Abstract
OBJECTIVES:
Precancerous and cancerous cells can trigger an immune response that may limit tumor development and can be used as a prognostic marker. The aims of the present study were to quantify the presence of B and T lymphocytes, macrophages and cells expressing inducible nitric oxide synthase (iNOS) in the cervical stroma of women with grade III cervical intraepithelial neoplasia (CIN III) or in the intratumoral and peritumoral tissue of women with stage I invasive carcinoma.
METHODS:
Cervical tissue specimens were obtained from 60 women (20 each from control tissues, CIN III and invasive carcinomas). The average ages in the control, CIN III and invasive groups were 43.9 (± 4.3), 35.5 (± 9.5), and 50 (± 11.2) years, respectively. The specimens were immunohistochemically labeled with antibodies to identify T lymphocytes (CD3), cytotoxic lymphocytes (CD8), B lymphocytes (CD20), macrophages (CD68) and iNOS. We evaluated the markers in the stroma above the squamocolumnar junction (control), at the intraepithelial lesion (CIN cases), and in the nfiltrating tumor. Two independent observers performed the immunohistochemical analysis.
RESULTS:
T lymphocytes, B lymphocytes, macrophages and iNOS were present more frequently (P<0.05) in the stroma of peritumoral invasive tumors compared to the controls and intratumoral invasive cancer samples. CD3+ and CD20+ lymphocytes were present more frequently in CIN III patients compared to samples from patients with intratumoral invasive cancer (P<0.05).
CONCLUSION:
High numbers of T and B lymphocytes, macrophages and iNOS-expressing cells in the peritumoral stroma of the invasive tumors were observed. Cell migration appeared to be proportional to the progression of the lesion.
Keywords: Cervical cancer, Nitric oxide, CIN III, Lymphocyte, Macrophages
INTRODUCTION
Chronic infection of the uterine cervix mucosa by human papillomavirus (HPV), most notably HPV16, is associated with malignant transformation of keratinocytes in metaplastic areas of the transformation zone. Despite evidence that HPV is the causative agent of cervical cancer and its precursors (squamous intra-epithelial lesions, SIL), HPV infection alone is not sufficient for tumor development. There is accumulating evidence that tumor and virus-infected cells are able to inhibit protective immune reactions.1 Although HPV DNA is detected in the majority of SIL and uterine cervical carcinomas, the persistence or progression of cervical lesions suggest that viral antigens are not adequately presented to the immune system.2 Invasive epidermoid cancer of the uterine cervix is preceded by cervical intraepithelial neoplasia (CIN) associated with HPV.3 CIN I is the characteristic response to HPV infection and rarely evolves to cancer; instead, it spontaneously resolves. In contrast, CIN II and III are potential precursor lesions, and approximately 12% of CIN III cases will progress to cancer if untreated.4
Lymphocytes can be divided into B cells, which are distinguished by the presence of CD20 on the cell surface, or T cells, which are distinguished by the presence of CD3 on the cell surface. T cells can be further divided into two classes: T-helper Lymphocytes (CD4+) and cytotoxic T Lymphocytes (CD8+). The stroma partially controls tumor growth and invasion and has a considerable influence on the immune response.5 CD4+ T Lymphocytes, CD8+ T Lymphocytes and B Lymphocytes have been observed in the stroma of the vaginal and cervical mucosae.6,7 Lymphocytic infiltrates at neoplasia sites determine the clinical prognosis of the infection (persistence, regression or progression). Previous studies on dysplastic cervical tissue using immunohistochemistry have demonstrated significant infiltrates of cytotoxic and auxiliary T and B lymphocytes in the stroma below the lesion.6 Several factors determine whether IL-12 or IL-4 will predominate and, therefore, whether a Th1 or Th2 type immunological response will occur in the female genital tract in response to infection with a specific infectious agent.8 According to Gonçalves and Donadi9, macrophage populations have been found in the cervical and vaginal mucosae in the absence of infection or inflammation. In CIN II/III patients, increased numbers of macrophages have been observed. The presence of these cells may indicate regression of the lesion. Currently, the role of macrophages in tumor immunology is the subject of scientific studies and is considered a promising target for future therapy.10
Nitric oxide (NO) is both a cytotoxic agent and a tumor growth promoter. Cytotoxic effects of NO may occur by direct damage to DNA subsequent to inhibition of enzymes involved in nucleic acid synthesis. In contrast, NO may favor tumor dissemination by promoting angiogenesis.11 In a study of NO activity, Weissler et al.12 concluded that nitric oxide synthase (NOS) levels were elevated in oral squamous cell carcinomas. Addicks et al.13 came to a similar conclusion in a prostate cancer study. Using an animal model for colon carcinoma, Jeannin et al.14 demonstrated that the presence of mononuclear cells decreased with increasing production of NO by splenic macrophages. Thus, NO production in tumors may be responsible for the frequently observed phenomenon of tumor-induced immunosuppression. Currently, the role of NO in HPV infection and carcinogenesis remains unclear.
