SUMMARY
Autosomal dominant polycystic kidney disease (ADPKD) results from loss-of-function mutations in PKD1 or PKD2. The products of these genes, the polycystins PC-1 and PC-2, form a transmembrane channel that is necessary for flow sensing by renal cilia. In C. elegans, the polycystin orthologs LOV-1 and PKD-2 function in sensory neurons that mediate male mating behavior. Here, we report that the novel single-pass membrane protein CWP-5 is necessary for polycystin signaling during the response step of mating behavior. As with the polycystins, CWP-5 localizes to neuronal cilia; this localization requires LOV-1. The response defect of cwp-5 mutants does not appear to result from disruption of ciliogenesis or polycystin localization. Instead, genetic and behavioral analyses indicate that CWP-5 represses a previously undescribed antagonistic effect of the polycystins on sensory function. Although cwp-5 does not have a primary-sequence ortholog in vertebrates, it has intriguing parallels with the autosomal recessive PKD gene FPC (also known as PKHD1). Together, this study identifies a new component of C. elegans polycystin signaling, demonstrates that the polycystins have a latent capacity to hinder sensory transduction, and suggests that aberrant functions of the polycystins could contribute to the pathogenesis of PKD.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is a common and potentially lethal disorder that is characterized by the growth of fluid-filled cysts that disrupt renal function (Torres et al., 2007). Virtually all cases of ADPKD arise from loss-of-function mutations in either PKD1 or PKD2, which are genes that encode the polycystin proteins PC-1 and PC-2. PC-2 is a non-selective, TRP (transient receptor potential)-family cation channel whose activity is thought to be regulated by PC-1, an 11-pass transmembrane protein with which PC-2 associates physically (Gonzalez-Perrett et al., 2001; Hanaoka et al., 2000). The polycystins localize to sensory cilia and intracellular structures in a variety of cell types, including renal epithelia. In cilia, the polycystin channel is necessary for the transduction of mechanical stimuli through ciliary bending (Nauli and Zhou, 2004).
Although ADPKD is inherited dominantly, its pathology is likely to arise from the reduction or loss of polycystin function at the cellular level (Harris and Torres, 2009; Torres et al., 2007). When one defective PKD1 or PKD2 allele is inherited, somatic mutation of the remaining wild-type allele is thought to account for most, or all, cyst formation. The means by which the loss of polycystin function in a single cell or clone of cells leads to cyst formation is not entirely clear. However, the prevailing view is that an insensitivity to ciliary polycystin signaling disrupts downstream signaling pathways, possibly involving cAMP, thereby triggering pathogenic changes in cell division and differentiation. Highlighting the importance of cilia in maintaining normal renal epithelial function, a related disorder, autosomal recessive PKD (ARPKD), results from inactivating mutations in PKHD1, the gene encoding fibrocystin/polyductin (FPC), a single-pass membrane protein that also localizes to renal epithelial cilia and other subcellular compartments (Harris and Torres, 2009). Although fibrocystin is likely to interact directly with the polycystin complex, the regulatory relationships between the polycystins and fibrocystin/polyductin are not well understood. In addition, many other ciliopathies, including Bardet-Biedel syndrome and Meckel syndrome, are also frequently associated with polycystic kidneys, further highlighting the connection between cilium function and cystic kidney disease.
In the nematode C. elegans, the polycystin orthologs LOV-1 (PC-1) and PKD-2 (PC-2) act in the dendritic ciliated endings of male-specific sensory neurons to mediate the transduction of mechanical and/or chemical signals that are important for male mating behavior (Barr et al., 2001; Barr and Sternberg, 1999). Loss of the gene encoding either protein significantly reduces the ability of males to carry out two key steps of this process. In the first step, called response behavior, males respond to hermaphrodite contact by placing the ventral side of the tail against their mate’s body, contracting posterior ventral muscles to maintain this posture, and initiating sustained tail-first locomotion along the hermaphrodite. The second polycystin-dependent step, called vulva location, requires the male to cease reverse locomotion upon encountering the vulval opening and to begin prodding the vulva with the spicules. Polycystin function in the response step is mediated primarily through the ray RnB neurons, whereas vulva location behavior depends on polycystin function in the hook HOB neuron (Barr et al., 2001; Barr and Sternberg, 1999; Liu and Sternberg, 1995). Although null lov-1, pkd-2 and lov-1; pkd-2 mutant males are significantly impaired in both response and vulva location, these behaviors are not eliminated, suggesting that a secondary, polycystin-independent pathway can also mediate the sensory signaling required for these steps (Barr et al., 2001; Barr and Sternberg, 1999). The polycystins are also expressed in the male-specific CEM head sensory neurons, which are important for detecting pheromones that attract males to potential mates. However, the evidence that polycystin function is important for pheromone response is conflicting (Chasnov et al., 2007; Srinivasan et al., 2008; White et al., 2007).
In both C. elegans and vertebrates, the two polycystins are thought to act in a single genetic pathway (Barr et al., 2001; Wu and Somlo, 2000). According to this model, the phenotypes and pathology associated with the loss of one polycystin should solely reflect the absence of signaling through the polycystin channel. However, several findings suggest that the polycystin pathway may be more complex: lov-1; pkd-2 double-mutant males display small but consistent improvements in mating behavior compared with either of the single polycystin mutants (Barr et al., 2001; Peden and Barr, 2005), and the phenotypes of Pkd1−/− and Pkd2−/− mice are not identical to each other (Lu et al., 2001; Wu et al., 1998). This indicates that the loss of one polycystin may cause disease through mechanisms beyond simple ablation of the function of the polycystin channel. A better understanding of these effects could provide important insight into the mechanisms of cyst formation, identify genes that modify the severity of disease, and provide new opportunities for therapy.
In this study we identify a novel C. elegans gene, cwp-5, that acts through an unexpected mechanism to regulate polycystin function. cwp-5 encodes a single-pass transmembrane protein that is specifically co-expressed with the polycystins in a characteristic set of male-specific neurons. As with the polycystins, CWP-5 localizes both to the cilia and cytoplasmic structures. cwp-5 mutants exhibit marked impairment in the response step of male mating behavior. The loss of cwp-5 does not further enhance the behavioral defects of lov-1; pkd-2 mutants, indicating that cwp-5 acts in the polycystin pathway. Interestingly, genetic interactions between cwp-5 and the polycystins indicate that both of the polycystins have a latent potential to obstruct sensory signaling, and that cwp-5 functions to repress this negative activity of the polycystins. Although vertebrates do not possess clear primary-sequence homologs of cwp-5, we propose that similar regulatory functions exist to modulate vertebrate polycystin function. Moreover, our results indicate that aberrant activities of the human polycystins could be important contributors to the pathogenesis of polycystic kidney disease.
