Abstract
BACKGROUND:
Glucose-insulin-potassium (GIK) therapy has been proposed to provide metabolic support to ischemic myocardium. A meta-analysis that included 1932 patients performed 10 years previously demonstrated that GIK therapy may have an important role in reducing mortality after acute myocardial infarction (AMI). Since then, many larger randomized trials investigating the role of GIK in the setting of AMI have been published; hence, the present study repeats the previous meta-analysis performed by the current authors to include these trials.
METHOD AND RESULTS:
A systematic MEDLINE search for all randomized, placebo-controlled studies of GIK therapy in the setting of AMI was conducted and a meta-analysis of the mortality data was performed. A total of 16 randomized trials from 1966 to 2008 were identified, with 28,374 patients included in the current meta-analysis. There was a total of 1367 deaths (9.6%) in the GIK group, with 1351 deaths (9.6%) in the control group. Meta-analysis did not reveal any benefit from GIK treatment (OR 1.0; 95% CI 0.9 to 1.1; P=0.9). Subgroup analysis of patients given high-dose GIK and in patients in whom reperfusion was not obtained did not demonstrate a benefit from GIK therapy.
CONCLUSION:
A meta-analysis of 16 randomized trials that spanned 40 years and involved more than 28,000 patients did not reveal any mortality benefit for ST segment elevation AMI using GIK therapy when data from the modern thrombolysis/primary percutaneous coronary intervention era were included.
Keywords: Insulin, Meta-analysis, Myocardial infarction
The concept of metabolic modulation in acute myocardial infarction (AMI) with glucose-insulin-potassium (GIK) infusion was initially proposed in the 1960s as a means of producing electrical stability in the myocardium during episodes of ischemia (1). Subsequently, it was proposed that GIK therapy provided metabolic support to ischemic myocardium. During episodes of cardiac ischemia, the myocardium converts from aerobic carbohydrate metabolism to anaerobic free fatty acid metabolism, which results in the production of metabolites and free radicals that are toxic to the myocardium, and are associated with ventricular arrhythmias (2) and decreased myocardial contractility (3). It was hypothesized that insulin and glucose infused as part of GIK therapy resulted in a shift from potentially harmful free fatty acid metabolism to glucose metabolism in the ischemic myocardium. Furthermore, suppression of plasma free fatty acid levels by insulin would further facilitate this effect (4). It is now more than 10 years since we published our meta-analysis evaluating the role GIK therapy on mortality after AMI (5), in which a total of 1932 patients from nine trials were evaluated. We demonstrated that the number of deaths was significantly lower in patients who received GIK (16.1%) than in patients who received placebo (21%). Based on the results of this meta-analysis, we concluded that GIK therapy most likely had a role in reducing in-hospital mortality following AMI. In our original meta-analysis, we argued that there was a need for a larger randomized trial to investigate the role of GIK as an adjunct to thrombolysis or primary percutaneous coronary intervention following AMI on mortality. Since then, many larger randomized trials investigating the role of GIK in the setting of AMI have been published. The Glucose-Insulin-Potassium Study-I (GIPS-I) (6) showed clinical benefit of GIK in ST segment elevation myocardial infarction (STEMI) patients without signs of heart failure; however, GIPS-II (7) could not confirm this finding. Two other recent large sample trials – the Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment (CREATE)-Estudios Cardiologicos Latinoamerica (ECLA)-II (8), which included more than 20,000 patients, and the Polish-Glucose-Insulin-Potasium trial (Pol-GIK) (9) trial comprising close to 1000 patients – showed no beneficial effects from GIK therapy. Although The Organization for the Assessment of Strategies for Ischemic Syndromes (OASIS-6) trial (10), a large, multicentre, randomized trial, was stopped prematurely after the enrollment of 2748 patients following publication of CREATE-ECLA II, it did not show any benefit from GIK therapy. We have therefore updated our meta-analysis to include these more recent trial data.
METHODS
A MEDLINE search (1996 to February 2008) was performed using the search term “myocardial infarction” or “AMI” and a set of terms for GIK including “glucose”, “insulin”, “glucose-insulin”, “GIK”, “GIP”, “PGI”, “glucose-insulin-potassium” and “metabolic support”. Furthermore, references and review articles obtained in this manner were scrutinized to ensure that all relevant studies were identified. Only controlled studies that documented 30-day mortality rates and were properly randomized were selected. Studies that were published before 1996 were the subject of the previous meta-analysis (5), and these studies were also included in the current meta-analysis. Studies were excluded if they used inadequate means of randomization, such as allocations based on date of birth, alternate numbers, admission to a particular unit or date of presentation. Trials that used retrospective group or historic cases as a control group, and those that limited the study to a particular subgroup were also excluded. Only trial-level data were used for the present meta-analysis.
