Abstract
The cancer preventive properties of grape products such as red wine have been attributed to polyphenols enriched in red wine. However, much of the studies on cancer preventive mechanisms of grape polyphenols have been conducted with individual compounds at concentrations too high to be achieved via dietary consumption. We recently reported that combined grape polyphenols at physiologically relevant concentrations are more effective than individual compounds at inhibition of ERα(−), ERβ(+) MDA-MB-231 breast cancer cell proliferation, cell cycle progression, and primary mammary tumor growth (Schlachterman et al., Transl Oncol 1:19–27, 2008). Herein, we show that combined grape polyphenols induce apoptosis and are more effective than individual resveratrol, quercetin, or catechin at inhibition of cell proliferation, cell cycle progression, and cell migration in the highly metastatic ER (−) MDA-MB-435 cell line. The combined effect of dietary grape polyphenols (5 mg/kg each resveratrol, quercetin, and catechin) was tested on progression of mammary tumors in nude mice created from green fluorescent protein-tagged MDA-MB-435 bone metastatic variant. Fluorescence image analysis of primary tumor growth demonstrated a statistically significant decrease in tumor area by dietary grape polyphenols. Molecular analysis of excised tumors demonstrated that reduced mammary tumor growth may be due to upregulation of FOXO1 (forkhead box O1) and NFKBIA (IκBα), thus activating apoptosis and potentially inhibiting NfκB (nuclear factor κB) activity. Image analysis of distant organs for metastases demonstrated that grape polyphenols reduced metastasis especially to liver and bone. Overall, these results indicate that combined dietary grape polyphenols are effective at inhibition of mammary tumor growth and site-specific metastasis.
Keywords: Breast cancer, Catechin, Metastasis, Quercetin, Resveratrol
Introduction
Breast cancer is the most commonly diagnosed form of cancer and the second major cause of death from cancer in women [1, 2]. Recent clinical advances have remarkably increased the survival rates from primary breast cancer; however, the prognosis of breast cancer patients is still limited by metastases that can occur years after initial diagnosis and potential cure. Malignant breast cancers often overexpress epidermal growth factor receptor (EGFR) isoforms such as Her-2 that further confound effective treatment of metastatic breast cancer [3]. Therefore, investigation of the effect of dietary alternatives and their mechanisms of action specifically on Her-2 over-expressing metastatic cancers can lead to alternative therapeutic strategies.
Grape skins and thus red wine, contain many polyphenols that have anticancer properties [4, 5]. Grape polyphenols have been implicated in cancer protection in numerous in vitro studies due to antioxidant and pro-apoptotic effects as well as inhibition of a number of tumorigenic pathways [6–8]. Combined grape polyphenols extracted from red wine have been shown to specifically inhibit the growth of breast cancer cells with low cytotoxicity towards normal mammary epithelial cells [9]. However, the effects of grape polyphenols on metastatic breast cancer remain to be investigated.
Resveratrol, quercetin, and catechin, grape polyphenols selected for this study, represent about 70% of the total polyphenols in red wine and have been shown to be the most effective anticancer compounds in red wine [8, 10]. Resveratrol is found in low, but significant amounts in red wine and comprises about 1% of total polyphenols [10, 11]. In breast cancer, resveratrol has been implicated in prevention of multistage carcinogenesis [12, 13]. Quercetin comprises about 6% of total polyphenols in red wine [10] and has been reported to decrease Her-2 expression [14]. Her-2 is often overexpressed in metastatic cancers including the MDA-MB-435 cell line that was used for this study. The monomeric form of catechin constitutes up to 65–70% of total red wine polyphenols and has been shown to delay tumor initiation [10, 15, 16]. Resveratrol, quercetin, and catechin are all viable chemopreventives because they are absorbed and metabolized rapidly in vivo and can be detected in plasma and urine samples in the intact form in humans and rodent models [17–20].
Individually, resveratrol, quercetin, or catechin induce cell cycle arrest and apoptosis in cancer cells [21–23], prevent breast carcinogenesis and cancer progression in rodent models [24–26], and inhibit angiogenesis [27]. Much of the data on the cancer preventive effects of grape polyphenols have been generated from estrogen receptor (ER) (+) tissue culture cell lines and rodent models using pharmacological concentrations of individual polyphenols [16, 24–26, 28, 29]. We previously reported that in ERα(−), ERβ(+) MDA-MB-231 breast cancer cells, resveratrol is inhibitory at high pharmacological concentrations and acts similar to estrogen by increasing cell functions and signaling relevant for metastasis at low dietary levels [30, 31]. However, the effects of combined grape polyphenols at low, dietary concentrations are only now beginning to be assessed.
Recently, we reported that combined resveratrol, quercetin, and catechin (RQC) treatment at physiologically relevant concentrations was more efficient than individual grape polyphenols at inhibition of cell proliferation, cell cycle progression, and primary mammary tumor growth of MDA-MB-231 cells [1]. In this study, we were limited in investigation of the role of grape polyphenols as potential metastasis preventives because the low metastatic MDA-MB-231 cell line formed only a few lung metastases. Most breast cancers preferentially metastasize to bone and liver, where ~80% of patients with advanced breast cancer develop bone cancer, causing severe morbidity and mortality [32]. Therefore, for the current study, we selected a bone metastatic variant of the highly metastatic cancer cell line, MDA-MB-435 [33], to test the effect of grape polyphenols on cell proliferation, cell cycle progression, apoptosis, cell migration, tumor growth, and metastatic progression. Mammary fat pad tumors were established in nude mice and we show that dietary grape polyphenols inhibit both primary tumor growth and metastatic cancer progression from the breast to bone and liver. Our results show that this inhibition may be due to upregulation of caspase 3 activity and expression of FOXO1 transcription factor and NFKBIA; molecules known to regulate cancer progression [34–36].