RESULTS
cwp-5 encodes a novel membrane protein present in the sensory cilia of the polycystin neurons
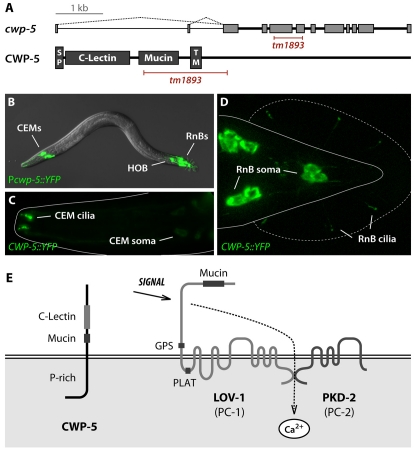
Using DNA microarrays, we previously identified four genes, cwp-1 through to cwp-4, that are specifically co-expressed with the C. elegans polycystins in a characteristic set of male-specific sensory neurons: sixteen ray RnB neurons (all RnB neurons apart from those of ray 6), four cephalic CEM neurons, and the tail HOB neuron (Portman and Emmons, 2004). In further analysis of this dataset, we studied the predicted gene F48C11.2 (Fig. 1A), which ranked in the first percentile of candidate ray-expressed genes. Because of its expression pattern (described below), we named this gene cwp-5. The predicted product of cwp-5 is a single-pass type I membrane protein. Transcriptome data (from www.wormbase.org) indicate that cwp-5 contains two alternative first exons that encode 11 or 14 amino acids; the use of either of these exons is predicted to generate a functional signal peptide. The extracellular region of the CWP-5 protein contains a divergent C-type lectin domain and a small mucin-like region of predicted O-glycosylation sites (Fig. 1A). Its 377-amino acid cytoplasmic domain lacks obvious similarity to other known proteins, but is rich in prolines throughout (17.5%). The predicted membrane topology of CWP-5 is shown in Fig. 1E.
Fig. 1.
cwp-5 is a novel gene expressed exclusively in male-specific sensory neurons. (A) The cwp-5 gene contains 11 exons and is located at +10.23 cM on the X chromosome. Transcriptome data indicate that cwp-5 has two alternative first exons (indicated with dotted lines) that encode 11 (F48C11.2a) or 14 (F48C11.2b) amino acids. Both CWP-5 products are predicted by SignalP 3.0 (Emanuelsson et al., 2007) to contain functional signal peptides (SP). CWP-5 is also predicted to contain a C-type lectin domain and a mucin-like region, as well as a single-pass transmembrane (TM) domain. The allele tm1893 is an in-frame insertion-deletion removing most of the mucin domain and the entire transmembrane domain to yield a truncated, potentially secreted, protein. The predicted structure of the mutant transcript was confirmed by sequencing the cwp-5(tm1893) cDNA. (B) Expression of a transcriptional cwp-5::YFP reporter using the upstream (F48C11.2b) promoter was observed in 21 male-specific sensory neurons: 4 CEM neurons in the head, 16 RnB-type ray neurons (all RnBs except for those of ray 6), and the hook neuron HOB. A translational reporter, CWP-5::YFP, in which the YFP sequence was inserted at the 3× end of cwp-5, should reflect both isoforms of CWP-5. This construct marked the same set of cells, with fluorescence visible in the cell bodies, dendrites and the cilia of CEM (C), RnB (D) and HOB (not shown) neurons. The cilia in the CEM and RnB neurons are roughly 4 and 1 μm in length, respectively. (E) A model for the membrane topology of CWP-5 and the polycystins in sensory cilia. Domains of each of these proteins are noted. As shown here, signaling through the polycystin complex is thought to be mediated by regulated Ca2+ entry.
We determined the expression pattern of cwp-5 using a transcriptional yellow fluorescent protein (YFP) reporter containing the upstream promoter as well as a C-terminal translational YFP fusion that should reflect the expression from both promoters. Expression of both of these transgenes was observed specifically in the RnB, HOB and CEM neurons of the male, and nowhere in the hermaphrodite (Fig. 1B–D), thereby following the same pattern that has been reported for the polycystins (Barr et al., 2001; Barr and Sternberg, 1999). The CWP-5::YFP fusion protein was clearly enriched in the sensory cilia of these neurons and was also detectable in the soma (Fig. 1 and data not shown).
cwp-5 is necessary for efficient male sensory behavior
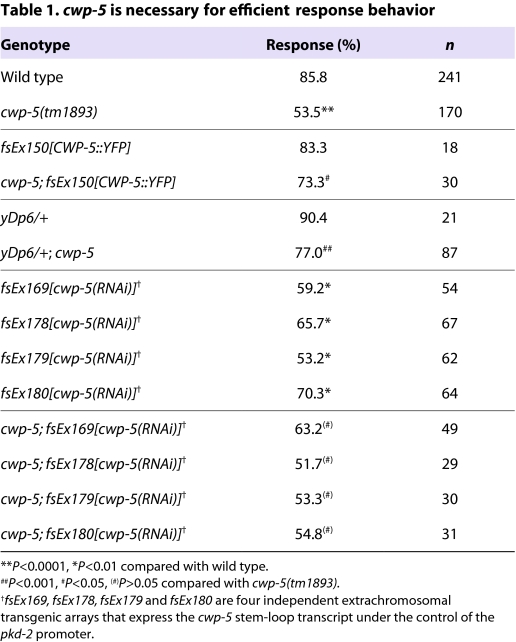
To determine whether the cwp genes have a role in polycystin signaling, we examined the behavior of males carrying three different deletion mutations: cwp-2&cwp-3(ok1366), cwp-4(tm727) and cwp-5(tm1893) (ok1366 is a deletion that removes substantial amounts of the neighboring cwp-2 and cwp-3 genes). These animals exhibited no apparent defects in body, tail or sensory ray morphology, and neither cwp-2&cwp-3 nor cwp-4 mutant males displayed defects in response or vulva location behavior (data not shown). However, cwp-5 mutant males had a significant and specific deficit in response behavior: 85.9% of wild-type (WT) males responded to hermaphrodites by backing and curling their tails (n=241), whereas cwp-5 males exhibited a response frequency of 53.5% (n=170), which is a significant reduction (P<10−4) (Table 1). By contrast, vulva location behavior in these animals was essentially unaffected (WT, 86.8%; cwp-5, 82.9%). Thus, unlike other genes that are important for male sensory behavior, cwp-5 appears to have a specific function in response behavior, indicating that the regulation or function of polycystin signaling may differ between the RnB and HOB neurons.
