (5) Anterior LA wall (8–16 electrodes); (5) Coronary sinus (8 electrodes) and (6) Right atrium (8–10 electrodes).
Electrogram Signal Processing
Signals were digitized at 1 kHz to 16-bit resolution on the physiologic recorder (Bard, Lowell, MA) and exported to a computer running custom software written in Labview (National Instruments, Austin, TX) and Matlab (Mathworks, Natick, MA). Recordings showing excessive baseline wander, evidence of poor contact, artifact or noise were excluded. Of the 44 bipolar electrodes available, on average 8 (range 8–10) were excluded due to poor atrial contact over the mitral valve and noise. Analysis was applied to sequential 8.192-second recordings using our custom software with manual verification of each recording period (8).
Evaluation of AF Cycle Length at Each Site
As we have described (8), a Hanning window was used to taper bipolar electrograms (5). Each signal was rectified, smoothed via low-pass filtering at 20 Hz then analyzed via an 8192-point (resolution 0.12 Hz) fast Fourier transformation to compute its power spectrum (5). The dominant frequency (DF) is the largest peak in the 3–12 Hz bandwidth, and was reported as its CL (i.e. 333 to 83 ms). Importantly, we verified the CL of very fast sites (DF > 10 Hz) using our validated correlation- autocorrelation method (8; 12), that reduces overestimation of rate due to double counting in spectra from bipolar electrograms (8; 13).
Evaluating AF Regularity at Each Sample Site
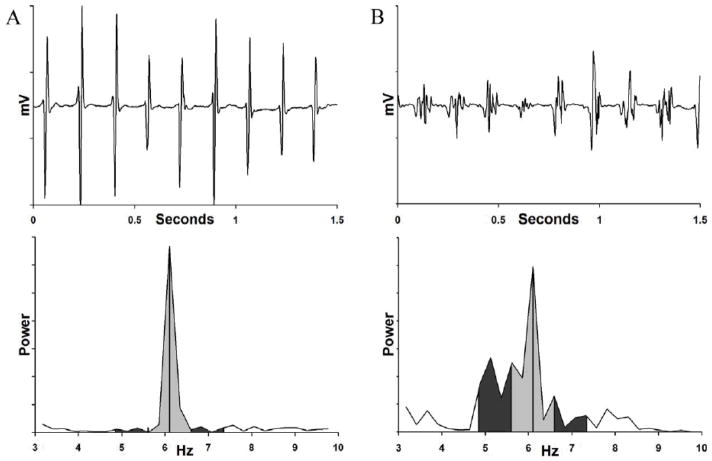
We measured regularity (i.e. cycle-to-cycle regularity of electrograms) as the peak area ratio (PAR, range 0–1) – the area ratio of the DF (1 Hz width) to the encompassing 2.5 Hz envelope (8; 12). We have previously described the use of this index (8; 12). Briefly, PAR nearer 1 reflects repeatable activation (a narrow peak, figure 2A), while lower values (a broad spectrum, figure 2B) reflect irreproducible activity over time (12; 14; 15). Notably, fractionated electrograms may not have a low PAR. First, fractionated electrograms may occur in a regular fashion, regardless of rate (16). Second, the frequency of fractionation is much higher than the dominant frequencies considered for this study (usually > 20 Hz, often 50 Hz and potentially higher (17).
Figure 2.
Regularity Measure (Peak Area Ratio, PAR) for (A) Regular Atrial Electrogram (Top) Produces Narrow FFT Spectrum (Bottom). The ratio of the 1 Hz envelope (light gray shaded area) surrounding the dominant frequency peak (DF = 6.1 Hz) to the 2.5 Hz envelope (dark gray and light gray regions combined) yields a peak area ratio (PAR) of 0.86. (B) PAR Calculation in Electrograms with Greater Temporal Irregularity (Top) Obtained Simultaneously in Same Patient. The Broad FFT Spectrum (Bottom) yields a PAR of 0.61, reflecting greater temporal variability than figure 3A. The presence or absence of electrogram fractionation is not reflected in PAR since frequencies associated with fractionation are much higher (~20–50Hz) than those used for the PAR metric (3–12 Hz).
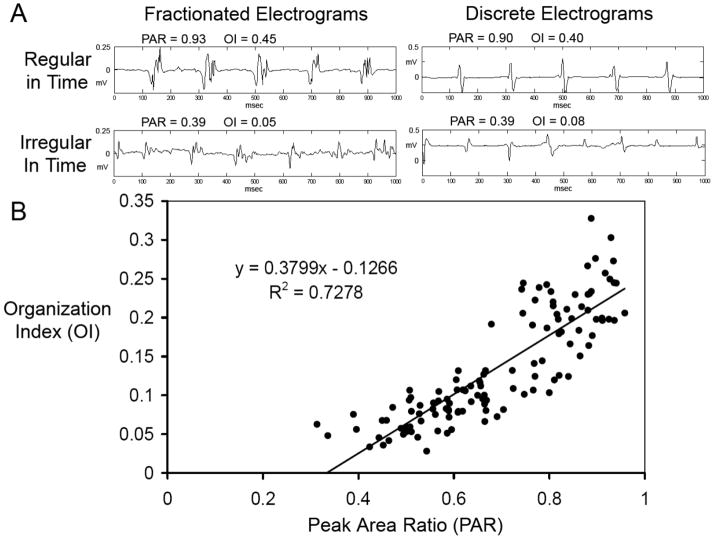
To validate results from PAR, figure 3A shows examples of electrograms that were concordantly classified as organized by PAR and the previously reported organization index (OI) (14). PAR and OI values were closely related (Figure 3B). However, they were not interchangeable. Using OI in our driver analysis algorithm did not identify drivers in any of our patients, including sites of AF termination found with the use of PAR.