Statistical analysis
For individual trials, the χ2 heterogeneity test (11) was used to calculate the significance, ORs and 95% CIs for the differences in mortality between the GIK and placebo group, except for trials in which the expected number of deaths was less than five. For trials in which the number of deaths was less than five, the Fisher’s exact test was used (12). Statistical analysis was performed using Arcus Quickstat (Research Solutions, United Kingdom).
RESULTS
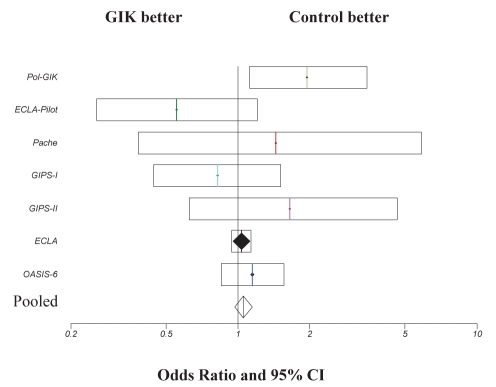
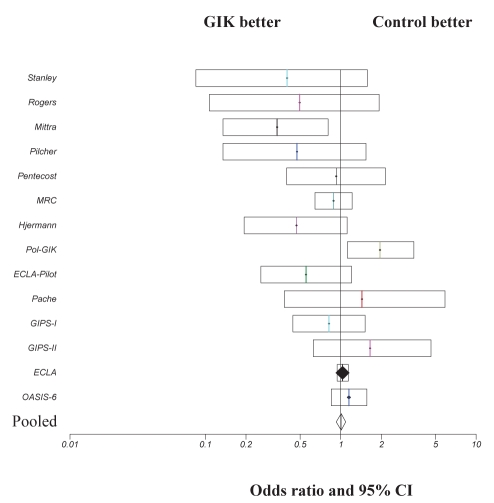
Since the original meta-analysis (5), a total of nine trials (6–10,13–16) were identified, of which seven (6–10,15,16) were included in the current analysis (Table 1). Two trials (13,14) were excluded because only diabetic patients were enrolled in the Diabetes Mellitus, Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI-2) trial (13) and only patients who were diabetic or presented with hyperglycemia were included in the other study (14). Similar exclusion criteria were applied in the original meta-analysis (5) in which the DIGAMI-1 trial was excluded for the same reason. Studies that were conducted between 1998 and 2005, and those included in the present meta-analysis were open design. Delays between the onset of chest pain and treatment varied from less than 12 h to less than 48 h, with treatment duration of between 12 h and 24 h in all studies. All trials reported 30-day mortality. A total of 26,442 patients were included in the current meta-analysis, with 13,293 patients in the GIK treatment group and 13,149 patients in the control group. Mortality rates for the control group and GIK treatment group for the individual trials are presented in Table 2. Pooled data demonstrate that the total number of deaths was 1213 (9.1%) in the GIK group and 1146 (8.7%) in the control group. GIK treatment did not significantly influence 30-day mortality post-AMI (OR 1.05; 95% CI 0.97 to 1.14; P=0.34) (Figure 1). Summation of these new data to the original meta-analysis data performed by Fath-Ourdoubadi and Beatt (5) on nine randomized trials (17–25) resulted in a total of 16 trials and 28,374 patients, with a total mortality of 1367 (9.6%) in the GIK group and 1351 (9.6%) in the control group. A repeat meta-analysis of these 16 randomized trials did not significantly alter the outcome (OR 1.0; 95% CI 0.9 to 1.1; P=0.9) (Figure 2).
TABLE 1.