Table 1.
cwp-5 is necessary for efficient response behavior
The tm1893 mutation deletes 730 bp from cwp-5 and replaces it with a 4-bp insertion. Because this change maintains the cwp-5 open reading frame, cwp-5(tm1893) is predicted to encode a secreted protein lacking the mucin and transmembrane domains but still bearing a C-type lectin domain (Fig. 1A). As a result, cwp-5(tm1893) could simply reduce or remove CWP-5 function, or it could actively interfere with a process required for normal behavior. Because cwp-5 is on the X chromosome, males are hemizygous for this locus. We therefore asked whether the cwp-5(tm1893) phenotype could be rescued by introduction of a wild-type cwp-5 gene. Consistent with the possibility that cwp-5(tm1893) reduces or eliminates cwp-5 function, we found that a translational CWP-5::YFP could rescue the response defect of cwp-5(tm1893) males (Table 1). In addition, a wild-type copy of cwp-5 provided by the small X-chromosome duplication yDp6 (Akerib and Meyer, 1994) was able to suppress the cwp-5(tm1893) phenotype (Table 1). Finally, cwp-5(RNAi), mediated by a RNA hairpin expressed in the polycystin neurons, reduced response behavior to a level comparable to that of cwp-5(tm1893) males (Table 1), indicating that tm1893 phenocopies the reduction of cwp-5 function. Because cwp-5(RNAi) caused no change in the behavior of cwp-5(tm1893) males (P>0.05), tm1893 is likely to be a strong loss-of-function or null allele (Table 1). Consistent with this possibility, we obtained no evidence that cwp-5(tm1893) behaved dominantly in tra-1(e1099); cwp-5/+ heterozygous XX pseudomales (data not shown).
cwp-5 promotes response behavior by blocking an inhibitory activity of the polycystins
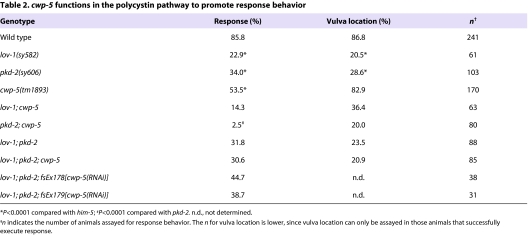
Because the loss of polycystin signaling does not eliminate male mating behavior, one or more secondary pathways for response and vulva location have been proposed to exist (Barr et al., 2001; Barr and Sternberg, 1999). To determine whether cwp-5 acts in the polycystin pathway or a secondary pathway, we examined genetic interactions between cwp-5, lov-1 and pkd-2. When polycystin signaling was eliminated with a lov-1; pkd-2 double-mutant background, we found that the loss of cwp-5 had no effect on response: 31.8% of lov-1; pkd-2 males displayed response behavior (n=88), and we observed essentially the same frequency, 30.6%, in the lov-1; pkd-2; cwp-5 triple mutants (n=85) (Table 2). In agreement with this, the cwp-5(RNAi) transgenes that impaired response behavior in wild-type animals (Table 1) failed to enhance the lov-1; pkd-2 defect (Table 2). These results indicate that cwp-5 functions in the polycystin pathway to promote response behavior.
Table 2.
cwp-5 functions in the polycystin pathway to promote response behavior
We found unexpected genetic interactions when we examined the phenotype of cwp-5(tm1893) mutants carrying null alleles of just one polycystin. In both cases, cwp-5(tm1893) clearly enhanced the response defects of single polycystin mutants (Table 2). Loss of cwp-5 function reduced the remaining response behavior of lov-1mutants by 37.6%. Moreover, it virtually eliminated the remaining response behavior of pkd-2 mutants, such that pkd-2; cwp-5 males almost never responded to hermaphrodites. Genetically, these results indicate that cwp-5 mutants reveal the potential of single polycystin subunits to hinder sensory transduction. By comparing the phenotype of the double mutants lov-1; cwp-5 (14.3% responders) or pkd-2; cwp-5 (2.5%) with that of the lov-1; pkd-2; cwp-5 triple mutant (30.6%), it is apparent that the enhanced behavioral deficits of the double mutants can be alleviated by removing the remaining functional polycystin. Thus, in cwp-5 mutants, a lone polycystin subunit can impede, rather than facilitate, normal sensory function.
Such lone polycystin subunits might also accumulate when the stoichiometry of normal polycystin expression is disrupted. To determine whether an excess of one polycystin over the other might disrupt sensory transduction in a wild-type background, we assayed the behavior of males stably expressing a PKD-2::GFP fusion protein driven by the pkd-2 promoter (myIs4). Although this myIs4 transgene rescues the mating defects of pkd-2(sy606) mutants (Bae et al., 2006), we found that it significantly reduced response in a wild-type background (60.4%, n=48, P<0.0002 compared with WT males). Thus, an excess of functional PKD-2 can interfere with ray neuron function, consistent with the notion that the polycystins can both promote and antagonize sensory signaling.
cwp-5 is not essential for ciliogenesis or cilium maintenance
We next explored the mechanisms by which CWP-5 promotes RnB sensory transduction and how a lone polycystin can inhibit it. Several possibilities can be imagined: CWP-5 could be required for normal ciliogenesis, correct trafficking of cilium proteins, the assembly of a functionally competent polycystin complex, or for transduction of the sensory signal itself. Although not all of these possibilities can be easily evaluated in C. elegans sensory neurons, we assessed the first two possibilities by examining ciliogenesis and protein trafficking in cwp-5 and polycystin mutants.