Figure 3.
(A) Examples of Fractionated (left) and Discrete (right) Electrograms with High (top) and Low (bottom) Temporal Regularity. Note that Peak Area Ratio (PAR) and Organizational Index (OI) metrics show similar levels of regularity, and are not directly related to fractionation. (B) High Correlation (R = 0.85, R2 = 0.73) between OI and PAR is shown in this plot of electrograms from five randomly selected patients.
Identification and Ablation of Potential Driver Sites
To address our hypothesis, we focused on evidence for single drivers as proposed in human AF by Sahadevan et al (6) and Sanders et al (7). A driver site was defined if: (1) it was the fastest of all atrial sites at that time point, (2) its PAR was greater than the average PAR of the neighboring sites, and (3) the average DF and PAR of the neighboring sites was greater than the average DF and PAR of distant sites.
By routine, all patients’ ablation included left and right wide area circumferential ablation and segmental pulmonary vein isolation in addition to additional lesions at the discretion of the clinician. Driver site analysis was performed separately in this study, and driver location was not available during the ablation. Thus, drivers which lay outside out the clinical lesion set were not ablated. However, we retrospectively combined NavX and Bard procedural data to determine at what point ablation lesions occurred at driver sites to determine the effect of driver site ablation on AF cycle length.
Simulation of Sequential, Rate-Based Mapping
For patients in whom ablation terminated AF (thus identifying a potential AF maintaining site), we compared results from our simultaneous mapping technique to maps created in a sequential manner with identical resolution. First, a stable 8.192 second recording was measured at one electrode. Then, after 10–30 seconds to simulate catheter movement, a subsequent 8.192 second electrogram was measured at an adjacent electrode. This cycle was repeated for all electrodes. FFT analysis was then performed and drivers identified using the same criteria as the simultaneous analysis. To minimize the impact of the actual sequential sequence chosen, we performed the simulation 1000 times, and evaluated the probability of successfully identifying the site (electrode pair) or region (within the atrium) of termination during ablation.
Patient Follow-up
By protocol, patients were discharged on antiarrhythmic medications, if tolerated. Antiarrhythmic medications were then stopped at 3 months post-procedure; thereafter, patients provided monthly 24 hour ambulatory ECG (Holter) recordings, as shown to produce optimal detection of intermittent AF (18). Antiarrhythmic medications could be restarted at the discretion of the clinician upon AF recurrence. Patients were also evaluated in clinic every 3 months to determine if they were in sinus rhythm. We report both the percentage of patients in whom a single procedure maintained sinus rhythm and the use of antiarrhythmic medications at 6 months.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD). The two-tailed t-test was used to compare continuous variables. Repeated measures analysis of variance was used to determine the significance of AF type (paroxysmal or persistent) upon step-down of CL and PAR from drivers and upon regional CLs. The Bonferroni correction was applied for planned multiple comparisons. Chi-squared and Fisher’s exact test were applied to contingency tables. A probability < 5 % was considered statistically significant. Statistical analysis was performed using NCSS 2007 (Kaysville, UT).
Results
Clinical characteristics of our population are shown in table 1. The only significant difference between groups was a greater left atrial diameter in persistent AF patients.
Baseline CL and Regularity Measurements in Persistent and Paroxysmal AF
A total of 10,298 electrode-intervals were analyzed. Compared to patients with paroxysmal AF, the population with persistent AF had shorter AF CL for all sites (186±23 vs 220±23, p=0.002) and non-significantly (NS) lower AF regularity (PAR 0.52±0.06 vs 0.57±0.13, p=NS). AF CL and regularity differed regionally between the two groups. As shown in table 2, sites in patients with persistent AF showed shortest CL in the CS (176±16 ms) and LA roof (187±24 ms), while pulmonary vein sites were most rapid for patients with paroxysmal AF (180±31 msec). Of note, there were no significant differences between spontaneous and induced AF CL (187±26 msec vs 199±25, p=NS), PAR (0.57±0.06 versus 0.59±0.09, p=NS) or percentage of patients with drivers (42% vs 50%, p=NS).
Table 2.