The aims of the present study were to identify and quantify the presence of CD3+ and CD8+ T lymphocytes, CD20+ B lymphocytes, CD68+ macrophages and inducible nitric oxide synthase (iNOS)-expressing cells in the stroma of women without neoplastic lesions of the uterine cervix and women with CIN III or stage I invasive carcinoma in the intratumoral and peritumoral tissue.
MATERIALS AND METHODS
Samples
Histological sections from the uterine cervix of 60 women were retrospectively analyzed. The women had been patients at the Gynecology and Obstetrics outpatient clinic of the Federal University of Triângulo Mineiro (UFTM) and had undergone cold-knife conization, loop electrosurgical excision, hysterectomy or Wertheim-Meigs surgical procedures. The project was approved by the Research Ethics Committee of Federal University of Triângulo Mineiro (UFTM). The histological sections were divided into three distinct sets: a control group of 20 healthy women without any histological evidence of CIN or invasive carcinoma who had undergone total abdominal hysterectomy due to benign uterine disease (leiomyoma) and did not present with cervical abnormalities; 20 women with a histological diagnosis of CIN III; and 20 women with a histological diagnosis of stage I invasive carcinoma according to the classification of the International Federation of Gynecology and Obstetrics (FIGO). The surgical specimens were obtained from the archives of the Surgical Pathology Division and the data used to characterize the samples were accessed by reviewing medical files. To maintain the confidentiality of the information sources, each patient was identified with a number, with numbers 1 to 20 assigned to women in the control group, 21 to 40, given to CIN III patients, and 41 to 60 assigned to invasive carcinoma patients. The inclusion criteria for the groups were that the women were not pregnant at the time of the biopsy, there was no description in the medical files of positive serological tests for HIV or any organic condition relating to immunosuppression, and the women had no previous history of radiotherapy for the treatment of gynecological neoplasia.
The age range was 35 to 52 years (mean 43.9 ± 4.3 in the control group); 22 to 63 years (mean 35.5 ± 9.5) in the CIN III group and 26 to 73 years (mean 50 ± 11.2) in the invasive carcinoma group. The age at which the subjects became sexually active was analyzed in only 19 patients in the control group because the medical file of one patient indicated that she was a virgin at the time of the surgery. The age at sexarche ranged from 12 to 30 years (mean 18.9 ± 4.3) in the control group, 13 to 24 years (17.6 ± 3.3) in the CIN III group and 12 to 23 years (16.4 ± 3.2) in the invasive carcinoma group. The number of pregnancies ranged from 0 to 8 (mean 2.75 ± 2) in the control group, 1 to 17 (4.35 ± 3.8) in the CIN III group and 1 to 13 (6.25 ± 3.9) in the invasive carcinoma group.