Design of the randomized placebo-controlled trials of glucose-insulin-potassium (GIK) therapy in acute myocardial infarction (AMI)
| Study (ref) | Year | Design (n) | Delay between onset of AMI and treatment | Exclusion criteria | GIK regimen | Treatment duration |
|---|---|---|---|---|---|---|
| ECLA pilot (16) | 1998 | Randomized, controlled, open (407) | <24 h | Severe renal impairment, hyperkalemia | High and low dose | 24 h |
| Pol-GIK (9) | 1999 | Prospective, randomized placebo-controlled, open (954) | <24 h | Pulmonary congestion, renal failure, diabetes requiring insulin | Low dose | 24 h |
| GIPS-I (6) | 2003 | Randomized, open (940) | <24 h | Thrombolysis, poor life expectancy | High dose | 12 h |
| Pache et al (15) | 2004 | Randomized, open (312) | <48 h | NA | High dose | 24 h |
| ECLA (8) | 2005 | Randomized, controlled, open (20,201) | <12 h | Type 1 diabetes mellitus, renal impairment, hyperkalemia | High dose | 24 h |
| GIPS-II (7) | 2006 | Randomized, controlled (889) | <24 h | Pulmonary congestion | High dose | 12 h |
| OASIS-6 (10) | 2007 | Randomized, controlled (2748) | <24 h but decreased to <12 h after 4300 patients recruited | Oral anticoagulants, moderate renal impairment | High dose | NA |
ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; NA Not available; OASIS The Organization for the Assessment of Strategies for Ischemic Syndromes trial; Pol-GIK Polish Glucose-Insulin-Potassium study
TABLE 2.
Thirty-day mortality in randomized trials of glucose-insulin-potassium (GIK) therapy in acute myocardial infarction
| Study (reference), year |
Patients, n |
Deaths (at 30 days), n (%) |
P | ||
|---|---|---|---|---|---|
| GIK | Control | GIK | Control | ||
| ECLA pilot (16), 1998 | 268 | 139 | 18 (6.7) | 16 (11.5) | 0.1 |
| Pol-GIK (9), 1999 | 494 | 460 | 44 (8.9) | 22 (4.8) | 0.01 |
| GIPS-I (6), 2003 | 476 | 464 | 23 (4.8) | 27 (5.8) | 0.5 |
| Pache et al (15), 2004 | 155 | 157 | 7 (4.5) | 5 (3.2) | 0.85 |
| ECLA (8), 2005 | 10,088 | 10,107 | 1004 (10) | 976 (9.7) | 0.45 |
| GIPS-II (7), 2006 | 444 | 445 | 13 (2.9) | 8 (1.8) | 0.26 |
| OASIS-6 (10), 2007 | 1368 | 1377 | 104 (7.6) | 92 (6.7) | 0.76 |
ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; OASIS The Organization for the Assessment of Strategies for Ischemic Syndromes trial; Pol-GIK Polish Glucose-Insulin-Potassium study
Figure 1).
Comparison of 30-day mortality outcome in patients treated with glucose-insulin-potassium (GIK) therapy. ORs and 95% CIs are presented for individual studies and pooled data. ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; OASIS Organization for the Assessment of Strategies for Ischemic Syndromes trial; Pol-GIK Polish GIK trial
Figure 2).
Comparison of 30-day mortality outcome in patients treated with glucose-insulin-potassium (GIK) therapy. ORs and 95% CIs are presented for individual studies and pooled data. ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; MRC Medical Research Council; OASIS Organization for the Assessment of Strategies for Ischemic Syndromes trial; Pol-GIK Polish GIK trial
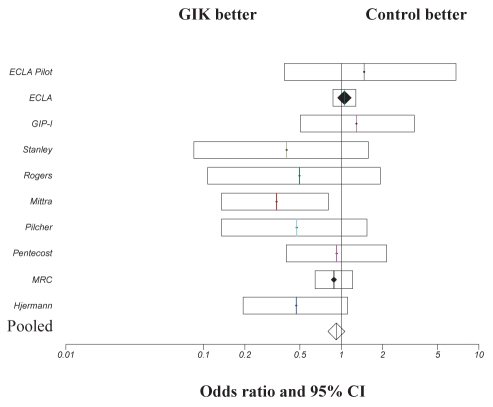
Most of the trial data analyzed in the Fath-Ourdoubadi and Beatt (5) meta-analysis were from an era when thrombolysis or primary percutaneous coronary intervention was not performed; therefore, the patients included in the present meta-analysis would represent AMI in which no reperfusion was achieved (with the exception of the study by Satler et al [25]). Consequently, the data from 1966 to 2007 were re-analyzed in the patient cohort in which reperfusion was not achieved to determine whether this influenced outcome. Studies in which reperfusion strategies were not used (16–23) and current studies (6,8,16) in which mortality data was presented in the nonreperfusion subgroup, were pooled. Analysis was performed on a total of 5716 patients, with 437 (15%) deaths in the GIK treatment group and 467 (16.4%) deaths in the control group. Figure 3 illustrates that no significant benefit was observed with GIK treatment in this subgroup (OR 0.92; 95% CI 0.80 to 1.06; P=0.27).