Regional Rate and Regularity (PAR) During AF
| AF CL | PAR | |||||
|---|---|---|---|---|---|---|
| Parox. | Persist. | p* | Parox. | Persist. | p* | |
| LA Roof | 221±23 | 187±24 | <0.001 | 0.57±0.13 | 0.52±0.06 | NS |
| Pulmonary Veins | 180±31 | 198±46 | NS | 0.74±0.11 | 0.59±0.13 | NS |
| LA Posterior Wall | 223±27 | 200±23 | NS | 0.57±0.12 | 0.50±0.05 | NS |
| MV Annulus | 223±19 | 211±25 | NS | 0.54±0.11 | 0.50±0.06 | NS |
| Coronary Sinus | 209±24 | 176±16 | NS | 0.61±0.09 | 0.56±0.05 | NS |
| Right Atrium | 225±28 | 198±26 | NS | 0.55±0.17 | 0.52±0.04 | NS |
after Bonferroni correction for multiple comparisons
Potential Driver Sites
Only a subset of sites with high AF rate (short CL) qualified as potential drivers. The majority of rapid sites were not classified as drivers because they failed to show a radial step-down in rate and regularity. Overall, potential driver sites had a significantly greater dominant frequency (7.0±1.5 vs 4.9±0.6 Hz, p<0.001) and peak area ratio (0.68±0.18 vs 0.55±0.13, p=0.02) than other sites during atrial fibrillation.
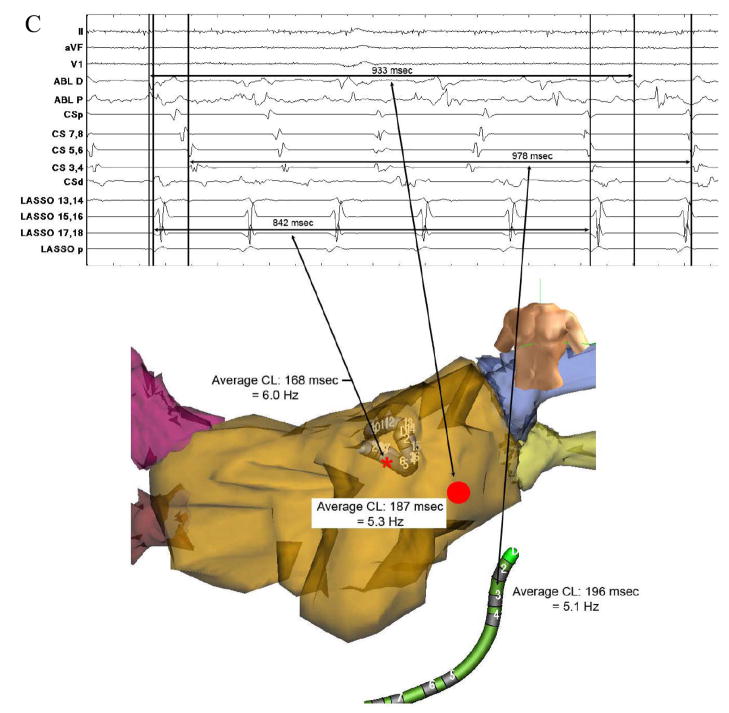
In patients with paroxysmal AF, 6 of 12 patients (50%) exhibited such sites. In patients with persistent AF, 5 of 12 (42%) exhibited a total of 8 potential driver sites. Of the 6 paroxysmal AF patients with drivers, 3 showed 2 separate potential driver sites at different time intervals and positions within the LA. Figure 4 shows electrograms (A) and FFT spectral analysis data (B) from a potential driver near the right superior pulmonary vein, identified by centrifugal analysis in a patient with paroxysmal AF, where ablation terminated AF. Figure 5 shows electrograms (A) and FFT data (B) from another patient in whom AF was maintained by regular focal atrial tachycardia at the base of the left atrial appendage that drove the remaining atrium into AF and that was also identified from centrifugal activation analysis. In this case, figure 5C, obtained during ablation shows a clear step-down in activation frequency and regularity (PAR) from the driver in the left atrial appendage (lasso catheter, 6 Hz), to the neighboring left atrium (5.3 Hz) to the coronary sinus (5.1 Hz). Activation rate maps for both patients are shown in figure 6.
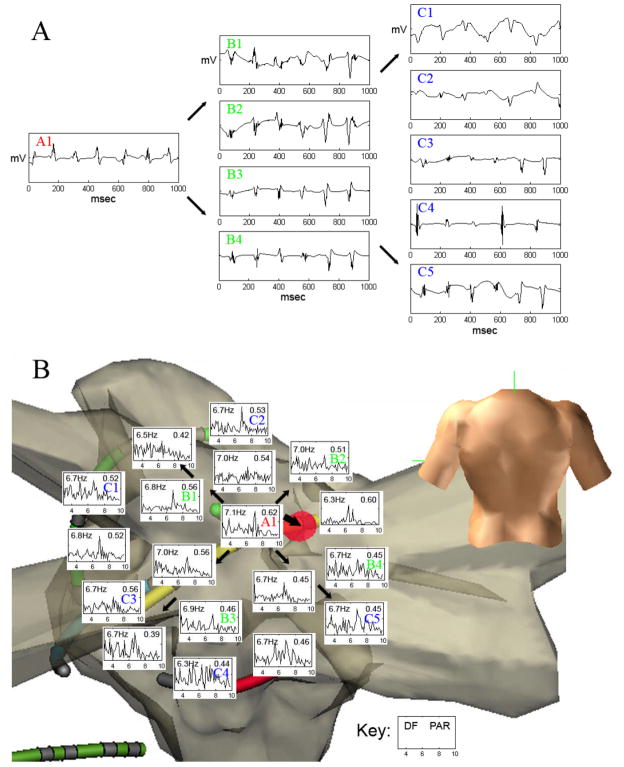
Figure 4.