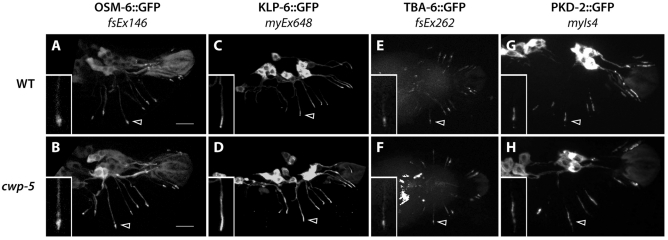
The structural integrity of RnB and HOB sensory cilia is known to be important for response behavior (Bae et al., 2006) (R. Lints and P. Koo, personal communication; D. Hurd, R.M.M., L. Nuñez and D.S.P., manuscript submitted). To assess cilium integrity in cwp-5 mutants, we first examined the localization of OSM-6, a cilium and transition zone marker whose mislocalization is a hallmark of ciliary assembly and maintenance defects (Barr and Sternberg, 1999; Collet et al., 1998; Ou et al., 2007). We observed robust ciliary localization of OSM-6::GFP in the rays of cwp-5 mutant males (Fig. 2A,B), often with two distinct domains of OSM-6::GFP in a single ray. This suggests that the sensory cilia of the RnA and RnB neurons, whose dendrites run parallel to each other inside each ray, were both intact (data not shown). We also found that the localization of two motor proteins that are important for cilium structure, KLP-6 and OSM-3 (Peden and Barr, 2005; Snow et al., 2004), was not altered in cwp-5 mutants (Fig. 2C,D and data not shown). Finally, the localization of TBA-6, an α-tubulin isoform that marks the cilia of the RnB neurons (D. Hurd, R.M.M., L. Nuñez and D.S.P., manuscript submitted), was not defective in cwp-5 mutants (Fig. 2E,F). Because both KLP-6 and TBA-6 allow specific visualization of RnB neuron sensory cilia, these results suggest that the loss of cwp-5 does not disrupt cilium assembly or maintenance.
Fig. 2.
cwp-5(tm1893) does not disrupt RnB cilium structure or PKD-2 localization. Images show the localization of the indicated green fluorescent protein (GFP) fusion protein in wild-type (A,C,E,G) and cwp-5(tm1893) (B,D,F,H) adult male tails. In all panels, arrowheads indicate the cilium of R3B (the RnB neuron of ray 3). Insets show high-magnification views of the R3B cilia. Bars, 10 μm (A,B). All panels show lateral views, with anterior to the left, except for the ventral views in E,F.
Behavioral defects in cwp-5 mutants are unlikely to result from defects in protein trafficking
A potential cause of the response defect of cwp-5 males is mislocalization of PKD-2, as is seen in animals bearing mutations in the kinesin-like gene klp-6 (Peden and Barr, 2005) and the phosphoinositide (PI) 5-phosphatase gene cil-1 (Bae et al., 2009). However, we found that the localization of PKD-2::GFP in cwp-5(tm1893) males was indistinguishable from that in wild-type males (Fig. 2G,H). Although we were unable to examine LOV-1 localization directly, mislocalization of this polycystin in cwp-5 mutants seems unlikely (see Discussion). Thus, we do not favor the possibility that polycystin mislocalization underlies the response defects of cwp-5 mutants.
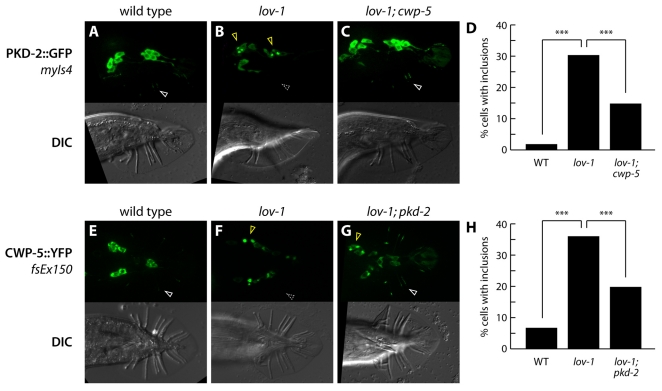
Previous work has shown that the ciliary trafficking of PKD-2 requires LOV-1 (Bae et al., 2006). In lov-1mutants, PKD-2 is largely absent from cilia and often accumulates in one or two perinuclear aggregates (Fig. 3A,B) (Bae et al., 2006). We therefore considered the possibility that the inhibitory activity of the polycystins, as manifested in lov-1; cwp-5 and pkd-2; cwp-5 mutants, stems from a disruption of protein trafficking. For example, PKD-2 trapped in these cytoplasmic bodies might disrupt the ciliary trafficking of other signaling components, thereby compromising sensory behavior mediated by a polycystin-independent pathway. In this case, we would expect the frequency or intensity of cytoplasmic PKD-2 bodies in lov-1 mutants to be enhanced by cwp-5(tm1893), since cwp-5(tm1893) enhances the response defect of lov-1 mutants. However, we observed the opposite result: the frequency of cytoplasmic PKD-2 inclusions was significantly reduced in lov-1; cwp-5 mutants compared with lov-1 single mutants (Fig. 3C,D). Interestingly, PKD-2 ciliary localization was often restored in these double mutants (Fig. 3C). Thus, the inhibitory effect of free PKD-2 is not likely to be a simple consequence of its aberrant perinuclear localization. Instead, these results indicate that, in the absence of lov-1, CWP-5 is important for efficient cytoplasmic sequestration of PKD-2 in the RnB neurons, which may help prevent its aberrant inhibitory functions.
Fig. 3.
Protein trafficking in cwp-5 and polycystin mutants. (A–C) Panels show fluorescence (above) and DIC (below) of young adult males of the indicated genotypes carrying the PKD-2::GFP fusion protein transgene myIs4 (Bae et al., 2006). White arrowheads indicate the localization of PKD-2::GFP to RnB cilia; the dashed gray arrowhead indicates the absence of ray cilium localization. Yellow arrowheads indicate aberrant perinuclear inclusions of PKD-2::GFP. (D) Quantitation of perinuclear inclusions of PKD-2::GFP in animals of the indicated genotypes. Individual cells were scored for the presence or absence of intense cytoplasmic inclusions by epifluorescence microscopy. In wild-type animals, 173 cells in 13 animals were scored; in lov-1 mutants, 285 cells in 23 animals were scored; in lov-1; cwp-5 mutants, 272 cells in 24 animals were scored. Statistical comparisons were made using Fisher’s exact test. Asterisks (***) indicate P<10−4. (E–G) Panels show fluorescence (above) and DIC (below) of young adult males of the indicated genotypes carrying the CWP-5::YFP fusion protein transgene fsEx150. White arrowheads indicate the localization of CWP-5::YFP to RnB cilia; the dashed gray arrowhead indicates the absence of ray cilium localization. Yellow arrowheads indicate aberrant perinuclear inclusions of CWP-5::YFP. (H) Quantitation of perinuclear inclusions of CWP-5::YFP in animals of the indicated genotypes. Individual cells were scored for the presence or absence of intense cytoplasmic inclusions by epifluorescence microscopy. In wild-type animals, 121 cells in 13 animals were scored; in lov-1 mutants, 393 cells in 32 animals were scored; in lov-1; pkd-2 mutants, 30 cells in 24 animals were scored. Statistical comparisons were made using Fisher’s exact test. Asterisks (***) indicate P<10−4.