Figure 3).
Comparison of 30-day mortality outcome in patients treated with glucose-insulin-potassium (GIK) therapy in whom reperfusion was not attained. ORs and 95% CIs are presented for individual studies and pooled data. ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; MRC Medical Research Council; OASIS Organization for the Assessment of Strategies for Ischemic Syndromes trial
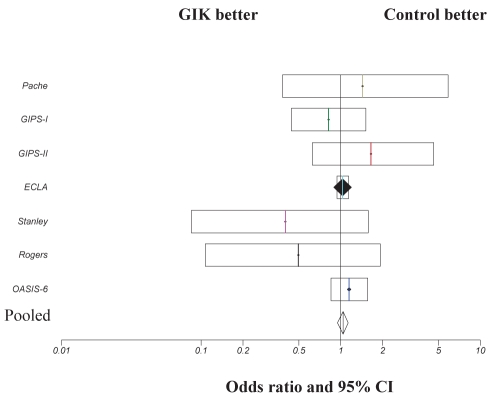
Finally, data were pooled from the trials in which high-dose GIK solution was used (6–8,15,22–25). A total of 25,369 patients were included in the meta-analysis (Figure 4). A mortality of 9.1% was observed in the high-dose GIK group and 9.1% in control group, with no observed benefit of high-dose GIK infusion on mortality observed (OR 1.04; 95% CI 0.95 to 1.13; P=0.43).
Figure 4).
Comparison of 30-day mortality outcome in patients treated with high-dose glucose-insulin-potassium (GIK) therapy. ORs and 95% CIs are presented for individual studies and pooled data. ECLA Estudios Cardiologicos Latinoamerica; GIPS Glucose-Insulin-Potassium Study; OASIS Organization for the Assessment of Strategies for Ischemic Syndromes trial
DISCUSSION
It is now 10 years since our most recent meta-analysis (5) that included 1932 patients, in which a 28% reduction in mortality post-AMI was demonstrated following treatment with GIK (P=0.004). Since publication of this meta-analysis, many larger randomized controlled trials investigating the effects of GIK on patients with AMI have been conducted. GIPS-I (6) showed clinical benefit of GIK in STEMI patients without signs of heart failure; however, GIPS-II (7) could not confirm this finding. The ECLA GIK pilot trial (16) involving 407 patients demonstrated a nonsignificant decrease in mortality following GIK therapy (6.7%) when compared with placebo (11.5%). Similarly, in the more recent CREATE-ECLA (8), a trial evaluating the effects of high-dose GIK on mortality in 20,000 patients with STEMI, showed no significant difference in mortality. The OASIS-6 trial (10), a large, multicentre, randomized trial, was stopped prematurely after having enrolled 2748 patients following publication of CREATE-ECLA; the OASIS-6 trial showed no benefit from GIK treatment. Our current meta-analysis of these more recent trials involving 26,442 patients demonstrates no significant difference between mortality in the GIK treatment group and the control group, even if the meta-analysis is repeated to include our original data.
In contrast, a recent analysis of the OASIS-6 and CREATE-ECLA trials combined trial populations and actually demonstrated an increased mortality associated with GIK treatment in the first three days. Although in agreement with our current data, this difference disappeared at 30 days (10). Interestingly, at 6 h and 24 h postrandomization, serum glucose levels were significantly greater in the GIK cohort than in the control group and, on average, the net fluid balance in the GIK cohort was 600 mL greater after 24 h.
Indeed, it is well recognized that admission glucose level is a stronger predictor of mortality in the setting of AMI (26,27). Analysis by Díaz et al (10) revealed that glucose and fluid balance were independent predictors of mortality at three days. Once these variables were accounted for, no excess mortality was observed at this time point in the GIK group. Clearly, this is a major limitation of all GIK studies performed to date in that any potential benefit incurred from metabolic modulation within the myocardium from GIK infusion may be offset by the detrimental effects of hyperglycemia and increased net fluid balance in the GIK arm of these trials.