Materials and methods
Cell culture
Human metastatic cancer cell lines MDA-MB-231 (ERα−, ERβ+) (American Type Culture Collection, Manassas, VA, USA) and a bone metastatic variant of MDA-MB-435 (ER−) stably expressing GFP were used for the study (kind gift of Dr. Danny Welch, The University of Alabama at Birmingham, AL, USA) [37]. Cells were cultured in DMEM with 10% heat-inactivated FBS as described in [1, 33].
Cell proliferation and cell cycle progression
MDA-MB-435 (2 × 105) cells in 5% charcoal-stripped FBS were treated every 48 h for 96 h with vehicle (0.2–0.5% DMSO), 0.5, 5, or 20 μM resveratrol, quercetin, or catechin or a combination RQC at 0.5, 5, or 20 μM each. Cells were fixed, nuclei stained with PI and cell proliferation quantified as the number of cells with intact nuclei. Cell cycle stage of MDA-MB-435 cells was determined by flow cytometry of PI-stained cells as previously described in [1], following treatment with 5 μM resveratrol, quercetin, or catechin or 5 μM RQC every 48 h for 96 h.
Caspase 3 activity assay
Apoptosis was analyzed by the caspase 3 activity of cell lysates following vehicle (0.2% DMSO) or 0.5 or 5 μM RQC for 48 h using a Caspase-3 Colorimetric Assay Kit as per manufacturer’s instructions (Sigma–Aldrich, St Louis, MO, USA). Briefly, the p-nitroaniline (pNA) moiety resulting from hydrolysis of acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase 3 activity was detected at 405 nm (εmM = 10.5) after incubating the reaction mixture at 37°C for 22 h. The concentration of the pNA released from the substrate was calculated from the absorbance values at 405 nm using a calibration curve prepared with pNA standards. Concentration of pNA was further converted to caspase 3 activity in μmol of pNA min−1 ml−1.
Annexin V staining
Apoptotic cells were detected by fluorescence microscopy of Annexin V-Cy3-18 stained cells as per manufacturer’s instructions (Sigma–Aldrich, St Louis, MO, USA). Briefly, MDA-MB-435 cells grown on coverslips were treated with vehicle (0.2% DMSO) or 5 μM RQC for 48 h and stained with Annexin V-Cy3-18 in binding buffer (10 mM HEPES/NaOH, pH 7.5, 0.14 M NaCl, 2.5 mM CaCl2) for 15 min at room temperature. Coverslips were washed in binding buffer and fixed with 3.7% paraformaldehyde prior to fluorescence microscopy. Images were digitally acquired from an Olympus inverted fluorescence microscope using Metamorph software (Molecular Devices, Sunnyvale, CA, USA) and quantified from ten random microscopic fields (20× mag.)/coverslip.
Cell migration
Equal numbers of viable quiescent GFP-tagged MDA-MB-231 or MDA-MB-435 cells (1 × 105) were placed in the top well of Transwell chambers where the bottom well contained vehicle (0.2% DMSO), 0.5 or 5 μM resveratrol, quercetin, or catechin or 0.5 or 5 μM RQC in serum-free and phenol red-free media. Following 8 h incubation, the cells on top of the membrane of the inner well were removed and the number of cells that migrated to the underside of the membrane through 8 μm diameter pores quantified following PI staining as described in [31].
Animals
Female athymic nu/nu mice, 5–6 week old (Charles River Laboratories, Inc., Wilmington, MA, USA) were maintained under pathogen-free conditions in Hepa-filtered cages under controlled light (12 h light and dark cycle), temperature (22–24°C), and humidity (25%). Throughout the experiment, the animals were provided with autoclaved AIN 76-A phytoestrogen-free diet (Tek Global, Harlan Teklad, Madison, WI, USA) and water ad libitum. This project was approved by the Universidad Central del Caribe Institutional Animal Care and Use Committee.
Tumor model
GFP-MDA-MB-435 cells (~1 × 106) in Matrigel (BD Biosciences, San Jose, CA, USA) were injected into the fourth right mammary fat pad of female nude mice under isoflurane inhalation to produce orthotopic primary tumors as described in [38]. After tumor establishment (1 week post-inoculation), the animals were randomly divided into experimental treatment groups. About 3–5 animals per group were eliminated due to failure of tumor take, small or too large tumor area in 1 week, or due to penetration of the peritoneum that resulted in immediate GFP fluorescence in the intestines. Mice with similar tumor area as quantified by integrated density of fluorescence images were selected for further study.
Diet administration
Nude mice (n = 10/experimental group) were orally gavaged either with vehicle (90% corn oil, 10% ethanol) or a combination of 5 mg/kg body weight (BW) resveratrol, 5 mg/kg BW quercetin, and 5 mg/kg BW catechin (RQC) in a 100 μl volume three times per week. The number of mice/group is in the range of previously published similar studies that demonstrated statistically significant differences in dietary treatments [39–41].
Whole body fluorescence image analysis
Mammary tumor growth was quantified as changes in intensity and integrated density of GFP-fluorescence as per our previously described methods [1, 42]. Anesthetized mice were imaged immediately following breast cancer cell inoculation and two times per week thereafter. A 300 Watt power source with two optical delivery systems fitted with excitation filters (470/40 nm) was used for whole body imaging of GFP fluorescence (LT99D2, Lightools Research, Encinitas, CA, USA). Images were captured with a Spot II charge-coupled device (CCD) camera (Diagnostic Instruments, Sterling Heights, MI, USA) mounted with a 530/25 nm emission filter (Chroma Technology, Rockingham, VT, USA).
Tumor fluorescence intensities were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The final images were acquired on day 77. Relative tumor area was calculated as the fluorescence intensity of each tumor on each day of imaging relative to the fluorescence intensity of the same tumor on day 1 of diet administration.