CWP-5 localization depends on the polycystins
The finding that cwp-5(tm1893) suppresses the frequency of aberrant cytoplasmic PKD-2 localization in lov-1 mutants (Fig. 3C,D) suggests that CWP-5 helps to stabilize or anchor the mislocalized PKD-2 seen in lov-1mutants. This raises the possibility that CWP-5 might functionally interact with the polycystins, potentially as part of a larger polycystin signaling complex. Consistent with this possibility, we found that the dendritic and ciliary localization of CWP-5::YFP in the ray neurons depends on lov-1 (Fig. 3E,F). Interestingly, in lov-1 mutants, CWP-5::YFP accumulated in perinuclear domains reminiscent of those seen with PKD-2::GFP. The simultaneous loss of both polycystins suppressed CWP-5 mislocalization to these structures (Fig. 3G,H). This indicates that the substrate for cytoplasmic sequestration in lov-1 mutants could be a CWP-5:PKD-2 complex, further supporting the possibility that CWP-5 functionally interacts with the polycystins.
DISCUSSION
The simplicity and genetic tractability of C. elegans male mating behavior (Barr and Garcia, 2006) provides a powerful system to investigate the mechanism and function of polycystin signaling. Using this model, we have identified CWP-5, a novel single-pass transmembrane protein that co-localizes with the polycystins in the sensory cilia of a specific set of sensory neurons in C. elegans adult males. We find that CWP-5 functions in the polycystin pathway to promote sensory behavior. Genetic interactions between cwp-5 and the polycystins revealed an unexpected aspect of the function and regulation of the C. elegans polycystins: both lov-1 and pkd-2 have the potential to hinder, rather than facilitate, sensory signaling, and cwp-5 represses these negative influences.
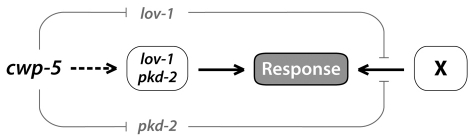
In the simplest genetic model for cwp-5 and polycystin function (Fig. 4), the canonical LOV-1/PKD-2 complex acts directly or indirectly to transduce a sensory stimulus. Because lov-1; pkd-2 mutants still exhibit significant response behavior (Barr et al., 2001), a secondary, as-yet-unidentified pathway (‘X’) also contributes to the transduction of stimuli that trigger this step. cwp-5 is unlikely to function in this secondary pathway, since loss of cwp-5 has no effect in a lov-1; pkd-2 mutant background. Instead, because the phenotype of lov-1; cwp-5 and pkd-2; cwp-5 double mutants is more severe than that of the triple mutant lov-1; pkd-2; cwp-5, we propose that wild-type CWP-5 acts to block a toxic activity of the remaining polycystin subunit. Because the enhanced response defects of these double mutants are suppressed by removing the remaining polycystin, we conclude that the polycystins act downstream of cwp-5. Since response defects are also apparent in cwp-5(tm1893) single mutants, it seems likely that CWP-5 actively functions to prevent the negative effects of free polycystins in wild-type animals. This possibility is also supported by the observation that the lov-1 single mutant phenotype is consistently more severe than that of the lov-1; pkd-2 double mutant (Table 1) (Barr et al., 2001; Peden and Barr, 2005), which suggests that PKD-2 can modestly inhibit sensory signaling in the absence of LOV-1, even when CWP-5 is present. Although our results cannot rule out the possibility that CWP-5 also has some functions downstream of, or in parallel with, the polycystins, we have no evidence that activation of CWP-5 by the polycystins is necessary for sensory signaling.
Fig. 4.
A genetic model for regulation of polycystin signaling by cwp-5. Our studies support a model in which CWP-5 facilitates polycystin signaling by repressing latent, toxic effects of the polycystins on sensory transduction. In wild-type animals, the sensory signals that elicit response behavior in C. elegans males are transduced both by the canonical LOV-1/PKD-2 polycystin complex, as well as by a secondary, as-yet-unidentified pathway (designated ‘X’), as shown in the central line. When cwp-5 function is lost in animals lacking both polycystins, no effect is seen, indicating that cwp-5 acts in the polycystin pathway. However, the loss of cwp-5 enhances the sensory defects of single polycystin mutants; this enhancement is dependent on the function of the remaining polycystin. The simplest genetic explanation for these observations is that cwp-5 mutants reveal that each of the polycystins has a latent capacity to interfere with the secondary pathway, as indicated in the upper and lower pathways. In wild-type animals, cwp-5 is likely to prevent the manifestation of this aberrant function; in addition, cwp-5 may also directly facilitate the response-promoting activity of the polycystins (dashed line). See Discussion for details.
The most likely site of action of CWP-5 is in the ray RnB neurons. Because RnB-specific rescue of cwp-5 is challenging, we have not demonstrated this directly. However, as the primary mediators of response behavior (Liu and Sternberg, 1995), the RnBs are the most likely site for cwp-5 function. Because cwp-5(tm1893) mutants exhibit wild-type vulva location behavior, we consider it unlikely that cwp-5 has an important role in the HOB neuron. However, since HOB can play a role in response behavior (Liu and Sternberg, 1995), it is possible that CWP-5 has a response-specific function in HOB in addition to a role in RnB. cwp-5 is not likely to act primarily in the CEM neurons, as cwp-5 mutant males have no defects in pheromone-response assays (R.M.M. and D.S.P., unpublished data). Moreover, ceh-30 mutant males, which specifically lack the CEM neurons, exhibit robust response behavior (R.M.M. and D.S.P., unpublished data), indicating that the CEMs are not likely to be important for response. These results also highlight an interesting cell-type specificity in the function of the C. elegans polycystin pathway, such that it is functionally specialized to respond to different stimuli in different cells. Although cwp-5 is expressed in all C. elegans polycystin neurons, it may play a role in this functional specialization. Consistent with this possibility, there are also cell-type differences in protein trafficking: Bae et al. have shown that the requirements for PKD-2 localization to cilia differ by neuron type (Bae et al., 2006), and we find that the frequency of CWP-5 mislocalization in lov-1 and lov-1; pkd-2 mutants differs between RnB, HOB and CEM neurons (R.M.M. and D.S.P., unpublished data). The mechanisms that tune these different cell types to respond to different stimuli in a polycyst-independent manner is a promising area for future study.