(A) Electrogram Recorded from Driver Site (A1) Near Right Superior Pulmonary Vein (red “A1” in part B below) with representative simultaneous electrograms from neighboring (B1-B4) and distant (C1-C5) sites in atrial fibrillation. Note significant decrease in rate at distant site C4 electrogram. (B) Radial Step-down (Thin Arrows) in Dominant Frequency (DF) and Regularity (Peak Area Ratio, PAR) from Driver Site (red “A1”) is seen in this spectral analysis to neighboring and distal (blue “B”) sites. Ablation at the driver site (thick arrow to red circle) terminated AF.
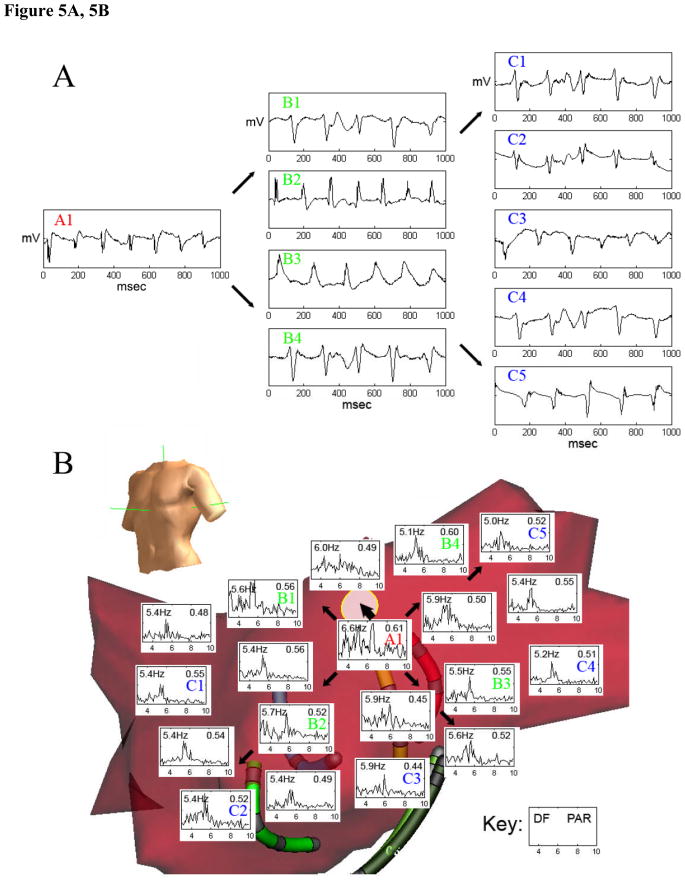
Figure 5.
(A) Electrograms from Regular Driver (red “A1”) were rapid and regular versus slower and irregular activation at neighboring (particularly B1, B3, and B4) and distant (C1–C5) sites. (B) Radial Step-Down (Thin Arrows) in Dominant Frequency (DF) and Regularity (PAR) from a Driver Site (red “A1”) identified by centrifugal analysis at the base of the left atrial appendage (thick arrow to white circle). (C, next page, top) Focal Atrial Tachycardia Identified within the Left Atrial Appendage During Ablation (lasso p) near the previously identified driver site. (C, next page, bottom) Electroanatomic Map Showing AF Slowing and Increasing Irregularity with distance (arrows) from the driver site (lasso, red star) to neighboring sites (ablation catheter, red circle) and distant sites (coronary sinus electrograms, CS). DF = dominant frequency, PAR = peak area ratio, CL = cycle length.
Figure 6.
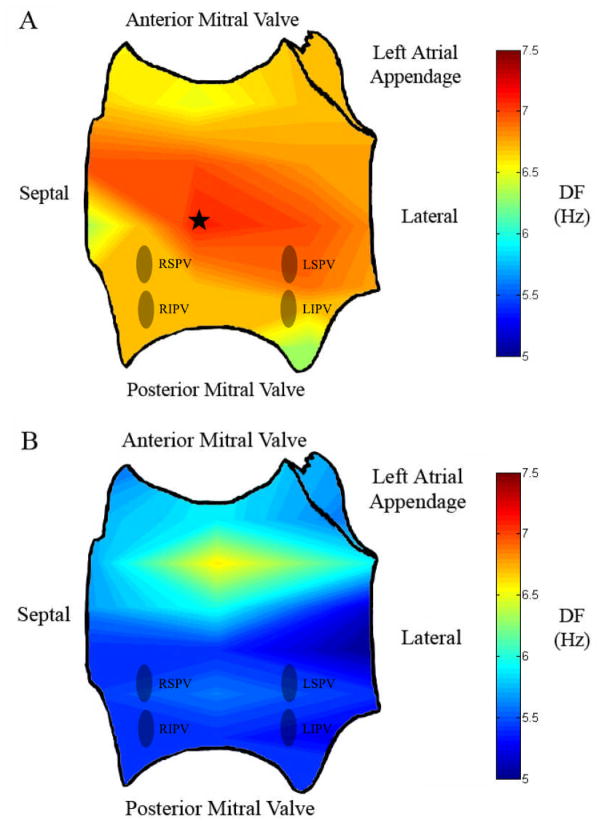
Left Atrial (LA) activation Rate Maps for Study Patients in Whom AF Terminated with Ablation. (A) Potential driver site (at star) in a patient with paroxysmal atrial fibrillation. Note the relatively gradual decrease in dominant frequency (DF) over a smaller range (7.1 Hz at maximum LA DF to a minimum of 6.3 Hz). (B) Potential driver site in a patient with persistent AF showing a greater gradient in LA DF (6.6 maximum to a minimum of 5.0). Interestingly, this patient had been treated with amiodarone (stopped 5 weeks prior to ablation), possibly explaining a relatively low average DF (as shown by the greater proportion of yellow/blue areas). RSPV = right superior pulmonary vein, RIPV = right inferior pulmonary vein, LSPV = left superior pulmonary vein, LIPV = left inferior pulmonary vein, DF = dominant frequency.
