Abstract
BACKGROUND:
An elevated level of homocysteine (Hcy) has been shown to be a cardiovascular risk factor in the majority of research studies. Recently, it was found to be associated with new risk factors such as inflammatory markers.
OBJECTIVES:
To investigate the distribution of plasma total Hcy (tHcy) and the levels of inflammatory markers in patients with acute coronary syndrome (ACS), and to evaluate the association between these parameters and the severity of the disease.
METHODS:
A total of 122 patients with ACS and 80 control subjects were recruited from the cardiac intensive care unit of the Military Hospital of Tunis, Tunisia. Lipid profile and the levels of tHcy, high-sensitivity C-reactive protein (HsCRP), interleukin (IL)-6, IL-8, IL-1β and tumour necrosis factor-alpha (TNFα) were determined for all participants. The distribution of these parameters were compared between groups and according to the number of diseased vessels in patients with ACS.
RESULTS:
ACS patients had significantly elevated levels of tHcy (P<0.01), HsCRP (P<0.001), IL-6 (P<0.001), TNFα (P<0.001), folates (P<0.05) and vitamin B12 (P<0.001), but lower high-density lipoprotein cholesterol (P<0.05) levels. The analysis of the association between these parameters and the number of diseased vessels showed significant differences in tHcy, HsCRP, IL-6 and TNFα, with positive correlations. Significantly negative correlations were found between the number of diseased vessels and folate (r=−0.34; P<0.01), and vitamin B12 (r=−0.22; P<0.01).
CONCLUSION:
Elevated levels of tHcy, IL-6, TNFα and HsCRP appear to be associated with a greater number of diseased arteries and, consequently, the severity of coronary artery disease.
Keywords: Acute coronary syndrome, Homocysteine, HsCRP, IL-1β, IL-6, IL-8, TNFα
An elevated homocysteine (Hcy) level is considered to be a risk factor for the development of atherosclerosis. It has been suggested that Hcy influences endothelial function leading to a prothrombotic environment, platelet activation and endothelial leukocyte interactions (1). In addition, Hcy enhances inflammatory responses that are recognized for their role in atherosclerotic disease (2,3). Recent studies (4,5) suggest that markers of inflammation may reflect different aspects of the atherothrombotic process and have a potential role in the prediction of risk for developing coronary artery disease (CAD). In fact, cytokines released from inflammatory cells may reflect the inflammatory process in atherosclerotic plaques. C-reactive protein (CRP) is another proinflammatory factor that has been implicated in the pathogenesis of CAD. Several studies (6) have demonstrated that CRP, measured at either presentation or discharge, may have prognostic value in patients with acute coronary syndrome (ACS). Importantly, biomarkers of different processes may be combined to enhance risk stratification above that of any single marker. The combined effect of Hcy and proinflammatory factors on CAD was recently studied, but not yet clarified.
In the present study, we investigated the distribution of plasma total Hcy (tHcy) and the levels of proinflammatory markers in patients with ACS, and evaluated the association between these parameters and disease severity.
METHODS
Population
A total of 122 patients hospitalized with ACS and 80 control subjects at the coronary intensive care unit of the Military Hospital of Tunis, Tunisia, were included in the present study.
ACS was defined either as unstable angina or as an acute myocardial infarction that was diagnosed based on the presence of chest pain and typical ischemic electrocardiographic changes. Stable angina was defined based on the presence of typical and stable chest pain during effort, a positive treadmill exercise test and obstructive coronary lesions as determined by coronary angiography. The extent of CAD was assessed by the number of diseased coronary vessels. Anthropometric and clinical data including body mass index, smoking status, alcohol consumption, systolic and diastolic blood pressure, and family history of cardiovascular disease were noted in the files of each patient. Seventy-two patients had hypertension confirmed by antihypertensive drug use or a recently noted blood pressure of greater than 140/90 mmHg. Fifty-nine patients had type 2 diabetes and 65 presented with dyslipidemia. None of the patients had an infectious disease, or renal or hepatic failure. Women who were postmenopausal (n=39) were not undergoing hormone replacement therapy. A total of 80 healthy subjects (43 men, 37 women) represented the control group recruited among medical and paramedical volunteers. Control subjects did not have a personal or family history of cardiovascular disease. None of the participants in either group were administered vitamin supplementation during the study period. All subjects provided informed, written consent to participate in the study, which was approved by the local ethics committee.