Signaling through the polycystin pathway requires the formation and maintenance of a primary sensory cilium, the appropriate trafficking of signaling molecules to the cilium, and the formation and function of a polycystin signaling complex (Bae and Barr, 2008). In principle, disruption of any of these steps could underlie the sensory defects that result from the loss of cwp-5. Our results do not support the possibility that disrupted ciliogenesis or protein trafficking contributes to the cwp-5 mutant phenotype: the localization of none of the four cilium markers that we examined was altered in cwp-5 mutants, nor was the localization of PKD-2 to the RnB neuron cilia. Because visualizing LOV-1 localization is technically difficult, we were unable to assess the localization of LOV-1 directly. However, we consider its mislocalization to be unlikely for the following reason: since lov-1 is necessary for the ciliary trafficking of PKD-2 (Fig. 2) (Bae et al., 2006), any mislocalization of LOV-1 would probably result in a secondary disruption of PKD-2::GFP ciliary trafficking. Because PKD-2::GFP localization appears normal in cwp-5(tm1893) mutants, we infer that lov-1 function is unlikely to be compromised, and that LOV-1 is not likely to be mislocalized in these animals. Nevertheless, we are unable to rule out the possibility that LOV-1 trafficking is disrupted in cwp-5 mutants.
Our results do indicate that CWP-5 has some involvement in protein trafficking: the cytoplasmic mislocalization of PKD-2 in lov-1 mutants is partially suppressed by cwp-5(tm1893). One possible explanation for this is that CWP-5 contributes to a surveillance mechanism that prevents inappropriate trafficking of lone polycystin subunits to the cilium, where they might disrupt ciliary function. This possibility is challenging to test directly in C. elegans. However, two results suggest that this may not be the most important role of CWP-5: first, its ciliary localization seems somewhat at odds with surveillance of trafficking as a primary function. In addition, the observation that cwp-5 single mutants have significant response defects would imply that CWP-5 prevents a substantial amount of misfolded or otherwise defective polycystins from reaching the cilium in wild-type animals. However, we detected no increase in ciliary PKD-2 (or decrease in cytoplasmic PKD-2) in cwp-5 mutants. An alternative interpretation for this result is that CWP-5 and PKD-2 comprise a complex that is the substrate for cytoplasmic inclusion by a surveillance pathway that is active in a lov-1 mutant. Since either component can be trafficked normally when the other is missing, this would suggest that CWP-5 and PKD-2 are the targets, rather than the components, of an important surveillance mechanism. Nevertheless, surveillance of polycystin trafficking by CWP-5 may be an important part of the mechanism by which this factor promotes sensory transduction.
A final possibility is that CWP-5 facilitates signaling through the polycystin complex more directly. At the molecular level, several functions can be imagined. First, CWP-5 could tether the polycystin complex to an extracellular matrix. In this model, deformation of this link as the cilium bends could be necessary for sensory signaling. In support of this idea, the cilia of the RnB neurons are surrounded by an electron-dense glycocalyx that could be important for putative mechanosensory capacities of these cells (Chow et al., 1995). Second, CWP-5 could be important for the assembly or stability of the polycystin complex. Third, CWP-5 could be a co-receptor for a signal transduced by the polycystins. Fourth, if the polycystin complex is not the primary transducer of a signal, CWP-5 could act between a primary receptor and the polycystin complex to facilitate signal transduction. In all of these possible models, CWP-5 may interact directly with the polycystin complex itself. Unfortunately, this is difficult to address directly in C. elegans. The colocalization of CWP-5 with the polycystins is consistent with the possibility of direct interaction, as are the findings that CWP-5 localization requires LOV-1, and that efficient sequestration of both PKD-2 and CWP-5 to cytoplasmic bodies in lov-1 mutants depends on each other. Interestingly, the extracellular N-terminus of LOV-1 contains a large mucin-like domain of predicted O-linked glycosylation sites that could serve as a site for direct interaction with the C-type lectin domain of CWP-5. Because the LOV-1 N-terminus is highly divergent from the comparable region of mammalian PC-1 (Barr and Sternberg, 1999), a functional mammalian CWP-5 ortholog that interacts with the PC-1 N-terminus may exist but be indiscernible by primary sequence alone. Consistent with this possibility, both polycystins are highly divergent in the diplogasterid nematode P. pacificus (Sommer, 2006), and no cwp-5 sequence ortholog is recognizable.
An important insight from our work is that the polycystins have the capacity to hinder, as well as facilitate, sensory behavior. How could a lone polycystin disrupt signaling through a secondary pathway? One possibility is that the polycystins mislocalized to cytoplasmic bodies could have an inhibitory effect. However, this mislocalization is suppressed by cwp-5 loss, whereas behavioral defects are enhanced. Thus, it seems more likely that these antagonistic effects are manifested in cilia. For example, when either LOV-1 or PKD-2 is absent, the remaining polycystin may be able to form complexes with other transmembrane proteins, particularly TRP channels, to generate species that interfere with sensory signaling. This possibility is especially interesting given the recent findings that mammalian PC-2 can interact with other classes of TRP channels to form functional heteromeric complexes (Bai et al., 2008; Kottgen et al., 2008). In addition, physiological studies have provided evidence for a mutually inhibitory relationship between mammalian PC-1 and PC-2 function in vitro (Delmas et al., 2004). The ability to measure Ca2+ signaling in vivo in C. elegans should help evaluate the physiological consequences of the loss of cwp-5 and the basis for the antagonistic effects of the polycystins. In addition, we now have a means to genetically dissect these inhibitory effects, as mutations in components of this pathway might restore the ability of pkd-2; cwp-5 males to exhibit response behavior.
Implications for polycystic kidney disease
In humans, ADPKD has been proposed to result from the disruption of the flow-sensing ability of primary cilia in renal epithelia (Nauli and Zhou, 2004). Because of the cellular-recessive mechanism of this disorder, cystogenic cells lack one polycystin subunit but contain two functional alleles encoding the other. Thus, our genetic dissection of polycystin signaling in C. elegans raises an interesting possibility for human pathology: in addition to the loss of positive signaling through the polycystin complex, aberrant inhibitory functions of the remaining polycystin may augment the severity of the ADPKD phenotype. Indeed, overexpression of Pkd1 in mice can cause polycystic kidney disease (Pritchard et al., 2000; Thivierge et al., 2006). Additional evidence demonstrating that disruption of the stoichiometry of the polycystin complex can have deleterious effects (Sharif-Naeini et al., 2009) appeared while this manuscript was in press. According to this view, interventions designed to block aberrant PC-1 or PC-2 activity might have therapeutic value. An important test of this prediction will be the examination of genetic interactions between Pkd1 and Pkd2 in mouse models: our model suggests that kidney-specific conditional loss of both Pkd1 and Pkd2 may cause less severe pathology than the loss of one polycystin alone.