Analysis of metastases
Following sacrifice, lungs, kidneys, livers, femurs, and hearts were excised and immediately stored in liquid N2. Stored organs were thawed and analyzed using an Olympus MV10 fluorescence macro zoom system microscope and images acquired with an Olympus DP71 digital camera. Each organ was imaged on both sides. The fluorescent lesions (green component of RGB images) were quantified for integrated intensity and area using Image J software. Pixel values ranging from 0 to 255 were detected and a signal cut off of 58 (approximately one standard deviation above the mean of the maximum noise) was used to separate background signal from GFP signal. To eliminate potential false positives, a minimum fluorescent area threshold of 0.003 mm2 was used (roughly four pixels). Areas identified as metastases were also validated by visual inspection and false positives eliminated from further analysis.
Real time reverse transcriptase (RT2)-PCR analysis
At the end of the study, solid primary tumors at the mammary fat pad were immediately stored in “RNA later” (Ambion, Austin, TX, USA). Total RNA extraction and DNAase treatment was performed using the Qiagen RNeasy Kit (Qiagen, Valencia, CA, USA) following manufacturer’s protocol. RNA concentration was detected using a NanoDrop (Thermo Scientific, Wilmington, DE, USA), while RNA integrity and quality analysis were evaluated using the Experion automated electrophoresis system (BioRad, Hercules, CA, USA). C-13 kit (SA Biosciences, Frederick, MD, USA) was used to synthesize cDNA from the extracted RNA (0.5 μg) and used to investigate gene expression profiles by the commercially-available phosphoinositide 3-kinase (PI3-K) Pathway Finder RT2 Profiler™ PCR arrays (SA Biosciences, Frederick, MD, USA). This RT2 Profiler™ PCR Array is designed to simultaneously profile the expression of 84 PI3-K pathway-specific genes, plus five housekeeping genes and seven RNA quality controls. The spreadsheets, gene tables, and template formulas included with the PCR array package were used to calculate relative changes in gene expression and perform statistical analyses. Reproducibility was maintained by using RNA from three tumors per treatment (three biological replicates).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analyses were done using Microsoft Excel or GraphPad Prism 5 software. Differences between means were determined using Student’s t-Test and two-way ANOVA.
Results and discussion
Effect of grape polyphenols on metastatic breast cancer cells in vitro
Previously, we demonstrated that a combination of resveratrol, quercetin, and catechin at 0.5, 5, or 20 μM reduced cell number significantly from control and was more efficient than individual compounds in the MDA-MB-231 ERα (−) ERβ (+) human low metastatic breast cancer cell line [1]. However, due to the low metastatic nature of this cell line, we did not observe adequate metastases in a nude mouse model to enable a statistical analysis of the role of grape polyphenols on metastasis. Therefore, we tested the effect of dietary RQC on a highly metastatic ER (−) cancer cell line, MDA-MB-435. The origin of the MDA-MB-435 cell line has been called into question by several recent microarray studies that show expression of melanoma-associated genes [43]. However, MDA-MB-435 cells express breast differentiation-specific proteins and secrete milk lipids [44]. Since the patient had no evidence of melanoma but was diagnosed with only a breast carcinoma; and, since melanocytes do not produce milk, the simplest conclusion is that MDA-MB-435 is a very poorly differentiated breast carcinoma. This cell line has been extensively used to investigate metastasis from mammary fat pad inoculations, and remains as one of few models available for experimental metastasis of breast cancer in nude mice [33, 45].
As shown in Fig. 1a, 0.5 or 5 μM treatment with resveratrol, quercetin, or catechin alone did not decrease MDA-MB-435 cell proliferation. Resveratrol or quercetin at high concentrations (20 μM) significantly inhibited cell proliferation by 80 and 60% while catechin alone increases cell proliferation significantly. Therefore, the effects on cell proliferation at 20 μM RQC appear to be an additive effect of resveratrol and quercetin. The combined RQC treatment significantly inhibited MDA-MB-435 cell proliferation by ~50, 80, and 90% at 0.5, 5, or 20 μM of each polyphenol (Fig. 1a). These compounds were more effective in the MDA-MB-231 cell line where both resveratrol and quercetin inhibited cell proliferation at 5 and 20 μM by ~60 and ~95%; while combined resveratrol, quercetin, and catechin (RQC) treatment at 0.5, 5, and 20 μM each inhibited cell proliferation by ~60, 85, and 98%, respectively, compared to vehicle controls [1]. Since combined RQC treatment induced a significant reduction on MDA-MB-435 cell proliferation, we then tested the cell cycle stage of MDA-MB-435 cells following 5 μM RQC treatment and found that these compounds arrested MDA-MB-435 cells at S phase as was shown before with MDA-MB-231 cells (Fig. 1b), [1]. However, unlike with the MDA-MB-231 cells, the S phase arrest of the MDA-MB-435 cells in response to RQC demonstrated a P value of 0.06.
Fig. 1.