In patients with paroxysmal AF with an identified potential driver, CL rose from 146±39 ms to 202±19 ms (adjacent; p<0.05 vs driver) to 225±26 ms (distant; p<0.05 vs driver). In parallel, PAR fell from 0.75±0.19 to 0.66±0.17 (p<0.05 vs driver) to 0.58±0.14 at distant sites (p<0.05 vs driver). In patients with persistent AF, CL rose from 138±20 ms at the driver to 187±19 ms at adjacent sites (p<0.05 vs driver) to 205±18 ms at distant sites (p<0.05 vs driver); PAR fell from 0.61±0.12 to 0.55±0.08 (p<0.05 vs driver) to 0.51±0.084 at distant sites (p<0.05 vs driver).
Spatial Distributions of Potential Drivers For Persistent and Paroxysmal AF
Potential drivers lay at the PVs more frequently in paroxysmal AF, where 7 of 10 drivers localized at or within the PVs, compared with 1 of 8 in persistent AF (p<0.05). In persistent AF, drivers localized to the CS (n=2), LA roof (n=2), mitral valve annulus (n=3), and pulmonary vein antrum (n=1).
Clinical Findings
As discussed above, in 2 patients where ablation terminated AF and showed clinical evidence for a driver, blinded analysis demonstrated drivers with radial step-down in rate and regularity. Additionally, driver sites were ablated during AF in 3 other patients that did not result in AF termination. AF CL increased by 43±17 msec when these sites were ablated, from 157±17 to 200±17 msec (p<0.05). Driver sites were present but not ablated during AF in 3 other patients, and AF CL was unchanged throughout the procedure (209±26 msec before versus 205±25 msec after ablation (p=NS)).
A total of 18 patients were discharged on antiarrhythmic medications, which were then stopped at 3 months. At 6 months follow-up, patients in whom drivers were found showed an 82% rate of sinus rhythm after a single procedure (1 requiring antiarrhythmic medication), versus 54% for those without drivers (4 on antiarrhythmic medications, p=NS for sinus rhythm and requirement for antiarrhythmic medication). Of patients with driver sites, patients in whom ablation traversed driver sites showed an 86% (6/7 in sinus rhythm, no patients on antiarrhythmic medications) single procedure success rate at 6 months, versus 75% (3/4 in sinus rhythm, with 1 patient requiring an antiarrhythmic medication) for those in whom ablation did not traverse driver sites (p=NS).
Comparison of Sequential versus Multisite Recordings
Simulated sequential mapping took 18±6 minutes for each patient. Notably, simulated sequential mapping was less effective at identifying the potential driver sites seen on simultaneous mapping. For the patients in whom ablation terminated AF, sequential mapping identified the site of AF termination in only 5.8% of simulations, and the region of AF termination in only 12.9% of simulations.
Discussion
This study provides evidence that human AF may be sustained by localized regions, that may serve as possible drivers for AF, and that may be detected clinically using spatial relationships in rate and regularity from simultaneous mapping from multiple electrodes. These data are consistent with the ‘focal source’ mechanism for AF reported in animal models. Although further studies are needed to confirm their prevalence in human AF, we identified a potential driver in 46% of patients, with radial step-down in rate and regularity to surrounding sites. In a subset of patients, ablation of driver sites during ongoing AF either terminated or slowed the arrhythmia. Potential drivers were more likely to arise near the PVs in paroxysmal AF than in persistent AF. Finally, sequential mapping was less likely to identify sites of AF termination than simultaneous mapping due to fluctuations in driver site dominant frequency and greater time requirement for each relational map.
Evidence for Potential Drivers as a Mechanism for Atrial Fibrillation
The concept of a driver for human AF was originally proposed by Lewis (19) and Scherf (20). Later, Scheussler et al (21) showed that acetylcholine may coalesce multi-loop reentry into a solitary circuit with decremental (‘fibrillatory’) conduction that sustains AF. Mandapati et al (22) subsequently confirmed vortex-like microreentry around small cores (<1 cm) in optically mapped sheep atria.