Biological assays (blood samples collected from subjects after 12 h of fasting)
tHcy:
Blood for measuring tHcy was collected in tubes containing EDTA and was kept on ice until centrifuged (3500 rpm/min for 15 min). tHcy concentrations were determined by using an automatic analyzer (Immulite DPC, USA) based on a competitive immunoassay and expressed in μmol/L.
Vitamin B12 and folate:
These levels were measured using the Immulite 2000 analyzer system (Immulite DPC, USA), which is based on a competitive immunoassay. Vitamin B12 is expressed in pg/mL and folates in ng/mL.
Lipid profile:
Total cholesterol (TC), triglycerides (TG) and high-density lipoprotein cholesterol (HDLc) were measured by using a colorimetric enzymatic method using the Technicon automatic analyzer system (RA-1000, Dade Behring, Germany). Low-density lipoprotein cholesterol (LDLc) levels were calculated by using the Friedwald formula for individuals with a TG level below 4.5 mmol/L:
High-sensitivity CRP (HsCRP):
Using an immunonephelometric method, HsCRP levels were determined on a BNII Nephelometer Analyzer (Dade Behring, Germany) and expressed in g/L.
Cytokines:
Interleukin (IL)-6, IL-1β, IL-8 and tumour necrosis factor-alpha (TNFα) levels were determined with an immunometric sequential chimiluminescent test on the Immulite 1000 Assay System (Immulite DPC, USA) and expressed in pg/mL.
Statistical analysis
Statistical analyses were performed using SPSS version 10.0 (SPSS Inc, USA) for Windows (Microsoft Corporation, USA). Continuous variables are presented as mean ± SD. The Kolmogorov-Smirnov test for the normality of the different parameters showed normal distribution. A comparison of means were performed by one-way ANOVA based on Fisher’s exact test statistics. Correlations between the different parameters were evaluated by calculation of the Spearman’s correlation coefficient. A P≤0.05 was considered to be statistically significant.
RESULTS
Demographic and biochemical characteristics of the patient and control groups are summarized in Table 1. Hypertension, dyslipidemia, family history of CAD and type 2 diabetes were frequent in the patient group. Patients had elevated tHcy (P<0.01), vitamin B12 (P<0.001), folates (P<0.05), HsCRP (P<0.001), IL-6 (P<0.001) and TNFα (P<0.001), but lower HDLc levels (P<0.05) (Table 1). No differences were found in TC, TG and LDLc levels or creatinine.
TABLE 1.