Immunohistochemical analysis
Histological sections (5 μm thick) were cut and stained using the hematoxylin-eosin technique. In our immunohistochemical study, we used antibodies against CD3, CD8, CD20, CD68 and iNOS to characterize the local immune response. The tonsil was used as a control. Histological sections (5 μm thick) were cut and placed on silanized slides (Sigma, A-3648, St. Louis, MO, USA). These slides were stained using the streptavidin-biotin-peroxidase technique. Briefly, the slides were kept in a glass chamber at 56 °C for 24 h before being deparaffinized in xylol 3 times for 5 min each, followed by dehydration in three baths of pure alcohol and one of 80% alcohol (ethanol) for 10 s each. Next, the slides were hydrated in phosphate-buffered saline (PBS) at pH 7.2 for 5 min. The excess buffer solution was immediately removed, and the edge of each section was carefully dried using absorbent paper. The slides were placed on a tray and 3% oxygenated water was added to each section for 10 min to block endogenous peroxidase activity. The slides were washed in PBS. The antigens were recovered by placing the slides in 5 cytological test tubes containing 10 mM citrate buffer solution (pH 6.0). The tubes were sealed with aluminum foil and placed in a steaming pan (ARNO) for 30 min. The tubes were then removed from the pan and placed on the bench to cool. The slides were then washed three times with PBS and incubated with their respective primary antibodies for 18 h in a damp chamber at 3 – 4 °C. The antibodies were diluted in bovine serum albumin (Sigma) according to the manufacturer’s recommendations. After overnight incubation, the slides were placed at room temperature for 15 min, washed in PBS and dried as before. Biotinylated secondary antibody (DAKO) was added to each slide for 30 min at room temperature in a damp chamber. The slides were washed in PBS and dried, and the streptavidin-biotin-peroxidase complex (DAKO) was added to the slide for 30 min at room temperature in a damp chamber. After washing in PBS, the slides were developed by adding diaminobenzidine (DAB; DAKO) for 5 min. The slides were then washed in running water and counterstained using Harris hematoxylin for 2 s. Finally, the slides were immersed in three baths of pure alcohol for 10 s each to remove excess water, followed by one bath of phenicated xylol and three baths of xylol for 5 min each. Coverslips were placed over the samples and they were sealed.
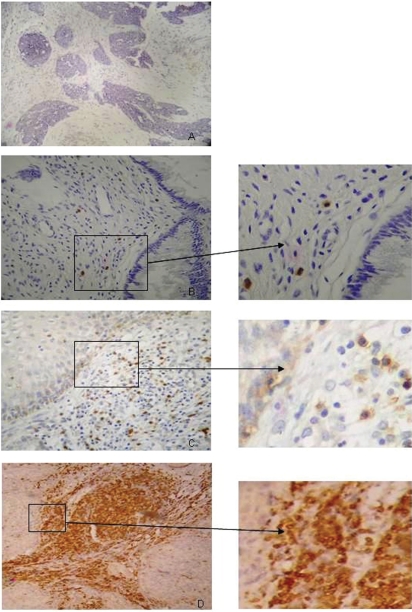
To count the lymphoid cells, we used the scoring criteria described previously by Sheaff et al.15 Briefly, the marked cells were graded as follows: 0 – absence of cells; 1 – rare cells; 2 – a moderate number of cells; 3 – many cells (maximum number of cells found for each analyzed marker; see Figure 1). For statistical purposes, we combined the 0 and 1 scores and 2 and 3 scores. The cells were initially observed at a low magnification (100x) to assess the general distribution. The samples were then examined in greater detail (magnification: 400x) to obtain the final score. Samples from the stroma above the squamocolumnar junction, intraepithelial lesion and infiltrating tumor were analyzed. The malignant tumors were separated into two distinct regions for counting labeled cells: intratumoral and peritumoral. Cells in the intratumoral region were considered to be located inside the islands of malignant cells. The peritumoral region was defined as the area bordering the invasive area. Necrotic areas were excluded.
Figure 1.
Samples of stained tissue sections. Histological analysis of tissue stained by CD3 immunohistochemistry shows positive cells (in brown; B, C and D) with a score of 0 (A), 1 (B), 2 (C) or 3 (D), as described in the “Materials and Methods” section. Magnification: 200x.
Statistical analysis
For all cases, the analysis was done by two independent observers. The agreement between the two observers was calculated using the Kappa coefficient. For all markers, the Kappa coefficient was 0.83. The final result was obtained after joint assessment of discordant cases to produce a single value by consensus. The variables were analyzed using GraphPad Instat software, version 4.0. The scores were compared using the chi-squared test with Yates correction. A P value of less than 0.05 was considered statistically significant.