Our study provides three lines of evidence to support this concept in human AF. First, radial decay in atrial rate and regularity itself suggests a driver, as reported in animals (2), since decrement to neighboring sites should not result from randomly colliding reentrant wavelets (2). Second, we found that when ablation terminated AF in a subset of patients, it did so at sites identified as drivers using this analysis. Third, when ablation crossed driver sites, AF cycle length slowed, while it did not slow in cases with drivers that were not ablated. Although this pilot study was not powered to detect outcome differences with statistical significance, the high 6 month single-procedural success rate when ablation intersected driver sites should be verified by larger, prospective studies.
Recent elegant ablation studies have used a 2 cm ‘flower’ catheter to identify radial activation as sources of human AF (15; 23; 24), although they did not survey the entire atrium simultaneously. Dibs et al also recently reported sites of rapid and regular atrial activation in human AF with a centrifugal decrease in rate and regularity, but did not correlate sites to clinical events (9). Our results agreed with the findings of Takahashi et al (25), showing that sites with a rate gradient with respect to surrounding tissue may be AF foci. This decrease in regularity (as measured by PAR in our study) may be due to wavefront breakdown, either due to collision with other wavelets at a distance equal to the activation space constant (26), or due to intermittent block as wavefronts advance from areas of short action potential duration to longer action potential duration at neighboring and distant sites (27). Conversely, sites of rapid AF without radial step-down may represent wavelet collision (26), artifact from measurement technique, or noise (8; 13; 28) rather than drivers.
Spatial Distribution of Drivers
Proposed drivers showed different locations in persistent and paroxysmal AF in our study, as suggested by prior human work. Our results extend the findings by Sahadevan et al. (6), who showed sites of high rate and regularity in 9 permanent AF patients at open-chest surgery, but did not examine paroxysmal AF that is more directly linked with the PVs.
Sanders et al (7) used sequential mapping to show that 50% of sites of highest rate in paroxysmal AF lay outside PVs, and that most of the highest AF sites in persistent AF were unrelated to ablation success. It would be interesting to determine if these sites failed to show a stepdown in regularity (8), and may therefore not meet our functional criteria for sources. An elegant report of paroxysmal AF reported radial step-down from right atrial sites, but did not examine the left atrium or patients with persistent AF (29).
In persistent AF, potential drivers in our study arose at LA sites including the roof and CS, but not the PVs This agrees with data by Sanders et al (7), who showed that rapid sites in persistent AF mostly lay away from the PVs. Thus, our results may explain the importance of the roof and CS for AF maintenance and the success of linear ablation in these regions (30–32).
Differences Between Our Measure of Irregularity and Fractionation
Fractionation refers to a complex shape of the local electrogram. As shown in many studies, such electrograms may occur at a very regular rate in AF (8) and may even occur in sinus rhythm (16). Conversely, irregularity in cycle-to-cycle timing may occur even without fractionation (6). Such irregularity may lead to decreased PAR. Pathophysiologically, Melby et al (33) have shown irregularity at distant sites due to intermittent conduction block in regions of scar. In our study, increasing irregularity at more distant sites (CS and LA electrodes) is clearly demonstrated in Figure 5C. Note that the coronary sinus electrograms vary both in morphology and timing to a much greater extent than LA sites, suggesting irregularity in both timing and wavefront direction.
Potential Clinical Implications
Regions of radial rate and regularity step-down may provide a new target for substrate modification by ablation. Identifying such sites using simultaneous multi-site mapping is preferable to sequential mapping, since AF reentrant wavelets likely migrate as suggested by Zlochiver et al. (10). Additionally, simultaneous mapping requires less time (1–2 minutes for multiple maps) than sequential mapping (18 minutes or more for a single map), and had a greater probability of detecting the site or region of termination than sequential mapping. Although prior studies have shown that complex fractionated atrial electrograms (CFAE) sites are stable over time (34; 35), we have found evidence that driver site dominant frequency fluctuates, making driver site detection dependent upon multiple observations.
Although our proof-of-concept study was not powered to detect a difference in outcome between ablating and not ablating potential driver sites, we did find non-significant differences to support this concept, and larger prospective studies are ongoing in our laboratory. Future studies should examine whether real-time identification and ablation of AF driver sites extends the observations of this study and improves outcome.