Effect of grape polyphenols on MDA-MB-435 cell proliferation and cell cycle progression. Cells in 5% serum and phenol red-free media were treated with vehicle, 0.5, 5, or 20 μM resveratrol, quercetin, or catechin, or a combination of 0.5, 5, or 20 μM each (RQC) every 48 h for 96 h. Data was quantified from PI-stained intact (non-apoptotic) nuclei. a Cell proliferation. Percentage of viable cells ± SEM for 20 microscopic fields/triplicate treatments is presented. b Cell cycle progression. Cell cycle stage following 5 μM treatment with individual resveratrol, quercetin, or catechin or combined RQC. An asterisk indicates statistical significance of P < 0.05 and three asterisks indicate P < 0.001 when compared to vehicle
The method we used to recover cells for analysis of cell cycle stage (i.e., trypsinization followed by centrifugation, fixing, staining, and washing) does not account for potentially apoptotic, non-adherent, or weakly adherent cells that may become removed during the repeated washings. Moreover, the observed increase in cells at S-phase does not correlate with the 80% decrease in cell number observed with 5 μM RQC (Fig. 1a, b). Therefore, we investigated the effect of RQC treatment on apoptosis of MDA-MB-435 cells by caspase 3 activity. This downstream effector caspase was selected to assess the effect of RQC on both receptor-regulated and mitochondrial apoptotic pathways. As shown in Fig. 2a, 5 μM RQC treatment increased caspase 3 activity by twofold at a P < 0.06 when compared to vehicle, while RQC at 0.5 μM did not affect caspase 3 activity in a significant manner. Similarly, Annexin V staining of MDA-MB-435 cells to detect phosphatidyl serines on the outer leaflet of the cell membrane indicated that at 48 h following 5 μM RQC, 44% of cells were significantly apoptotic (P < 0.01) compared to only 6.8% of vehicle-treated cells (Fig. 2b). Resveratrol and quercetin at high concentrations have been implicated in apoptosis of cancer cell lines by inducing caspase activity and inhibition of cell survival via PI3K/Akt pathways [23, 46, 47]. In MDA-MB-231 cells, by western blotting with total Akt and phospho-Aktser-473 antibodies, 5 μM RQC (15 min) was found to decrease Akt activity by ~50% compared to vehicle (data not shown). Therefore, the observed decrease in breast cancer cell numbers in response to RQC treatment is thought to be due to a block in cell cycle progression, increased apoptosis, and reduced cell survival signaling.
Fig. 2.
Effect of grape polyphenols on apoptosis of MDA-MB-435 cells. Apoptosis of MDA-MB-435 cells was detected by caspase 3 activity assays (a) or fluorescence microscopy for Annexin V staining (b) following 48 h incubation with vehicle or RQC. a Average caspase 3 activity in μmol of pNA min−1 ml−1 relative to vehicle (n = 4 ± SEM) as quantified from absorbance at 405 nm of the pNA released by caspase 3 activity. b Percentage of cells undergoing apoptosis was calculated by Image J analysis of brightfield (total number of cells) and red fluorescence (apoptotic cells stained with Annexin V-Cy3) from ten random microscopic fields/coverslip. Averages ± SEM are shown for two separate experiments with duplicates for each experiment (n = 4). An asterisk indicates statistical significance (P < 0.05) when compared to vehicle
Since directed cell migration has been implicated with metastatic efficiency, we tested the effect of individual and combined grape polyphenols on cell migration. Migration assays were performed using Transwell chambers where individual resveratrol, quercetin, or catechin or combined RQC treatment was added to the bottom well while the inner well contained serum-starved MDA-MB-231 or MDA-MB-435 cells. In MDA-MB-231 cells, resveratrol and quercetin at 0.5 μM increased cell migration in a statistically significant manner (Fig. 3a). The effect of resveratrol is similar to our previous results that reported low concentrations of resveratrol to act comparable to estrogen and increase cell migration [31]. None of the other grape polyphenols significantly changed breast cancer cell migration. At 0.5 and 5 μM, combined RQC treatment significantly reduced MDA-MB-231 cell migration by ~60% when compared to vehicle controls; whereas, 0.5 μM combined RQC treatment reduced MDA-MB-435 cell migration by ~20%, and 5 μM RQC significantly reduced cell migration by 40% (Fig. 3).
Fig. 3.
Effect of grape polyphenols on breast cancer cell migration. Quiescent MDA-MB-231 (a) or MDA-MB-435 (b) cells were placed on the top well of Transwell chambers in serum-free, phenol red-free media and the number of cells that migrated through the membrane of the top well in response to various treatments was quantified relative to control. Data are quantified from analysis of 25 microscopic fields/treatment (n = 3 ± SEM). The bottom well contained the following for 8 h: vehicle, 0.5 or 5 μM resveratrol, quercetin, catechin or a combination of 0.5 or 5 μM each (RQC). An asterisk indicates statistical significance (P < 0.05) when compared to vehicle
The lower response of the inhibitory effect of RQC treatment in the ER (−) MDA-MB-435 cells compared to the ERβ (+) MDA-MB-231 cells may be due to grape polyphenols acting as antiestrogenic compounds in the MDA-MB-231 cells. Also, since MDA-MB-435 cells are Her2++ it is possible that combined grape polyphenols are not as efficient at inhibiting the increased Her-2 signaling in this highly aggressive cancer cell line. However, mechanistic studies need to be conducted to further address the differences in response to grape polyphenols between these two cancer cell lines.
Effect of grape polyphenols on mammary tumor growth in vivo
To test the effect of resveratrol, quercetin, and catechin on metastatic breast cancer progression in vivo, we established mammary fat pad tumors from GFP-tagged highly meta-static MDA-MB-435 cancer cells as previously described [42]. As quantified from the integrated density of fluorescent images, mice (n = 10 per group) with similar initial tumor volumes (vehicle group = 9,036.6 ± 654 and RQC group = 9,825 ± 501) were selected for further study.
One week following tumor establishment, mice were gavaged with vehicle (90% oil, 10% ethanol) or 5 mg/kg BW RQC three times a week. This dietary concentration was selected based on our previous study where administration of 0.5, 5, or 20 mg/kg BW RQC demonstrated that the inhibitory effect on mammary tumor growth plateaued at 5 mg/kg BW [1]. Tumor progression was quantified by fluorescence image analysis twice a week. The relative tumor area was calculated as the fluorescence intensity of each tumor on day of imaging relative to the fluorescence intensity of the same tumor on day 1 of diet administration as described in [1]. As shown in Fig. 4a, tumor growth remained linear and similar for both vehicle and RQC treated mice for 60 days. After 60 days, the RQC-treated mice demonstrated reduced tumor growth compared to vehicle. At the end of the study on day 77, the mice following RQC diet demonstrated smaller tumors that were reduced by ~37% in a statistically significant manner (Fig. 4b). Previously, we reported a 69% decrease in MDA-MB-231 mammary tumor growth with 5 mg/kg BW RQC treatment [1]. The present data demonstrates that, as with the in vitro effects, dietary RQC treatment of nude mice with MDA-MB-435 mammary tumors results in a significant inhibition of primary tumor growth but this effect is less compared to the effect of RQC treatment on MDA-MB-231 mouse mammary xenografts.