RESULTS
Table 1 presents the results of the histological analysis for the markers of T lymphocytes (CD3+), cytotoxic T lymphocytes (CD8+), B lymphocytes (CD20+), macrophages (CD68+) and iNOS. The number of samples analyzed for some markers is less than 20 because no more lesions were found in the histological analysis or the marker did not work satisfactorily, in which case that patient’s samples were excluded. The statistical analysis showed there was a significant presence of T lymphocytes (CD3+ and CD8+), B lymphocytes, macrophages and iNOS in the stroma of peritumoral invasive tumors compared to the controls and intratumoral invasive cancer samples. The same trends were observed among patients with CIN III, except with respect to CD3+ lymphocytes and iNOS. More CD3+ lymphocytes were present in CIN III patients than in control patients or those with invasive cancer. CD20+ was found more frequently in CIN III patients than in those with invasive cancer.
Table 1.
Distribution of CD3, CD8, CD20, CD68 and iNOS in the cervical stroma of control women, patients with CIN III and patients with invasive cervical cancer [n (%)]
| Control | CIN III | Invasive |
||
|---|---|---|---|---|
| Peritumoral** | Intratumoral•,•• | |||
| CD3 | 6/13 (31.6/68.4) | 16/3 (84.2/15.8)* | 19/0 (100/0) | 1/18 (5.2/94.8) |
| CD8 | 4/16 (20/80) | 8/11 (42.1/57.9) | 16/2 (88.8/11.2) | 3/15 (16.7/83.3) |
| CD20 | 3/16 (15.7/84.3) | 5/14 (26.3/73.7) | 18/0 (100/0) | 0/18 (0/100) |
| CD68 | 1/14 (6.6/93.4) | 1/14 (6.6/93.4) | 8/7 (53.3/46.7) | 1/14 (6.7/93.3) |
| iNOS | 6/14 (30/70) | 7/9 (43.7/56.3) | 15/5 (75/25) | 5/15 (25/75) |
P < 0.002, CD3+ compared with control;
P < 0.01, all markers compared with CIN III (except CD3 and iNOS) and control;
P < 0.005, all markers compared with peritumoral markers;
P < 0.05, CD3+ and CD20+ compared with CIN III. Note: N for some markers is under 20 because no more lesions were found by histological analysis or the staining was insufficient.
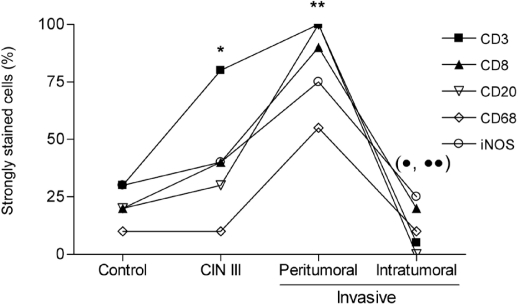
Overall, there was an increase in CD3+ T lymphocytes in CIN III patients and in the peritumoral stroma of patients with invasive tumors (Figure 2). There was significant labeling of all markers analyzed in the peritumoral stromal microenvironment; however, all markers were decreased in invasive cancer patients compared to CIN III patients.
Figure 2.
Distribution of immunohistochemically labeled cells. T lymphocytes (CD3+ and CD8+), B lymphocytes (CD20+), macrophages (CD68+) and cells that express iNOS from patients in the control group, the CIN III group and the group with invasive carcinomas of the uterine cervix in the peritumoral stroma and the intratumoral microenvironment were detected by immunohistochemistry as described in the “Materials and Methods” section. * P < 0.002, CD3+ compared with control; ** P < 0.01, all markers compared with CIN III (except CD3 and iNOS) and control; • P < 0.005, all markers compared with peritumoral; •• P < 0.05, CD3+ and CD20+ compared with CIN III.
DISCUSSION
Our data demonstrated a significant increase in CD3+ T lymphocytes in CIN III; other cell types did not show this trend. This finding is in agreement with previously published data.18 Our evaluation revealed no significant difference in the number of CD8+ T lymphocytes present in CIN III patients compared to controls. Therefore, these cells may be helper or regulatory T lymphocytes present in the lesion site, as reported in another study, which demonstrated that regulatory T cells have a pivotal role in HPV-induced lesions.21 The development of effective immunotherapies depends on our understanding of the interactions between the immune system and the neoplasia and the changes that occur during the progression and regression of the lesion.