It is notable that CWP-5 shares several features with fibrocystin/polyductin (FPC), the product of the PKHD1 gene; mutations in this gene cause the autosomal recessive form of PKD (ARPKD) (Onuchic et al., 2002; Ward et al., 2002). As with CWP-5, FPC is a single-pass transmembrane protein whose loss causes a phenotype similar to polycystin mutants. FPC may be a component of the polycystin complex (Wu et al., 2006) and has been implicated in flow-mediated calcium signaling in renal epithelia (Wang et al., 2007). Heterozygosity of Pkd1 enhances the severity of cystic defects in Pkhd1 null mice (Garcia-Gonzalez et al., 2007), and loss of Pkhd1 enhances the cystic phenotype of Pkd2 mutant animals (Kim et al., 2008), reminiscent of the genetic interactions between cwp-5 and the polycystins. However, FPC has been implicated in ciliogenesis (Kim et al., 2008), whereas this does not seem to be the case for CWP-5. Because CWP-5 and FPC share no primary sequence similarity, it is unlikely that they are functionally interchangeable orthologs. However, our results raise the possibility that some aspects of FPC and CWP-5 function may be analogous, and that therapies designed to block toxic polycystin functions may have value in ARPKD. Our results point to specific genetic tests in mouse models that could be carried out to investigate this possibility.
METHODS
Nematode culture and strains
Animals were cultured on nematode growth media (NGM) agar plates seeded with E. coli OP50, as described previously (Brenner, 1974). The following mutants were used in this work: linkage group (LG) II: lov-1(sy582); LG III: pha-1(e2123ts), tra-1(e1099); LG IV: pkd-2(sy606), unc-31(e169); LG V: him-5(e1490), cwp-4(tm727), cwp-2&cwp-3(ok1366); LG X: cwp-5(tm1893); LG unknown: yDp6(X;A) (Akerib and Meyer, 1994). Standard genetic methods were used to construct strains containing multiple mutations (Brenner, 1974); PCR genotyping was used to follow the presence of deletion alleles that lacked obvious phenotypes. The cwp-5(tm1893) and cwp-4(tm727) deletion alleles were obtained from the Japanese National Bioresource Project (S. Mitani, Tokyo, Japan). The cwp-2&cwp-3(ok1366) deletion was obtained from the C. elegans Gene Knockout Consortium (Oklahoma). All strains were out-crossed to wild-type animals at least five times prior to the behavioral assays. Unless stated otherwise, all strains carried him-5(e1490) to increase the number of males in self-fertilizing cultures.
Transgenes and transgenic strains
To produce the cwp-5b::YFP transcriptional reporter, 1.2 kb of sequence upstream of the predicted F48C11.2b start site was amplified and fused to YFP using overlap-extension PCR (Boulin et al., 2006). To produce the CWP-5::YFP translational reporter, DNA from 1.2 kb upstream of the predicted F48C11.2b start site to the penultimate codon of CWP-5 was amplified and fused in-frame to YFP. Fused PCR products were co-injected with pBX1 into a pha-1(e2123ts); him-5(e1490) strain (Granato et al., 1994), or co-injected with unc-122::GFP into young adult him-5 hermaphrodites. Several stable transgenic lines were obtained for each construct.
myIs4[PKD-2::GFP] and myEx648[Pklp-6::KLP-6::GFP] were generously provided by Maureen Barr (Rutgers University, NJ). OSM-6::GFP DNA was kindly supplied by Robert Herman (University of Minnesota) and was co-injected into young adult hermaphrodites, at 50 ng/μl, with unc-122::GFP to generate fsEx146. The TBA-6::YFP fusion construct fsEx262 will be described elsewhere (D. Hurd, R.M.M., L. Nuñez and D.S.P., manuscript submitted).
To produce the cwp-5 stem-loop RNA interference (RNAi) construct, two overlapping fragments of cwp-5 (571 bp and 639 bp) were amplified by standard PCR techniques. The two fragments were directionally cloned end-to-end into pPD49.78, then subsequently moved into pPD49.26 behind the pkd-2 promoter. The construct was sequenced and injected into young adult him-5 hermaphrodites at 50 ng/μl.
Microscopy
All images, except for those in Fig. 2A,B, were acquired with Nomarski DIC and epifluorescence optics using a Zeiss Axioplan 2 and Axioskop software. A Leica confocal microscope was used to produce the images in Fig. 2A,B. Image files were imported into Adobe Photoshop CS3 for adjustment of black, white and contrast levels.
Behavioral assays
Male behavioral assays were carried out according to described protocols (Hart, 2006; Liu and Sternberg, 1995). In all assays, the experimenter was blinded to the genotype of the animals until all observations were complete. Male nematodes were isolated at the L4 larval stage and allowed to reach sexual maturity overnight (16–20 hours). Young adult males were placed on a 1-cm lawn of E. coli OP50 with ∼8 paralyzed unc-31(e169) hermaphrodites and observed for a 10-minute period. For a given strain, the response frequency was calculated as the percentage of males that exhibited response behavior, defined as sustained backing along the body of the hermaphrodite with the male tail in a ventrally arched posture, within 10 minutes of being placed on the bacterial lawn. Vulva location efficiency was calculated as the ratio of the number of successful vulva location events to the number of times that the male tail sensilla encountered the vulval region. Unless noted otherwise, all comparisons were made using Fisher’s exact test (GraphPad Prism 4.0). Since the outcome of each assay is a binary choice rather than a continuous distribution, results are reported without any associated error values.
Acknowledgments
We are grateful to Kwi Yeon Lee and Stefani McGregor for expert technical assistance; to members of our laboratory for discussion and critical reading of the manuscript; to Maureen Barr and Young-Kyung Bae for important insights and reagents; to Paul Sternberg for helpful discussions; to Robert Herman for the OSM-6::GFP construct; to Shohei Mitani and the National Bioresource Project of Japan for providing cwp-5(tm1893); to Daryl Hurd for the images in Fig. 2A and 2B; to Scott Emmons, in whose laboratory the initial microarray screen was carried out; and to an anonymous reviewer for helpful insights on protein trafficking models. The Caenorhabditis Genetics Center, which is supported by NCRR, provided most of the strains used in this work. This research was supported by a Research Grant from the PKD Foundation (to D.S.P.) and by NIH grants R21 DK071645 (to D.S.P.) and F32 DK075270 (to R.M.M.). Deposited in PMC for release after 12 months.