Fig. 4.
Effect of grape polyphenols on the growth of MDA-MB-435 mammary fat pad tumors. MDA-MB-435 cells (106) in Matrigel were inoculated at the mammary fat pad of nude mice. One week following injection, mice were fed vehicle or a combination of 5 mg/kg BW Res, 5 mg/kg BW Quer, and 5 mg/kg BW Cat (RQC) three times a week by oral gavage. Whole body fluorescence images were acquired two times a week. a Average relative tumor area as a function of days following injection. Relative tumor area was calculated as the area of fluorescence, measured by fluorescence intensity on each day of imaging as a function of the fluorescence intensity of the same tumor on day 1. b GFP-MDA-MB-435 tumors following vehicle or RQC diets at day 77. Representative digital images and mammary tumor area as quantified from digital images acquired on day 77, made relative to same tumor image on day 1. Asterisk denotes statistical significance at P < 0.05
At the end of the study (77 days), there were no statistically significant differences in body weights from mice treated with vehicle (24.35 g ± 1.6) or RQC (24.04 g ± 1.7). This is similar to our previous report where 118 days of dietary RQC treatment at concentrations as high as 25 mg/kg BW did not significantly change mouse weights from vehicle controls [1]. Therefore, the decrease in tumor area at the end of the study was not due to toxic effects of dietary RQC. This data indicates that combined grape polyphenols can be safe and effective therapeutics and preventives of primary tumor growth of ER (−) breast cancer.
To initiate a molecular analysis of the effect of grape polyphenols on breast cancer, we analyzed changes in expression of PI3-K pathway genes because this pathway is a central regulator of cancer cell survival and invasion [48]. Interestingly, real-time PCR arrays for PI3-K pathway genes from tumor extracts revealed that expression of FOXO1 transcription factor was upregulated significantly by 1.87-fold in mouse mammary tumors following RQC treatment (Table 1). FOXO factors have been shown to function as tumor suppressors in a variety of cancers. They are actively involved in promoting apoptosis in a mitochondria-independent and -dependent manner by inducing the expression of death receptor ligands and pro-apoptotic Bcl-2 family members [35]. Forkhead proteins have been shown to be important for the anticancer activities of resveratrol [49]. This is the first time that elevation of death promoting genes have been implicated in vivo for a combination of dietary grape polyphenols in reducing mammary tumor growth. Since FOXO1 can be inactivated via Akt-mediated phosphorylation, elevation of FOXO1 transcripts may not necessarily result in increased protein activity. Interestingly, AKT1 expression was also decreased by threefold in the PCR array but this was not statistically significant. Moreover, RQC treatment of breast cancer cells significantly reduces active phospho Akt1 (p-Akt ser473) levels in 15 min without affecting total Akt1 levels (data not shown). The relative contribution of decreased phospho Akt1 levels and increased FOXO1 levels and caspase 3 activity to RQC-mediated effects on cell survival and apoptosis is currently under investigation.
Table 1.
Effect of RQC treatment on expression of PI3-kinase pathway genes
| Gene | Fold difference RQC/vehicle | P value |
|---|---|---|
| FOXO1, forkhead box 01A | 1.87 | 0.007 |
| NFKBIA, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | 1.70 | 0.041 |
| TLR4, toll-like receptor 4 | 1.91 | 0.003 |
Only genes that demonstrated >1.5-fold difference and P value of <0.05 from RT2 PCR arrays are shown
NfκB transcription factor that regulates tumorigenic and immunomodulatory signaling is a potential target for the chemopreventive activity of grape polyphenols, resveratrol, and quercetin [50–53]. NFKBIA, which codes for IκB, the subunit that sequesters NfκB in an inactive state, was also upregulated significantly by 1.7-fold in mammary tumors following RQC treatment (Table 1). This data indicates that inhibition of NfκB signaling may contribute to the observed reduced mammary tumor growth and metastasis by grape polyphenols. However, IκB proteins can become inactivated via phosphorylation-induced, proteasome-mediated degradation by IκB kinase (IKK) activity. Therefore, increased NFKBIA gene expression by RQC treatment may not reflect increased stable protein levels. Future experiments will determine the stability and phosphorylation status of IκB in response to RQC. In addition, we also found a significant increase in Toll-like receptor 4 (TLR4) expression that have been implicated in cancer progression (Table 1). However, TLRs may also stimulate apoptosis under certain conditions [54] and can be negatively regulated by PI3-K signaling [55]. Therefore, the significance of this result warrants further investigation.
Previous in vivo studies have also supported a role for grape polyphenols in cancer prevention. Grape juice, grape seed extract, and red wine have been shown to significantly reduce cancer in rodent models [56–58]. Grape skin extract, which is concentrated in red wine, was recently shown to contain more growth inhibitory effects than grape pulp, juice, or seeds on mouse mammary tumor growth [59]. Since the effect of grape polyphenols on cancer metastasis remains to be evaluated, we next analyzed the effect of dietary grape polyphenols on breast cancer metastasis.
Effect of grape polyphenols on metastasis
Our macro imaging system easily detected surface primary tumors, local invasion into the circulatory system, lymphatics, and metastatic tumors in the GI tract through the skin of nude mice. However, the resolution of this imaging system allows detection of only ~104 GFP-tagged cells and is thus limited in sensitivity for detection of micrometastases. Therefore, fluorescent metastatic lesions were quantified by microscopy following surgical removal of organs. Only eight mice/treatment were used for analysis of metastasis due to early death of two from the vehicle group and one from the RQC group. This number is similar to a previous study that reported the effect of dietary genistein on metastasis from MDA-MB-435 mammary tumors [39]. All of the mice following vehicle or RQC treatment presented with lung metastases. Therefore, lungs were further analyzed by Image J for quantification of the area of fluorescence. The number of metastatic lesions/lung was reduced in RQC-treated mice in a statistically significant manner when compared to vehicle treatment. However, the area of fluorescence calculated from these lesions was not statistically different for mice treated with vehicle or RQC (Fig. 5a, b, c; Table 2). Therefore, we conclude that RQC treatment does not block metastases to the lung from this cancer cell variant.