The local and systemic immune responses in uterine cervical cancer patients and the corresponding precursor lesions have previously been studied, as has the migration of these cells to the tumor; however, the role specific cell types and nitric oxide in the local response is not yet clear. The immune response is extremely important in restraining these neoplastic cells, and failures in this system may lead to cancer progression.16 It has been hypothesized that the lesions undergo gradual transformation into tumors and that the transformation depends on the type of tumor and the mediators of inflammation that are produced within the tumor microenvironment.17 Recently, our group demonstrated that CIN III patients had more CD3+ T lymphocyte infiltrates, a factor that was related to a greater chance of recurrence.18 In uterine cervical tumors, systemic abnormalities19 or abnormalities in the tumor microenvironment may control the local immune response and interfere with disease progression.20
NO has a role in inducing specific and nonspecific immunity and an immune response to a variety of extracellular parasites and some tumor cells. NO produced by iNOS may regulate T cell proliferation, cytokine production and apoptosis.22 We found this enzyme in both pre-neoplastic lesions and invasive lesions. Data in the literature demonstrate that NO derived from macrophages may inhibit the lymphocyte response.23 NO produced by human tumor cells may, in turn, favor tumor growth by paralyzing the function of immune T cells.22,23 Chen et al.24 demonstrated that the overexperssion of iNOS in cervical cancer patients was associated with decreased survival and a greater propensity for metastasis. Another study demonstrated that iNOS expression had an inverse relationship with macrophage density and tumor progression, suggesting that this mediator could modulate the function of macrophages and promote tumor progression.25 These data suggest that NO may have a role in the differentiation or inductive response phase of CD8+ T lymphocytes. Therefore, NO may partially inhibit lymphocyte and macrophage migration into the tumor, thereby helping the neoplastic cells escape the immune response. In addition, NO could act in neoplastic cells, as suggested by Kawanishi et al,26 who demonstrated that infection by high-risk HPV types promotes iNOS-dependent DNA damage, which leads to dysplastic changes and carcinogenesis.
It is intriguing that, although CIN III does not constitute an invasive neoplasia, patients with CIN III had statistically significant CD3+ T lymphocyte infiltrates in the underlying stroma compared to the control group. Moreover, despite the large concentration of peritumoral inflammatory infiltrates, little or no infiltrate was found inside the tumors. CIN III is a systemic disease27 that produces cytokines capable of stimulating the migration of CD3+ T lymphocytes. One of these cytokines (IL-10) is significantly increased in CIN III patients compared to controls.28 Early infiltration of CIN lesions by highly cytotoxic effector cells protects against progression.29 IL-10 inhibits the production of IL-12 and IFN-γ, both of which are key to the Th1-type response. Inhibition of interferon (IFN)-γ production in CIN III patients may explain the decreased abundance of CD68+ macrophages and iNOS in CIN III patients as compared to the control patients, considering that activated macrophages are the main inducers of iNOS. Therefore, in CIN III patients, the Th2-type response may dominate. In this way, the immune response to CIN III would occur through production of antibodies and not through cellular immunity.
If invasive disease develops from CIN III, the large influx of peritumoral CD20+ B lymphocytes in these patients may be the result of Th2 predominance at an early disease stage. The behavior of CD8+ T lymphocytes, macrophages and iNOS-expressing cells in patients with invasive disease is similar to control patients, and the elevation of CD8+ T cells is proportionally lower than the elevation of CD3+ T lymphocytes. A likely explanation for this finding is that from the moment of invasion, macrophages migrate to the tumor site and induce iNOS expression, with consequent NO production. The induction of NO may inhibit the proliferation of CD8+ T lymphocytes. Although there is an increase in T and B lymphocytes, macrophages and iNOS in the peritumoral area, these cells do not penetrate the intratumoral region.
A high number of B and T lymphocytes were found in the stroma of human immunodeficiency virus (HIV+)/CIN patients compared to HIV-/CIN patients or normal tissue.30,31 Our results showed that very little CD20 is present in CIN III patient tissue and that CD20 is absent in intratumoral tissue; however, it is present at increased levels in the peritumoral stroma. Some studies have questioned the importance of B lymphocytes and, consequently, the importance of anti-HPV antibodies in CIN patients. Petter et al.32 demonstrated that, despite the presence of anti-HPV antibodies, HIV+ patients had aggressive CIN, suggesting that the cellular immune response and not the humoral immune response is important for controlling and resolving these neoplasias.