Our original meta-analysis showing a benefit from GIK therapy was performed on studies conducted in an era when revascularization and thrombolysis were not routinely used in the treatment of AMI. Therefore, it is possible that GIK therapy benefits patients in whom revascularization is not achieved. Experimental evidence suggests a beneficial effect of GIK by shifting myocardial free fatty acid metabolism toward glucose metabolism. This consumes less oxygen and produces less toxic byproducts (28). Furthermore, reperfusion injury may be reduced by limiting apoptosis of postischemic myocardial cells. However, once adequate reperfusion has been established, GIK may be of only limited value because maximal myocardial salvage has already occurred. This potential benefit of treatment with GIK in patients in whom reperfusion is not achieved is not supported by our meta-analysis – there was no improvement in 30-day mortality rates post-treatment with GIK. It has also been argued that many trials do not show a beneficial effect of low-dose GIK solution on reducing mortality because the doses of glucose and insulin used are not sufficient to suppress free fatty acid levels (29). However, in the pilot ECLA study (16), no significant difference in mortality was observed between ‘low-dose’ and ‘high-dose’ GIK treatment. Indeed, our meta-analysis of trials that used only high-dose GIK infusion did not demonstrate any benefits on mortality rates.
The discrepancy in the outcome between the meta-analysis of nine smaller trials performed 10 years previously and the current meta-analysis involving six significantly larger trials is difficult to explain. It may relate to a publication bias of smaller trials for which neutral studies are less likely to be published compared with similar studies with positive results. Furthermore, our meta-analysis is heavily weighted toward the CREATE-ECLA trial, which contributed 71.2% of the patients to the current analysis. Even when patients from the CREATE-ECLA trial are excluded, repeat meta-analysis of the dataset does not show any benefit of GIK in the setting of AMI (data not shown).
Our original meta-analysis was performed on studies in which beta-blockade and antiplatelet therapy was not routinely used, resulting in an increased ischemic burden in those patients and a greater metabolic insult to the myocardium. Under these conditions, it is possible that GIK therapy has more of a myocardial protective role. Indeed, beta-blockade itself may shift metabolism from free fatty acid oxidation to glucose oxidation (30); therefore, its routine use in the management of AMI in the current era may reduce the benefit from therapies such as GIK. There is good experimental evidence from animal models that GIK should be given early post-AMI, so as to reach the ischemic myocardium before reperfusion. In several animal species including rat (31), baboon (32) and rabbit (33), GIK was protective when started only a few minutes after coronary ligation. It is believed that early administration of GIK post-AMI can slow the rate of myocardial injury during ischemia and, consequently, substantially increase the extent of myocardial salvage when effective reperfusion occurs. However, in these animal models of ischemia, antiplatelet therapy, beta-blockade and revascularization therapy are not used in the context of AMI; therefore, relevance to clinical practice remains uncertain. In the CREATE-ECLA trial (8), which was by far the largest overall contributor to patient numbers in the current meta-analysis, 83% of patients had reperfusion therapy at a median time of 3.85 h after symptom onset, and randomization to GIK or control groups occurred almost 1 h later (median 4.7 h postsymptom onset) after which GIK was started mostly ‘within the next hour’ (ie, up to 2 h post reperfusion) (29). Importantly, these data would suggest that a key component of the CREATE-ECLA trial was commonly violated because the trial explicitly prescribed initiation of GIK infusion before reperfusion. Clearly, such a delayed and often postreperfusion administration of GIK minimized its potential to reduce mortality. Of note, however, in a subgroup analysis of OASIS-6 and CREATE ECLA, it appeared that time between symptom onset and randomization to a treatment arm did not significantly influence outcome, even when symptom onset occurred at less than 2 h (10). In contrast, GIK infusion was started within 15 min to 20 min after admission in the GIPS-1 study (6), which was approximately 30 min earlier than the average door to balloon time (and hence reperfusion) of 45 min. Interestingly, in this study, a benefit of GIK was demonstrated in the subgroup without signs of heart failure (Killip class 1), although this observation was not reproduced in the later GIPS-II study (7). Clearly, the timing of GIK in relation to reperfusion may have an important role in its potential for benefit. The ongoing National Institutes of Health-sponsored Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care (IMMEDIATE) study of prehospital initiation of GIK therapy for acute coronary syndrome patients aims to address this issue (www.immediatetrial.com/). It is anticipated that 15,540 patients will be included in this study and the primary end point will be 30-day and one-year mortality. Secondary end points will include in-hospital cardiac arrest, progression of unstable angina pectoris to AMI, infarct size and heart failure. In contrast to other GIK trials, this trial will test the efficacy of GIK in all acute coronary syndromes rather than STEMI alone.