Fig. 5.
Effect of grape polyphenols on lung metastasis. Following necropsy, lungs were excised from mice with GFP-MDA-MB-435 mammary tumors that received either vehicle or RQC diets and analyzed for metastases by fluorescence microscopy followed by quantitative image analysis. a Green fluorescence image of a representative lung demonstrating analysis of traced fluorescence area. b Lung metastatic efficiency expressed as average area of fluorescence from lungs of vehicle or RQC treated mice ±SEM (n = 8). c Average number of fluorescent metastatic foci/lung for vehicle or RQC treated mice. Asterisk denotes statistical significance at P < 0.05
Table 2.
Distant metastases in mice following vehicle or combined resveratrol, quercetin, and catechin (RQC) treatment
| Number of mice with metastases (N = 8) | Number of metastatic lesions/organ with metastases | |||
|---|---|---|---|---|
| Veh | RQC | Veh | RQC | |
| Bone | 5 | 2 | 11.4 | 3 |
| Heart | 4 | 1 | 1.5 | 1 |
| Kidney | 3 | 3 | 15.7 | 1.33 |
| Liver | 8 | 2 | 22 | 11 |
| Lung | 8 | 8 | 34.7 | 15.7 |
| Lymph node | 3 | 3 | 2 | 1.7 |
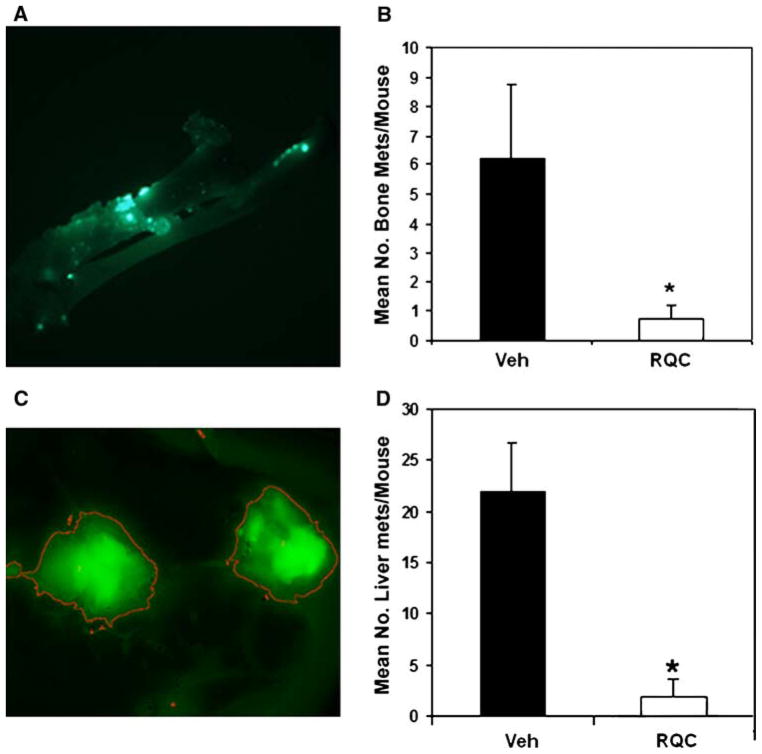
The MDA-MB-435 cell line used in this study was selected as a bone metastatic variant by intracardiac injection in nude mice [33]. Since breast cancers preferentially metastasize to the bone [60], this trend was simulated by inoculating the MDA-MB-435 bone metastatic variant into the mammary fat pad. The ability of the GFP-MDA-MB-435 mammary tumors to invade bone was investigated by fluorescent image analysis of excised, cleaned femurs from mice on vehicle control or RQC diets as described in [33]. In vehicle controls, 5/8 mice presented with bone metastases while only 2/8 mice following RQC treatment demonstrated fluorescent metastatic foci in femurs. Of the mice with bone metastases, the number of metastatic lesions were higher for vehicle treated mice compared to RQC treatments in a statistically significant manner (Fig. 6a, b; Table 2). Similarly, the number of mice with liver metastases and the number of metastatic lesions/liver were also significantly lower in RQC treated mice compared to vehicle controls (Fig. 6c, d; Table 2). The mean fluorescent area or integrated density for a single metastatic lesion were similar for bone or liver metastases from vehicle or RQC treated mice (data not shown); however, very few the RQC-treated mice presented bone or liver metastases and exhibited lower numbers of metastatic foci/organ.
Fig. 6.
Effect of grape polyphenols on bone and liver metastasis. Following necropsy, femurs and livers were excised from mice with GFP-MDA-MB-435 mammary tumors that received either vehicle or RQC diets and analyzed for metastases by fluorescence microscopy followed by quantitative image analysis. a Green fluorescence image of a representative femur from vehicle treated mouse. b Average number of fluorescent metastatic foci/femur for vehicle or RQC treated mice. c Green fluorescence image of a representative liver from vehicle treated mouse. d Average number of fluorescent metastatic foci/liver for vehicle or RQC treated mice. Asterisk denotes statistical significance at P < 0.05
Table 2 also shows that less RQC treated mice presented with heart metastases when compared to vehicle treated mice. However, the number of metastatic foci/heart in RQC treated mice was only slightly less when compared to vehicle. When kidneys were examined for metastases, the number of mice with kidney metastases did not change but there were more metastatic lesions/kidney in the vehicle treated mice. Only three mice for vehicle or RQC treatments demonstrated lymph node metastases. Since the numbers of mice with heart, kidney, or lymph node metastases were low even for vehicle treatments, it is not possible to analyze these results in a statistically significant manner or derive definitive conclusions on the effect of RQC treatment compared to controls.