Clinical and biochemical features of acute coronary syndrome (ACS) patients compared with healthy controls
| Healthy controls (n=80) | ACS patients (n=122) | P | |
|---|---|---|---|
| Sex (male/female), n/n | 43/37 | 77/45 | – |
| Age, years | 57.02±4.32 | 63.86±10.07 | <0.01 |
| BMI, kg/m2 | 26.29±3.57 | 26.61±3.84 | NS |
| Type 2 diabetes, n | 0 | 59 | – |
| Hypertension, n | 0 | 72 | – |
| Dyslipidemia, n | 0 | 65 | – |
| Family history of CAD, n | 4 | 31 | – |
| Alcohol, n | 2 | 3 | NS |
| Smoking, n | 31 | 71 | <0.05 |
| TC, mmol/L | 4.67±1.37 | 4.60±1.30 | NS |
| HDLc, mmol/L | 1.08±0.25 | 0.99±0.32 | <0.05 |
| TC/HDLc ratio | 4.40±1.24 | 4.81±1.36 | <0.05 |
| TG, mmol/L | 1.38±1.02 | 1.45±0.80 | NS |
| LDLc, mmol/L | 2.93±1.17 | 2.93±1.06 | NS |
| Creatinine, μmol/L | 80.97±12.57 | 82.51±14.43 | NS |
| tHcy, μmol/L | 13.95±6.09 | 17.67±8.32 | <0.01 |
| Vitamin B12, pg/mL | 259.61±130.05 | 361.27±228.12 | <0.001 |
| Folate, ng/mL | 5.60±2.64 | 6.55±3.18 | <0.05 |
| HsCRP, g/L | 3.78±1.38 | 14.64±9.81 | <0.001 |
| Interleukin-6, pg/mL | 2.32±1.42 | 11.56±8.23 | <0.001 |
| Interleukin-1β, pg/mL | 5.00±0.00 | 5.26±2.26 | NS |
| Interleukin-8, pg/mL | 5.0±0.00 | 5.37±2.25 | NS |
| TNFα, pg/mL | 6.81±4.67 | 11.18±6.83 | <0.001 |
Data presented as mean ± SD unless indicated otherwise. BMI Body mass index; CAD Coronary artery disease; HDLc High-density lipoprotein cholesterol; HsCRP High-sensitivity C-reactive protein; LDLc Low-density lipoprotein cholesterol; NS Not significant; TC Total cholesterol; TG Triglycerides; tHcy Plasma total homocysteine; TNFα Tumour necrosis factor-alpha
As shown in Table 2, tHcy, IL-6, TNFα and HsCRP differed significantly according to the number of diseased vessels. Patients with three diseased vessels presented with elevated levels compared with those with two, and those with one diseased vessel. Patients with three diseased vessels presented with significantly higher LDLc and creatinine but lower TG levels.
TABLE 2.
Comparison of biological parameters according to the number of diseased arteries in acute coronary syndrome patients
|
Diseased vessels, n |
||||
|---|---|---|---|---|
| 1 (n=50) | 2 (n=33) | 3 (n=39) | P | |
| Plasma total homocysteine, μmol/L | 13.93±4.89 | 16.27±5.46 | 23.65±10.37 | <0.001*† |
| Folate, ng/mL | 7.02±3.99 | 7.35±3.33 | 5.27±2.65 | <0.01*† |
| Vitamin B12, pg/mL | 387.84±241.27 | 372.30±213.77 | 317.87±221.77 | NS |
| Creatinine, μmol/L | 80.16±15.46 | 81.15±13.77 | 86.69±12.98 | <0.05* |
| Interleukin-6, pg/mL | 8.76±5.21 | 9.95±6.89 | 16.52±10.14 | <0.001*† |
| Interleukin-1β, pg/mL | 5.30±2.04 | 5.01±0.00 | 5.75±3.23 | NS |
| Interleukin-8, pg/mL | 5.57±1.22 | 5.82±1.56 | 6.00±1.56 | NS |
| Tumour necrosis factor-alpha, pg/mL | 8.29±4.12 | 8.81±4.54 | 16.90±7.73 | <0.001*† |
| High-sensitivity C-reative protein, g/L | 10.87±7.26 | 14.00±8.13 | 20.03±11.57 | <0.01*† |
| Total cholesterol, mmol/L | 4.62±1.47 | 4.32±1.22 | 4.81±1.09 | NS |
| High-density lipoprotein cholesterol, mmol/L | 1.01±0.29 | 0.89±0.29 | 1.04±0.37 | NS |
| Total cholesterol/high-density lipoprotein cholesterol ratio | 4.60±1.07 | 4.97±1.28 | 4.94±1.72 | NS |
| Low-density lipoprotein cholesterol, mmol/L | 2.96±1.22 | 2.62±0.94 | 3.15±0.96 | <0.05* |
| Triglycerides, mmol/L | 1.39±0.64 | 1.71±1.02 | 1.31±0.75 | <0.05* |
Data presented as mean ± SD unless indicated otherwise.