All GIK trials to date have investigated the role of GIK in STEMI. However, there are very little trial data investigating whether GIK plays a role in the treatment of other acute coronary syndromes. Yazici et al (34) studied the effects of high-dose GIK in a randomized study of 52 consecutive patients presenting with non-STEMI who underwent percutaneous coronary intervention. They demonstrated that GIK infusion initiated 24 h before coronary stenting for non-STEMI resulted in less myocardial damage as determined by postprocedure troponin I levels. Clearly, the role of GIK in non-STEMI warrants further investigation and it is hoped that the ongoing IMMEDIATE trial will provide further information regarding this issue.
CONCLUSIONS
Our meta-analysis performed on 16 randomized trials that spanned more than 40 years and included more than 28,000 patients does not demonstrate any benefits from GIK on mortality post-ST segment elevation and does not support its use as a treatment strategy in AMI. Ongoing trials may clarify whether earlier initiation of GIK therapy is associated with benefit in acute coronary syndromes.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Sodi-Pallares D, Testelli MR, Fishleder BL, et al. Effects of an intravenous infusion of potassium-glucose-insulin solution on electrocardiographic signs of myocardial infarction: A preliminary clinical report. Am J Cardiol. 1962;9:166–81. doi: 10.1016/0002-9149(62)90035-8. [DOI] [PubMed] [Google Scholar]
- 2.Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112:305–11. doi: 10.1016/s0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 3.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155–8. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 4.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–8. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 5.Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: An overview of randomized placebo-controlled trials. Circulation. 1997;96:1152–6. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- 6.Van der Horst IC, Zijlstra F, van’t Hof AWJ, et al. Zwolle Infarct Study Group Glucose-insulin-potassium infusion inpatients treated with primary angioplasty for acute myocardial infarction: The glucose-insulin-potassium study: A randomized trial. J Am Coll Cardiol. 2003;42:784–91. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 7.Timmer JR, Svilaas T, Ottervanger JP, et al. Glucose-insulin-potassium infusion in patients with acute myocardial infarction without signs of heart failure: The Glucose-Insulin-Potassium study II. J Am Coll Cardiol. 2006;47:1730–1. doi: 10.1016/j.jacc.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SR, Yusuf S, Diaz R, et al. CREATE-ECLA Trial Group Investigators Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: The CREATE-ECLA randomised controlled trial. JAMA. 2005;293:437–46. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 9.Ceremuzynski L, Budaj A, Czepiel A, et al. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: Results of a randomized multicenter Pol-GIK trial. Cardiovasc Drugs Ther. 1999;13:191–200. doi: 10.1023/a:1007787924085. [DOI] [PubMed] [Google Scholar]
- 10.Díaz R, Goyal A, Mehta SR, et al. Glucose-insulin-potassium therapy in patients with ST-segment elevation myocardial infarction. JAMA. 2007;298:2399–405. doi: 10.1001/jama.298.20.2399. [DOI] [PubMed] [Google Scholar]
- 11.Armitage P, Berry G. Statistical Methods in Medical Research. Oxford: Blackwell; 1987. [Google Scholar]
- 12.Bailey NTJ. Mathematics, Statistics and Systems for Health. New York: Wiley; 1997. [Google Scholar]
- 13.Malmberg K, Ryden L, Wedel H, et al. DIGAMI-2 Investigators Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): Effects on mortality and morbidity. Eur Heart J. 2005;26:650–61. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 14.Cheung NW, Wong VW, Mclean M. The hyperglycemia: Intensive Insulin Infusion In Infarction (HI-5) Study. A randomized controlled trial of insulin infusion therapy for myocardial infarction. Diabetes Care. 2006;29:765–70. doi: 10.2337/diacare.29.04.06.dc05-1894. [DOI] [PubMed] [Google Scholar]