To our knowledge, this is the first report of an inhibitory effect of grape polyphenols on breast cancer metastasis. The differential effects of RQC on site-specific metastases indicate that RQC treatment did not inhibit metastatic cancer cells from being released to the lymphatics or the vascular system from the primary tumors at the mammary fat pad. RQC treatment also did not block the entry of cells into the lungs, where all of the mice in the study presented with lung metastases. Interestingly, subsequent metastases to the bone and liver were reduced by RQC treatment, indicating that these compounds may affect establishment of further metastases either by regulation of exit from the lung vasculature or at the entry points of localized signaling at liver and bone microenvironments. Our intriguing data that demonstrates upregulation of NFKBIA levels in mammary tumors following RQC treatment implicates inhibition of NfκB signaling by dietary grape polyphenols as a potential pathway that regulates breast cancer progression. Interestingly, NfκB signaling has been associated with bone and liver metastasis [61–63]. The mechanistic basis for these interesting possibilities and the ability of grape polyphenols to specifically inhibit components of the bone and (or) liver molecular signature are currently under investigation.
Acknowledgments
We acknowledge the excellent technical assistance of Alexander Schlachterman, Felix Valle, and Alina De La Mota-Peynado with the animal protocols. This research was supported by grant numbers AICR IIG 03-31-06 and DoD/BCRP W81XWH-07-1-0330 to SD; DoD/BCRP W81XWH-08-01-0258 to LCP; NCCR/NIH 2G12RR003035, S06GM050695, and G11HD052352 to UCC; and NIH/RCMI G12-RR03051 and MBRS-RISE 5R25GM061838-08 to UPR-MSC. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR, NICHD, NIGMS or the National Institutes of Health.
Contributor Information
Linette Castillo-Pichardo, Department of Biochemistry, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, PR, USA.
Michelle M. Martínez-Montemayor, Department of Anatomy and Cell Biology, School of Medicine, Universidad Central del Caribe, P.O. Box 60327, Bayamón, PR 00960-6032, USA
Joel E. Martínez, Department of Anatomy and Cell Biology, School of Medicine, Universidad Central del Caribe, P.O. Box 60327, Bayamón, PR 00960-6032, USA
Kristin M. Wall, Department of Anatomy and Cell Biology, School of Medicine, Universidad Central del Caribe, P.O. Box 60327, Bayamón, PR 00960-6032, USA
Luis A. Cubano, Department of Anatomy and Cell Biology, School of Medicine, Universidad Central del Caribe, P.O. Box 60327, Bayamón, PR 00960-6032, USA
Suranganie Dharmawardhane, Email: surangi@uccaribe.edu, Department of Biochemistry, School of Medicine, University of Puerto Rico, Medical Sciences Campus, San Juan, PR, USA. Department of Anatomy and Cell Biology, School of Medicine, Universidad Central del Caribe, P.O. Box 60327, Bayamón, PR 00960-6032, USA.
References
- 1.Schlachterman A, Valle F, Wall KM, Azios NG, Castillo L, Morell L, Washington AV, Cubano LA, Dharmawardhane SF. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori. 2008;94:370–383. doi: 10.1177/030089160809400314. [DOI] [PubMed] [Google Scholar]
- 4.Peregrin T. Wine—a drink to your health? J Am Diet Assoc. 2005;105:1053–1054. doi: 10.1016/j.jada.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 5.de Lorimier AA. Alcohol, wine, and health. Am J Surg. 2000;180:357–361. doi: 10.1016/S0002-9610(00)00486-4. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Park EJ, Pezzuto JM. Botanicals in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:231–255. doi: 10.1023/A:1021254725842. [DOI] [PubMed] [Google Scholar]
- 8.Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–441. doi: 10.1002/1097-4644(20000901)78:3<429::AID-JCB8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85:65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- 10.Faustino RS, Sobrattee S, Edel AL, Pierce GN. Comparative analysis of the phenolic content of selected Chilean, Canadian and American Merlot red wines. Mol Cell Biochem. 2003;249:11–19. doi: 10.1023/A:1024745513314. [DOI] [PubMed] [Google Scholar]
- 11.Nigdikar SV, Williams NR, Griffin BA, Howard AN. Consumption of red wine polyphenols reduces the susceptibility of low-density lipoproteins to oxidation in vivo. Am J Clin Nutr. 1998;68:258–265. doi: 10.1093/ajcn/68.2.258. [DOI] [PubMed] [Google Scholar]
- 12.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 13.Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles JM, Lopez-Soriano FJ. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett. 2007;245:144–148. doi: 10.1016/j.canlet.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Jeong JH, An JY, Kwon YT, Li LY, Lee YJ. Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J Cell Biochem. 2008;105:585–595. doi: 10.1002/jcb.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 16.Ebeler SE, Brenneman CA, Kim GS, Jewell WT, Webb MR, Chacon-Rodriguez L, MacDonald EA, Cramer AC, Levi A, Ebeler JD, Islas-Trejo A, Kraus A, Hinrichs SH, Clifford AJ. Dietary catechin delays tumor onset in a transgenic mouse model. Am J Clin Nutr. 2002;76:865–872. doi: 10.1093/ajcn/76.4.865. [DOI] [PubMed] [Google Scholar]
- 17.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomark Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 18.Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2002;35:119–124. doi: 10.1016/S0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 19.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 20.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 21.Nifli AP, Kampa M, Alexaki VI, Notas G, Castanas E. Polyphenol interaction with the T47D human breast cancer cell line. J Dairy Res. 2005;72(Spec No):44–50. doi: 10.1017/S0022029905001172. [DOI] [PubMed] [Google Scholar]
- 22.Kim YA, Choi BT, Lee YT, Park DI, Rhee SH, Park KY, Choi YH. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol Rep. 2004;11:441–446. [PubMed] [Google Scholar]