The local immune response and migration of these cells to the tumor has been studied in uterine cervical cancer patients and precursor lesions, but the role of specific cells and NO in the local response is not yet clear. Apparently, cell migration induced by the neoplasia is directly proportional to the progression of the lesion, but migration is decreased in the intratumoral environment. Moreover, increased iNOS expression suggests that large quantities of NO are produced during carcinogenesis, principally in the peritumoral area, suggesting that this mediator may have an important role in carcinogenesis. Taken together, our data demonstrate that there are high numbers of CD3+, CD8+ and CD20+ lymphocytes, macrophages and iNOS-expressing cells in the peritumoral stroma of patients with invasive tumors.
Acknowledgments
The authors would like to thank the Research and Project Financing Board (FINEP), the Research Support Foundation of the State of Minas Gerais (PAPEMIG), the National Council for Scientific and technological Development (CNPq) and Research and Teaching Foudation of Uberaba (FUNEPU) for financial support.
REFERENCES
- 1.Caberg JH, Hubert PM, Begon DY, Herfs MF, Roncarati PJ, Boniver JJ, et al. Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes. Carcinogenesis. 2008;29:1441–7. doi: 10.1093/carcin/bgn145. [DOI] [PubMed] [Google Scholar]
- 2.Herfs M, Herman L, Hubert P, Minner F, Arafa M, Roncarati P, et al. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol Immunother. 2009;58:603–14. doi: 10.1007/s00262-008-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley MA. Human papillomavirus vaccines. Rev Med Virol. 2006;16:139–49. doi: 10.1002/rmv.498. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, et al. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res. 2004;64:6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- 5.Sahebali S, Van den Eynden G, Murta EF, Michelin MA, Cusumano P, Petignat P, et al. Stromal issues in cervical cancer: a review of the role and function of basement membrane, stroma, immune response and angiogenesis in cervical cancer development. Eur J Cancer Prev. 2010;19:204–15. doi: 10.1097/CEJ.0b013e32833720de. [DOI] [PubMed] [Google Scholar]
- 6.Bell MC, Edwards RP, Partridge EE, Kuykendall K, Conner W, Gore H, et al. CD8+ T lymphocytes are recruited to neoplastic cervix. J Clin Immunol. 1995;15:130–36. doi: 10.1007/BF01543104. [DOI] [PubMed] [Google Scholar]
- 7.White HD, Yeaman GR, Givan AL, Wira CR. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37:30–8. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 8.Witkin SS, Giraldo P, Linhares I, Ledger WJ. Individual immunity and susceptibility to female genital tract infection. Am J Obst Gynecol. 2000;183:252–6. doi: 10.1067/mob.2000.104258. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves MA, Donadi EA. Immune cellular response to HPV: current concepts. Braz J Infect Dis. 2004;8:1–9. [PubMed] [Google Scholar]
- 10.Heller DS, Hameed M, Cracchiolo B, Wiederkehr M, Scott D, Skurnick J, et al. Presence and quantification of macrophages in squamous cell carcinoma of the cervix. Int J Gynecol Cancer. 2003;13:67–70. doi: 10.1046/j.1525-1438.2003.13035.x. [DOI] [PubMed] [Google Scholar]
- 11.Brennan PA, Downie IP, Langdon JD, Zaki GA. Emerging role of nitric oxide in cancer. Br J Oral Maxillofacial Surg. 1999;37:370–3. doi: 10.1054/bjom.1999.0201. [DOI] [PubMed] [Google Scholar]
- 12.Rosbe KW, Prazma J, Petrusz P, Mims W, Ball SS, Weissler MC. Immunohistochemical characterization of nitric oxide synthase activity in squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 1995;113:541–9. doi: 10.1177/019459989511300504. [DOI] [PubMed] [Google Scholar]
- 13.Klotz T, Bloch W, Jacobs G, Niggemann S, Engelmann U, Addicks K. Immunolocalization of inducible and constitutive nitric oxide synthases in human bladder cancer. Urology. 1999;54:416–9. doi: 10.1016/s0090-4295(99)00212-5. [DOI] [PubMed] [Google Scholar]
- 14.Lejeune P, Lagade CP, Onier N, Pinard D, Oshima H, Jeannin JF. Nitric oxide involvement in tumor-induced immuno-supression. J Immunol. 1994;152:5077–83. [PubMed] [Google Scholar]