Comparison between patients with two and those with three diseased vessels;
Comparison between patients with one and those with three diseased vessels. NS Not significant
The Spearman’s correlation test demonstrated significant positive correlation between the number of diseased vessels and tHcy (r=0.49; P<0.01), IL-6 (r=0.32; P<0.01), TNFα (r=0.51; P<0.01) and HsCRP (r=0.39; P<0.01) levels. A significantly negative correlation was found between the number of diseased vessels and folate (r=−0.34; P<0.01) and vitamin B12 (r=−0.22; P<0.01).
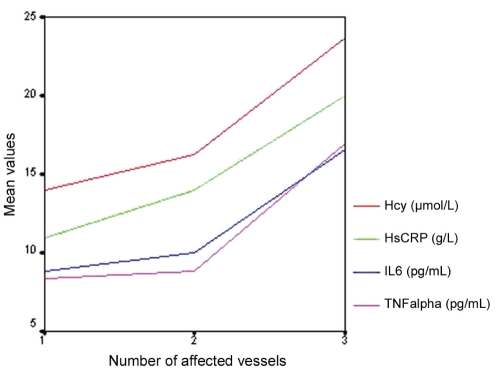
tHcy, HsCRP, IL-6 and TNFα levels increased with the number of affected vessels (Figure 1).
Figure 1).
Distribution of plasma homocysteine (tHcy), high-sensitivity C-reactive protein (HsCRP), interleukin-6 (IL-6) and tumour necrosis factor (TNF)-alpha mean levels according to the number affected vesssels
DISCUSSION
The main results of our study were the increased concentrations of tHcy, IL-6, TNFα and HsCRP in patients with ACS compared with control subjects and, second, the distinct distribution of these parameters according to the number of diseased vessels.
Several epidemiological studies (7–9) have identified moderately elevated concentrations of tHcy as a potentially modifiable risk factor for coronary artery disease, which may contribute to the development of atherosclerosis (10). One of the major causes of hyperhomocysteinemia (HHcy) is a deficiency in vitamin B12 and folates – important cofactors in Hcy metabolism. Despite a normal vitamin status, patients in the present study had elevated levels of tHcy. This result prompted us to investigate other causes or factors that lead to HHcy. Elevated tHcy was demonstrated to be a predictor of next cardiac event in patients with ACS (11) and represents an independent risk factor for recurrent ACS in a free form (12). Facila et al (13) showed that moderately elevated tHcy concentration measured at admission is a strong predictor of all-cause mortality in patients admitted with non-ST segment elevation ACS. The possible mechanism by which Hcy promotes atherosclerosis is unclear; however, inflammatory markers have been recently implicated. In a recent study (14), Hcy was demonstrated to contribute to the initiation and progression of vascular disease by activating monocytes, resulting in the secretion of cytokines that amplify the inflammatory response. The demonstration of a relationship among Hcy, inflammation and autoimmunity intriguingly expands the spectrum of the possible pathogenetic implications for HHcy in the course of arterial disease (15). In our study, we found that tHcy correlated with IL-6, TNFα and HsCRP levels, but not with IL-8 and IL-1β. Enhanced inflammation may be associated with Hcy-related cardiovascular disease, possibly involving IL-6-related mechanisms (16). High circulating concentrations of IL-6 are independent correlates of HHcy and may explain, at least in part, the association between Hcy and atherosclerosis (17). Recently, evidence of the presence of TNFα, IL-6 and IL-8 in atherosclerotic lesions has been obtained (18,19). In fact, in response to infection or tissue inflammation, IL-6, IL-1 and TNFα stimulate the production of CRP. IL-6 is a regulator of CRP and has a key role in the initiation of inflammation. CRP attracts monocytes, activates complement and induces a decrease in endothelial function (20). HsCRP (21) and IL-6 are considered new biomarkers of cardiovascular disease (8), and increased IL-6 and HsCRP levels are strongly associated with the inflammatory system and the course of clinical and hemodynamically significant CAD (22). In recent studies (23), elevated HsCRP was significantly correlated with clinical symptoms in patients with ACS and represents an effective utility for CRP in risk stratification (24).