- 15.Pache J, Kastrati A, Mehilli J, et al. A randomized evaluation of the effects of glucose-insulin-potassium infusion on myocardial salvage in patients with acute myocardial infarction treated with reperfusion therapy. Am Heart J. 2004;148:1–6. doi: 10.1016/j.ahj.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Diaz R, Paolasso EA, Piegas LS, et al. Metabolic modulation of acute myocardial infarction. The ECLA (Estudios Cardiologicos Latinoamerica) Collaborative Group. Circulation. 1998;98:2227–34. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 17.Mittra B. Potassium, glucose, and insulin in treatment of myocardial infarction. Lancet. 1965;2:607–9. doi: 10.1016/s0140-6736(65)90516-7. [DOI] [PubMed] [Google Scholar]
- 18.Medical Research Council Working Party on the Treatment of Myocardial Infarction Potassium, glucose, and insulin treatment for acute myocardial infarction. Lancet. 1968;2:1355–60. [PubMed] [Google Scholar]
- 19.Pilcher J, Etishamudin M, Exon P, et al. Potassium, glucose and insulin in myocardial infarction. Lancet. 1967;1:1109. [Google Scholar]
- 20.Pentecost BL, Mayne NM, Lamb P. Controlled trial of intravenous glucose, potassium, and insulin in acute myocardial infarction. Lancet. 1968;1:946–8. doi: 10.1016/s0140-6736(68)90903-3. [DOI] [PubMed] [Google Scholar]
- 21.Hjermann I. A controlled study of per oral glucose, insulin and potassium treatment in myocardial infarction. Acta Med Scand. 1971;190:213–8. doi: 10.1111/j.0954-6820.1971.tb07419.x. [DOI] [PubMed] [Google Scholar]
- 22.Heng MK, Norris RM, Singh BN, et al. Effects of glucose and glucose-insulin-potassium on haemodynamics and enzyme release after acute myocardial infarction. Br Heart J. 1977;39:748–57. doi: 10.1136/hrt.39.7.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanley AWH, Prather JW. Glucose-insulin-potassium, patient mortality and the acute myocardial infarction: Results from a prospective randomized study. Circulation. 1978;57(Suppl II):II-62. (Abst) [Google Scholar]
- 24.Rogers WJ, McDaniel HG, Mantle JA, et al. Prospective randomized trial of glucose-insulin-potassium in acute myocardial infarction: Effects of hemodynamics, short and long-term survival. J Am Coll Cardiol. 1983;1:628. doi: 10.1016/0002-9149(79)90081-x. [DOI] [PubMed] [Google Scholar]
- 25.Satler LF, Green CE, Kent KM, et al. Metabolic support during coronary reperfusion. Am Heart J. 1987;114:54–8. doi: 10.1016/0002-8703(87)90306-1. [DOI] [PubMed] [Google Scholar]
- 26.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–86. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 27.Goyal A, Mahaffey KW, Garg J, et al. Prognostic significance of the change in glucose level in the first 24 h after acute myocardial infarction: Results from the CARDINAL study. Eur Heart J. 2006;27:1289–97. doi: 10.1093/eurheartj/ehi884. [DOI] [PubMed] [Google Scholar]
- 28.Stanley AW, Moraski RE, Russell RO, et al. Effects of glucose-insulin-potassium on myocardial substrate availability and utilisation in stable coronary artery disease: Studies on myocardial carbohydrates, lipid and oxygen arterial coronary sinus differences in patients with coronary artery disease. Am J Cardiol. 1975;36:929–37. doi: 10.1016/0002-9149(75)90085-5. [DOI] [PubMed] [Google Scholar]
- 29.Apstein CS, Opie LH. A challenge to the metabolic approach to myocardial ischaemia. Eur Heart J. 2005;26:956–9. doi: 10.1093/eurheartj/ehi200. [DOI] [PubMed] [Google Scholar]
- 30.Wallhaus TR, Taylor M, DeGrado H, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2000;103:2441–6. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 31.de Leiris J, Opie LH, Lubbe WF. Effects of free fatty acid and glucose on enzyme release in experimental myocardial infarction. Nature. 1975;253:746–7. doi: 10.1038/253746a0. [DOI] [PubMed] [Google Scholar]
- 32.Opie LH, Bruyneel K, Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975;52:49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Eberli FR, Weinberg EO, Grice WN, et al. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–81. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 34.Yazici M, Demircan S, Durna K, et al. Effect of glucose-insulin-potassium infusion on myocardial damage due to percutaneous coronary revascularization. Am J Cardiol. 2005;96:1517–20. doi: 10.1016/j.amjcard.2005.07.060. [DOI] [PubMed] [Google Scholar]