- 15.Georgiannos SN, Renault A, Goode AW, Sheaff M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134:827–34. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, Ho CS, Laherty R, Maxwell T, Walker D, Gardiner RA, et al. Population of HLA-DR+ Immature cells accumulates in the blood dendritic cell compartment of patients with different types of cancer. Neoplasia. 2005;7:1112–22. doi: 10.1593/neo.05442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Sem Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Maluf PJ, Michelin MA, Etchebehere RM, Adad SJ, Murta EF. T lymphocytes (CD3) may participate in the recurrence of cervical intraepithelial neoplasia grade III. Arch Gynecol Obstet. 2008;278:525–30. doi: 10.1007/s00404-008-0621-8. [DOI] [PubMed] [Google Scholar]
- 19.Bais AG, Beckmann I, Lindemans J, Ewing PC, Meijer CJ, Snijders PJ, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58:1096–100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 21.Loddenkemper C, Hoffmann C, Stanke J, Nagorsen D, Baron U, Olek S, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009;100:1112–7. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blesson S, Their YJ, Gaudin C, Stancou R, Kolb JP, Moreau JL, et al. Analysis of the mechanisms of human cytotoxic T lymphocyte response inhibition by NO. Int Immunol. 2002;14:1169–78. doi: 10.1093/intimm/dxf081. [DOI] [PubMed] [Google Scholar]
- 23.Medot-Pirenne M, Heilman MJ, Saxena M, Mcdermott PE, Mills CD. Augmentation of an antitumor CTL response in vivo by inhibition of suppressor macrophage nitric oxide. J Immunol. 1999;163:5877–82. [PubMed] [Google Scholar]
- 24.Chen HHW, Su WC, Chou CY, Guo HR, Ho SY, Que J, et al. Increased expression of nitric oxide synthase and cyclooxygenase-2 is associated with poor survival in cervical cancer treated with radiotherapy. Int J Rad Onc Biol Phys. 2005;63:1093–100. doi: 10.1016/j.ijrobp.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Mazibrada J, Rittà M, Mundini M, Andrea M, Azzimonti B, Borgogna C, et al. Interaction between inflammation and angiogenesis during different stages of cervical carcinogenesis. Gynecol Oncol. 2008;108:112–20. doi: 10.1016/j.ygyno.2007.08.095. [DOI] [PubMed] [Google Scholar]
- 26.Hiraku Y, Tabata T, Ma N, Murata M, Ding X, Kawanishi S. Nitrative and oxidative DNA damage in cervical intraepithelial neoplasia associated with human papilloma virus infection. Cancer Sci. 2007;98:964–72. doi: 10.1111/j.1349-7006.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes PC, Jr, Garcia CB, Micheli DC, Cunha FQ, Murta EF, Tavares-Murta BM. Circulating neutrophils may play a role in the host response in cervical cancer. Int J Gynecol Cancer. 2007;17:1068–74. doi: 10.1111/j.1525-1438.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 28.Murta BMT, Resende AD, Cunha FQ, Murta EFC. Local profile of cytokines and nitric oxide in patients with bacterial vaginosis and cervical intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2008;138:93–9. doi: 10.1016/j.ejogrb.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Woo YL, Sterling J, Damay I, Coleman N, Crawford R, van der Burg SH, et al. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG. 2008;115:1616–22. doi: 10.1111/j.1471-0528.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 30.Bell MC, Schmidt-Grimminger D, Turbat-Herrera E, Tucker A, Harkins L, Prentice N, et al. HIV1 Patients Have Increased Lymphocyte Infiltrates in CIN Lesions. Gynecol Oncol. 2000;76:315–9. doi: 10.1006/gyno.1999.5716. [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves MAG, Soares EG, Donadi EA. The influence of human papillomavirus type and HIV status on the lymphomononuclear cell profile in patients with cervical intraepithelial lesions of different severity. Infect Agent Cancer. 2009;4:11. doi: 10.1186/1750-9378-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petter A, Heim K, Guger M, Ciresa-Ko Nig A, Christensen N, Sarcletti M, et al. Specific serum IgG, IgM and IgA antibodies to human papillomavirus types 6, 11, 16, 18 and 31 virus-like particles in human immunodeficiency virus-seropositive women. J Gen Virol. 2000;81:701–8. doi: 10.1099/0022-1317-81-3-701. [DOI] [PubMed] [Google Scholar]