In our study, we did not find a correlation between tHcy and IL-8 levels, although similar studies (25) demonstrated that the contribution of Hcy to the initiation and progression of atherosclerosis may be due to the stimulation of IL-8 expression, and that the induction of IL-8 in response to atheroprone hemodynamics may maintain lower levels of vascular cellular adhesion molecule-1, preventing further inflammation from localizing to vessel walls during atherogenesis (26).
As demonstrated by many research studies, lipid profile also has an important role in atherosclerosis. The interaction between lipid and inflammation processes defines the principal pathogenesis. In fact, atherogenic lesions have a large lipid nucleus, with signs of active inflammation and macrophage accumulation at the site of plaque rupture (27). In the present study, we found elevated LDLc levels in patients with multiple affected vessels and lower HDLc levels in patients compared with controls, but no association between lipids and the studied cytokines was found. Korhonen et al (28) demonstrated that high levels of LDLc are associated with an increased risk of CAD – the more vessels obstructed, the higher the serum LDLc level. Serum lipid profile – in particular, the serum levels of TC and HDLc – is a strong determinant of atherosclerosis, while smoking, elevated blood glucose and reduced physical activity are independent atherosclerotic risk factors for cardiovascular events. In our study, smoking, diabetes mellitus and dyslipidemia were more prevalent in patients than in controls, and are factors that can accelerate the proliferation of atherosclerosis. The etiology of CAD is complex. Although classic risk factors increase development of this disease, recently studied biomarkers also demonstrate an important role.
CONCLUSION
Hcy and proinflammatory markers appear to play an important role in the initiation of ACS. However, despite the impressive gains in the understanding of atherosclerosis, many questions persist regarding the use of these markers in clinical practice. Well-designed studies using large sample sizes are required to characterize the interaction between different inflammatory markers.
Acknowledgments
This work was supported by a grant from the Ministry of Defense Military Hospital of Tunis Department of Biochemistry. The authors thank all participants of the study.
REFERENCES
- 1.Pasterkamp G, Algra A, Grobbee DE, Banga JD, van der Graaf Y. Homocysteine and the stage of atherosclerotic disease: A study in patients suffering from clinically silent and clinically manifest atherosclerotic disease. Eur J Clin Invest. 2002;32:309–15. doi: 10.1046/j.1365-2362.2002.00986.x. [DOI] [PubMed] [Google Scholar]
- 2.Poddar R, Sivasubramanian N, Dibello PM, Robinson K, Jacobson D. Homocysteine induces expression and secretion of monocyte chemo attractant protein-1 and interleukin-8 in human aortic endothelial cells: Implication for vascular disease. Circulation. 2001;103:2717–23. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- 3.Wang G, Siow YL. Homocysteine induces monocyte chemoattractant protein-1 expression by activating NF-kappa B in THP-1 macrophages. Am J Physiol Heart Circ Physiol. 2001;280:H2840–H7. doi: 10.1152/ajpheart.2001.280.6.H2840. [DOI] [PubMed] [Google Scholar]
- 4.Zhong-qun Y, Goran KH. Innate immunity, macrophage activation and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 5.Saadeddin SM, Habbab MA, Ferns GA. Markers of inflammation and coronary artery disease. Med Sci Monit. 2002;8:5–12. [PubMed] [Google Scholar]