Limitations
First, we cannot unequivocally claim that sites are ‘drivers’ from these results without observing, in a large prospective trial, that their elimination terminates AF. Nevertheless, we did terminate AF by ablating at such sites in a subset of patients, and found AF CL prolongation when ablation crossed driver sites in others. In addition, evidence of radial activation has been used as sufficient evidence for drivers in animal studies (36), also motivating further study of these sites as ablation targets. Second, ongoing studies should include the possibility of 2 or more co-existing drivers. Third, this study is small and was not powered to detect outcome differences, that may explain the statistically similar ablation success in patients in whom ablation included driver sites compared to those in whom it did not. Fourth, we acknowledge that mapping in this human study was of lower resolution than in animal studies and may explain the absence of detected drivers in some patients. This limitation stems from our desire to use contact electrodes and safety concerns, although this is the highest resolution simultaneous endocardial contact mapping study yet reported in human AF at ablation. Nevertheless, future studies, during surgical ablation in the operating room, may further improve this resolution. Fifth, observations of multiple driver sites within individual patients are not statistically independent, and these observations require verification in larger studies. Sixth, we pooled driver, neighbor, and distant sites to increase statistical power to detect differences. However, distant sites had non-equal distances from the driver site. Seventh, our methods differ from prior work, possibly explaining disparity in the appearance of spectral data. Future work should examine the sensitivity of such analyses to different sampling durations and electrogram filtering. Eighth, future studies should examine the relationship between DF and regional activation patterns. Ninth, the use of an 8mm ablation catheter for mapping reduces the resolution of the bipole for sampling pulmonary veins, however, the majority of drivers were identified with the Constellation catheters. Finally, our study lacked women, reflecting the composition of a Veterans’ Affairs patient population. Although sex differences in AF are not clear, studies in both genders are required.
Conclusions
We found evidence to support the hypothesis that human AF may be maintained by sites of highest rate and regularity with decrement to surrounding atrium. In a subset of patients such sites co-localized with clinically identified AF-maintaining sites where ablation terminated or slowed AF. Proposed drivers lay within or near PVs in paroxysmal but not persistent AF. Finally, simultaneous mapping was superior to sequential mapping in the identification of driver sites. Future studies should establish prospectively whether ablation of such targets improves outcome.
Acknowledgments
We are indebted to Paul Clopton, MS, for guiding statistical analysis, and to Kathleen Mills, BA for coordinating this study. We thank Elizabeth Greer, RN, Stephanie Yoakum, NP, Donna Cooper, RN, Stanley Keys, CVT, and Anthony Moyeda, CVT, for their assistance in this research study. We also thank Amir Schricker, MD, for his work on the atrial activation rate maps in Figure 6. This study was supported, in part, by an American College of Cardiology Foundation/Merck Fellowship to DEK, and by grants from the National Heart, Lung, and Blood Institute (HL 70529, HL83359) and the Doris Duke Foundation to SMN.
Abbreviations
- AF
Atrial Fibrillation
- CL
Cycle length
- CS
Coronary Sinus
- DF
Dominant Frequency
- FFT
Fast Fourier Transform
- LA
Left Atrium
- PAR
Peak Area Ratio
- PV
Pulmonary Vein
- RA
Right Atrium
References
- 1.Berenfeld O, Zaitsev AV. The muscular network of the sheep right atrium and frequency-dependent breakdown of wave propagation. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1053–1061. doi: 10.1002/ar.a.20106. [DOI] [PubMed] [Google Scholar]
- 2.Ryu K, Shroff SC, Sahadevan J, Martovitz NL, Khrestian CM, Stambler BS. Mapping of atrial activation during sustained atrial fibrillation in dogs with rapid ventricular pacing induced heart failure: evidence for a role of driver regions. J Cardiovasc Electrophysiol. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 3.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavee C, Takahashi Y, Rotter M, Pasquie JL, Garrigue S, Clementy J, Jais P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]
- 5.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 6.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 7.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 8.Narayan SM, Krummen DE, Kahn AM, Karasik PL, Franz MR. Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol. 2006;29:1209–1218. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 9.Dibs SR, Ng J, Arora R, Passman RS, Kadish AH, Goldberger JJ. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: multisite electrogram analysis and surface electrocardiographic correlations--a pilot study. Heart Rhythm. 2008;5:686–693. doi: 10.1016/j.hrthm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns HJ, Davies DW, Kay GN, Prystowsky EN, Sutton R, Waldo AL, Wyse DG. International consensus on nomenclature and classification of atrial fibrillation: A collaborative project of the Working Group on Arrhythmias and the Working Group of Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2003;14:443–445. doi: 10.1046/j.1540-8167.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe BL, Kahn AM, Feld GK, Hassankhani A, Narayan SM. Separating atrial flutter from atrial fibrillation with apparent electrocardiographic organization using dominant and narrow F-wave spectra. J Am Coll Cardiol. 2005;46:2079–2087. doi: 10.1016/j.jacc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Ng J, Kadish AH, Goldberger JJ. Effect of electrogram characteristics on the relationship of dominant frequency to atrial activation rate in atrial fibrillation. Heart Rhythm. 2006;3:1295–1305. doi: 10.1016/j.hrthm.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Everett THt, Moorman JR, Kok LC, Akar JG, Haines DE. Assessment of global atrial fibrillation organization to optimize timing of atrial defibrillation. Circulation. 2001;103:2857–2861. doi: 10.1161/01.cir.103.23.2857. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Sanders P, Jais P, Hocini M, Dubois R, Rotter M, Rostock T, Nalliah CJ, Sacher F, Clementy J, Haissaguerre M. Organization of frequency spectra of atrial fibrillation: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:382–388. doi: 10.1111/j.1540-8167.2005.00414.x. [DOI] [PubMed] [Google Scholar]