Footnotes
AUTHOR CONTRIBUTIONS
R.M.M. and D.S.P. conceived and designed the experiments. R.M.M. performed the experiments and analyzed the data. R.M.M. and D.S.P. wrote the paper.
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Akerib C, Meyer B. (1994). Identification of X chromosome regions in Caenorhabditis elegans that contain sex-determination signal elements. Genetics 138, 1105–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Barr M. (2008). Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans. Front Biosci. 13, 5959–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae Y, Qin H, Knobel K, Hu J, Rosenbaum J, Barr M. (2006). General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133, 3859–3870 [DOI] [PubMed] [Google Scholar]
- Bae YK, Kim E, L’Hernault SW, Barr MM. 2009). The CIL-1 PI 5-phosphatase localizes TRP polycystins to cilia and activates sperm in C. elegans. Curr Biol. 19, 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai C, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. (2008). Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 9, 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW. (1999). A polycystic kidney-disease gene homologue required for male mating behaviour in C elegans. Nature 401, 386–389 [DOI] [PubMed] [Google Scholar]
- Barr MM, Garcia LR. (2006). Male mating behavior. In WormBook (ed. The C. elegans Research Community). WormBook [doi/10.1895/wormbook.1.78.1]. http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. (2001). The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 11, 1341–1346 [DOI] [PubMed] [Google Scholar]
- Boulin T, Etchberger JF, Hobert O. (2006). Reporter gene fusions. In WormBook (ed. The C. elegans Research Community). WormBook [doi/10.1895/wormbook.1.106.1]. http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnov J, So W, Chan C, Chow K. (2007). The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci USA 104, 6730–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow KL, Hall DH, Emmons SW. (1995). The mab-21 gene of Caenorhabditis elegans encodes a novel protein required for choice of alternate cell fates. Development 121, 3615–3626 [DOI] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. (1998). Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148, 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Nauli S, Li X, Coste B, Osorio N, Crest M, Brown D, Zhou J. (2004). Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 18, 740–742 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. (2007). Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc. 2, 953–971 [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez M, Menezes L, Piontek K, Kaimori J, Huso D, Watnick T, Onuchic L, Guay-Woodford L, Germino G. (2007). Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet. 16, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano A, Zotta E, Batelli M, Harris P, Reisin I, Arnaout M, Cantiello H. (2001). Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98, 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. (1994). pha-1, a selectable marker for gene transfer in C elegans. Nucleic Acids Res 22, 1762–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia A, Piontek K, Tsiokas L, Sukhatme V, Guggino W, Germino G. (2000). Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990–994 [DOI] [PubMed] [Google Scholar]
- Harris PC, Torres VE. (2009). Polycystic kidney disease. Annu Rev Med. 60, 321–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A. (2006). Behavior. In WormBook (ed. The C. elegans Research Community). WormBook [doi/10.1895/wormbook.1.87.1]. http://www.wormbook.org
- Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen X, et al. (2008). Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 19, 455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottgen M, Buchholz B, Garcia-Gonzalez M, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, et al. (2008). TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 182, 437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. (1995). Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14, 79–89 [DOI] [PubMed] [Google Scholar]
- Lu W, Shen X, Pavlova A, Lakkis M, Ward C, Pritchard L, Harris P, Genest D, Perez-Atayde A, Zhou J. (2001). Comparison of Pkd1-targeted mutants reveals that loss of polycystin-1 causes cystogenesis and bone defects. Hum Mol Genet. 10, 2385–2396 [DOI] [PubMed] [Google Scholar]
- Nauli S, Zhou J. (2004). Polycystins and mechanosensation in renal and nodal cilia. BioEssays 26, 844–856 [DOI] [PubMed] [Google Scholar]
- Onuchic L, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, et al. (2002). PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 70, 1305–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G, Koga M, Blacque O, Murayama T, Ohshima Y, Schafer J, Li C, Yoder B, Leroux M, Scholey J. (2007). Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell 18, 1554–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden E, Barr M. (2005). The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr Biol. 15, 394–404 [DOI] [PubMed] [Google Scholar]
- Portman DS, Emmons SW. (2004). Identification of C. elegans sensory ray genes using whole-genome expression profiling. Dev Biol. 270, 499–512 [DOI] [PubMed] [Google Scholar]
- Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, et al. (2000). A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum Mol Genet. 9, 2617–2627 [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering HJA, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. (2009). Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 [DOI] [PubMed] [Google Scholar]
- Snow J, Ou G, Gunnarson A, Walker M, Zhou H, Brust-Mascher I, Scholey J. (2004). Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol. 6, 1109–1113 [DOI] [PubMed] [Google Scholar]
- Sommer R. (2006. Pristionchus pacificus. In WormBook (ed. The C. elegans Research Community). WormBook [doi/10.1895/wormbook.1.102.1]. http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn H, Teal P, Malik R, Edison A, Sternberg P, Schroeder F. (2008). A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454, 1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge C, Kurbegovic A, Couillard M, Guillaume R, Cote O, Trudel M. (2006). Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol. 26, 1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres V, Harris P, Pirson Y. (2007). Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang J, Nauli S, Li X, Starremans P, Luo Y, Roberts K, Zhou J. (2007). Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 27, 3241–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C, Hogan M, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham J, Bacallao R, Ishibashi M, et al. (2002). The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 30, 259–269 [DOI] [PubMed] [Google Scholar]
- White J, Nicholas T, Gritton J, Truong L, Davidson E, Jorgensen E. (2007). The sensory circuitry for sexual attraction in C. elegans males. Curr Biol. 17, 1847–1857 [DOI] [PubMed] [Google Scholar]
- Wu G, Somlo S. (2000). Molecular genetics and mechanism of autosomal dominant polycystic kidney disease. Mol Genet Metab. 69, 1–15 [DOI] [PubMed] [Google Scholar]
- Wu G, D’Agati V, Cai Y, Markowitz G, Park J, Reynolds D, Maeda Y, Le T, Hou HJ, Kucherlapati R, et al. (1998). Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93, 177–188 [DOI] [PubMed] [Google Scholar]
- Wu Y, Dai X, Li Q, Chen C, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, et al. (2006). Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet. 15, 3280–3292 [DOI] [PubMed] [Google Scholar]