- 6.Jian-Jun L, Chun-Hong F. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med Hypotheses. 2004;62:499–506. doi: 10.1016/j.mehy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 8.Saeed S, Faramarz F, Mojtaba S, Gholamreza D, Abbasali K, Tehran Heart Center Homocysteine, vitamin B12 and folate levels in premature coronary artery disease. BMC Cardiovasc Dis. 2006;6:38. doi: 10.1186/1471-2261-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wouter De R, Rudi GJW, Willem J, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: Population based observational cohort study. BMJ. 2008;337:A3083. doi: 10.1136/bmj.a3083. (Abst) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanratty G, McGrath LT, McAuley DF, Young IS, Johnston GD. The effects of oral methionine and homocysteine on endothelial function. Heart. 2001;85:326–30. doi: 10.1136/heart.85.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsin-Bang L, Chih-Pei L, Wen-Tsai L, Tao-Cheng W, Shing-Jong L, Jaw-Wen C. Circulating mononuclear superoxide production and inflammatory markers for long-term prognosis in patients with cardiac syndrome X. Free Rad Biol Med. 2006;40:983–91. doi: 10.1016/j.freeradbiomed.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Martijn GH, van O, Bimmer EPMC, et al. Prognostic value of free plasma homocysteine levels in patients hospitalized with acute coronary syndrome. Am J Cardiol. 2008;102:135–9. doi: 10.1016/j.amjcard.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Facila L, Nunez JE, Sanchis J, et al. Early determination of homocysteine levels in acute coronary syndromes, is it an independent prognostic factor? Int J Cardiol. 2005;100:275–9. doi: 10.1016/j.ijcard.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Su SJ, Huang LW, Pai LS, Liu HW, Chang KL. Homocysteine at pathophysiological concentrations activates human monocyte and induces cytokine expression and inhibits macrophage migration inhibitory factor expression. Nutrition. 2005;21:994–1002. doi: 10.1016/j.nut.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Pietro EL, Pier LC, Enrico S, et al. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmunity Rev. 2007;6:503–9. doi: 10.1016/j.autrev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Holven KB, Aukrust P, Retterstol K, et al. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand J Clin Lab Invest. 2006;66:45–54. doi: 10.1080/00335510500429821. [DOI] [PubMed] [Google Scholar]
- 17.Gori AM, Corsi AM, Fedi S, et al. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nut. 2005;82:335–41. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 18.Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 1996;127:263–71. doi: 10.1016/s0021-9150(96)05968-0. [DOI] [PubMed] [Google Scholar]
- 19.Roberto HH, Carlos RZ, Fabiano C, Juliano LF, Jose AR, Carlos V. Serial changes in plasma levels of cytokines in patients with coronary artery disease. Vasc Health Risk Manag. 2005;1:245–50. [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir O, Gundogdu F, Karakelleoglu S, et al. Comparison of serum levels of inflammatory markers and allelic variant of interleukin-6 in patients with acute coronary syndrome and stable angina pectoris. Coron Artery Dis. 2008;19:15–9. doi: 10.1097/MCA.0b013e3282f27bf7. [DOI] [PubMed] [Google Scholar]
- 23.Arroyo-Espliguero R, Mollichelli N, Avanzas P, et al. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur Heart J. 2003;4:2006–11. doi: 10.1016/j.ehj.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 24.Correia LC, Lima JC, Rocha MS, D’Oliveira Junior A, Pericles Esteves J. Does high-sensitivity C-reactive protein add prognostic value to the TIMI-Risk Score in individuals with non-ST elevation acute coronary syndromes? Clin Chim Acta. 2007;375:124–8. doi: 10.1016/j.cca.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Geisel J, Jodden V, Obeid R, Knapp JP, Bodis M, Herrmann W. Stimulatory effect of homocysteine on interleukin-8 expression in human endothelial cells. Clin Chem Lab Med. 2003;41:1045–8. doi: 10.1515/CCLM.2003.161. [DOI] [PubMed] [Google Scholar]
- 26.Hastings NE, Feaver RE, Lee MY, et al. Human IL-8 regulates smooth muscle cell VCAM-1 expression in response to endothelial cells exposed to atheroprone flow. Arterioscler Thromb Vasc Biol. 2009;29:725–31. doi: 10.1161/ATVBAHA.109.184382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross R. Atherosclerosis: An inflammatory disease. N Engl J Med. 2003;340:115–23. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 28.Korhonen T, Savolaienen MJ, Ikaheimo M, et al. Association of lipoprotein cholesterol and triglycerides with the severity of coronary artery disease in men and women. Atherosclerosis. 1996;127:213–20. doi: 10.1016/s0021-9150(96)05958-8. [DOI] [PubMed] [Google Scholar]