- 16.Lellouche N, Buch E, Celigoj A, Siegerman C, Cesario D, De Diego C, Mahajan A, Boyle NG, Wiener I, Garfinkel A, Shivkumar K. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 2007;50:1324–1331. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 17.Lin YJ, Higa S, Kao T, Tso HW, Tai CT, Chang SL, Lo LW, Wongcharoen W, Chen SA. Validation of the frequency spectra obtained from the noncontact unipolar electrograms during atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1147–1153. doi: 10.1111/j.1540-8167.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler PD, Koehler JL, Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445–1452. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Lewis T. The Mechanism and Graphic Registration of the Heart Beat. London, UK: Shaw and Sons; 1925. [Google Scholar]
- 20.Scherf D. Studies on auricular tachycardia caused by aconitine administration. Proc Soc Exp Biol Med. 1947;64:233–239. doi: 10.3181/00379727-64-15754. [DOI] [PubMed] [Google Scholar]
- 21.Schuessler RB, Grayson TM, Bromberg BI, Cox JL, Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992;71:1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 22.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 23.Haissaguerre M, Hocini M, Sanders P, Sacher F, Rotter M, Takahashi Y, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16:1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 24.Rostock T, Rotter M, Sanders P, Takahashi Y, Jais P, Hocini M, Hsu LF, Sacher F, Clementy J, Haissaguerre M. High-density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006;3:27–34. doi: 10.1016/j.hrthm.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y, O’Neill MD, Hocini M, Dubois R, Matsuo S, Knecht S, Mahapatra S, Lim KT, Jais P, Jonsson A, Sacher F, Sanders P, Rostock T, Bordachar P, Clementy J, Klein GJ, Haissaguerre M. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol. 2008;51:1003–1010. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 26.Botteron GW, Smith JM. Quantitative assessment of the spatial organization of atrial fibrillation in the intact human heart. Circulation. 1996;93:513–518. doi: 10.1161/01.cir.93.3.513. [DOI] [PubMed] [Google Scholar]
- 27.Xie F, Qu Z, Garfinkel A, Weiss JN. Electrophysiological heterogeneity and stability of reentry in simulated cardiac tissue. Am J Physiol Heart Circ Physiol. 2001;280:H535–545. doi: 10.1152/ajpheart.2001.280.2.H535. [DOI] [PubMed] [Google Scholar]
- 28.Berntsen RF, Cheng A, Calkins H, Berger RD. Evaluation of spatiotemporal organization of persistent atrial fibrillation with time- and frequency-domain measures in humans. Europace. 2009;11:316–323. doi: 10.1093/europace/eun307. [DOI] [PubMed] [Google Scholar]
- 29.Lin YJ, Tai CT, Kao T, Tso HW, Huang JL, Higa S, Yuniadi Y, Huang BH, Liu TY, Lee PC, Hsieh MH, Chen SA. Electrophysiological characteristics and catheter ablation in patients with paroxysmal right atrial fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 30.Tada H, Yamada M, Naito S, Nogami A, Oshima S, Taniguchi K. Radiofrequency catheter ablation within the coronary sinus eliminates a macro-reentrant atrial tachycardia: Importance of mapping in the coronary sinus. J Interv Card Electrophysiol. 2006;15:35–41. doi: 10.1007/s10840-006-6310-2. [DOI] [PubMed] [Google Scholar]
- 31.Wit AL, Boyden PA. Triggered activity and atrial fibrillation. Heart Rhythm. 2007;4:S17–23. doi: 10.1016/j.hrthm.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jais P, O’Neill MD, Takahashi Y, Jonsson A, Hocini M, Sacher F, Sanders P, Kodali S, Rostock T, Rotter M, Clementy J, Haissaguerre M. Stepwise catheter ablation of chronic atrial fibrillation:importance of discrete anatomic sites for termination. J Cardiovasc Electrophysiol. 2006;17(Suppl 3):S28–36. [Google Scholar]
- 33.Melby SJ, Lee AM, Zierer A, Kaiser SP, Livhits MJ, Boineau JP, Schuessler RB, Damiano RJ., Jr Atrial fibrillation propagates through gaps in ablation lines: Implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008;5:1296–1301. doi: 10.1016/j.hrthm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherr D, Dalal D, Cheema A, Nazarian S, Almasry I, Bilchick K, Cheng A, Henrikson CA, Spragg D, Marine JE, Berger RD, Calkins H, Dong J. Long- and short-term temporal stability of complex fractionated atrial electrograms in human left atrium during atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:13–21. doi: 10.1111/j.1540-8167.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 35.Verma A, Wulffhart Z, Beardsall M, Whaley B, Hill C, Khaykin Y. Spatial and temporal stability of complex fractionated electrograms in patients with persistent atrial fibrillation over longer time periods: relationship to local electrogram cycle length. Heart Rhythm. 2008;5:1127–1133. doi: 10.1016/j.hrthm.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 36.Sun H, Velipasaoglu EO, Wu DE, Kopelen HA, Zoghbi WA, Spencer WH, 3rd, Khoury DS. Simultaneous multisite mapping of the right and the left atrial septum in the canine intact beating heart. Circulation. 1999;100:312–319. doi: 10.1161/01.cir.100.3.312. [DOI] [PubMed] [Google Scholar]