- 23.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anti-cancer Res. 2006;26:1177–1181. [PubMed] [Google Scholar]
- 24.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15–25. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Dechsupa S, Kothan S, Vergote J, Leger G, Martineau A, Beranger S, Kosanlavit R, Moretti JL, Mankhetkorn S. Quercetin, Siamois 1 and Siamois 2 induce apoptosis in human breast cancer MDA-MB-435 cells xenograft in vivo. Cancer Biol Ther. 2007;6:56–61. doi: 10.4161/cbt.6.1.3548. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Cao R, Brakenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/S0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 28.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 29.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61:7456–7463. [PubMed] [Google Scholar]
- 30.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia (New York, NY) 2005;7:128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia (New York, NY) 2007;9:147–158. doi: 10.1593/neo.06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 33.Phadke PA, Mercer RR, Harms JF, Jia Y, Frost AR, Jewell JL, Bussard KM, Nelson S, Moore C, Kappes JC, Gay CV, Mastro AM, Welch DR. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12:1431–1440. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krajewski S, Krajewska M, Turner BC, Pratt C, Howard B, Zapata JM, Frenkel V, Robertson S, Ionov Y, Yamamoto H, Perucho M, Takayama S, Reed JC. Prognostic significance of apoptosis regulators in breast cancer. Endocr Relat Cancer. 1999;6:29–40. doi: 10.1677/erc.0.0060029. [DOI] [PubMed] [Google Scholar]
- 35.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes SM, Rodriguez FV, Sanchez PI, Perona R. The role of the NFkappaB signalling pathway in cancer. Clin Transl Oncol. 2008;10:143–147. doi: 10.1007/s12094-008-0171-3. [DOI] [PubMed] [Google Scholar]
- 37.Welch DR, Harms JF, Mastro AM, Gay CV, Donahue HJ. Breast cancer metastasis to bone: evolving models and research challenges. J Musculoskelet Neuronal Interact. 2003;3:30–38. [PubMed] [Google Scholar]
- 38.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 39.Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65:3396–3403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 40.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma PC-3 tumor xenograft. Carcinogenesis. 2007;28:2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 41.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14:300–308. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 42.Carlson AL, Hoffmeyer MR, Wall KM, Baugher PJ, Richards-Kortum R, Dharmawardhane SF. In situ analysis of breast cancer progression in murine models using a macroscopic fluorescence imaging system. Lasers Surg Med. 2006;38:928–938. doi: 10.1002/lsm.20409. [DOI] [PubMed] [Google Scholar]
- 43.Lacroix M. MDA-MB-435 cells are from melanoma, not from breast cancer. Cancer Chemother Pharmacol. 2009;63:567. doi: 10.1007/s00280-008-0776-9. [DOI] [PubMed] [Google Scholar]
- 44.Sellappan S, Grijalva R, Zhou X, Yang W, Eli MB, Mills GB, Yu D. Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res. 2004;64:3479–3485. doi: 10.1158/0008-5472.CAN-3299-2. [DOI] [PubMed] [Google Scholar]
- 45.Price JE, Zhang RD. Studies of human breast cancer metastasis using nude mice. Cancer Metastasis Rev. 1990;8:285–297. doi: 10.1007/BF00052605. [DOI] [PubMed] [Google Scholar]
- 46.Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh SH, Kim SH, Kwon H, Park Y, Kim KS, Song CW, Kim J, Kim MH, Yu HJ, Henkel JS, Jung HK. Epigallocatechin gallate protects nerve growth factor differentiated PC12 cells from oxidative-radical-stress-induced apoptosis through its effect on phosphoinositide 3-kinase/Akt and glycogen synthase kinase-3. Brain Res Mol Brain Res. 2003;118:72–81. doi: 10.1016/j. molbrainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Cantley LC. The role of phosphoinositide 3-kinase in human disease. Harvey Lect. 2004;100:103–122. [PubMed] [Google Scholar]
- 49.Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem. 2007;282:19385–19398. doi: 10.1074/jbc.M702452200. [DOI] [PubMed] [Google Scholar]
- 50.Terra X, Valls J, Vitrac X, Merrillon JM, Arola L, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, Blay M. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J Agric Food Chem. 2007;55:4357–4365. doi: 10.1021/jf0633185. [DOI] [PubMed] [Google Scholar]
- 51.Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Salguero PM. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005;115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 52.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemo-resistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Mediavilla V, Crespo I, Collado PS, Esteller A, Sanchez-Campos S, Tunon MJ, Gonzalez-Gallego J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Wolska A, Lech-Marańda E, Robak T. Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cell Mol Biol Lett. 2008 doi: 10.2478/s11658-008-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/S1471-4906(03)00139-X. [DOI] [PubMed] [Google Scholar]
- 56.Martinez C, Vicente V, Yanez J, Alcaraz M, Castells MT, Canteras M, Benavente-Garcia O, Castillo J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol Histopathol. 2005;20:1121–1129. doi: 10.14670/HH-20.1121. [DOI] [PubMed] [Google Scholar]
- 57.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 58.Kim H, Hall P, Smith M, Kirk M, Prasain JK, Barnes S, Grubbs C. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004;134:3445S–3452S. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]
- 59.Morre DM, Morre DJ. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006;238:202–209. doi: 10.1016/j.canlet.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 60.Kapoor P, Suva LJ, Welch DR, Donahue HJ. Osteoprotegrin and the bone homing and colonization potential of breast cancer cells. J Cell Biochem. 2008;103:30–41. doi: 10.1002/jcb.21382. [DOI] [PubMed] [Google Scholar]
- 61.Ignatoski KM, Escara-Wilke JF, Dai JL, Lui A, Dougall W, Daignault S, Yao Z, Zhang J, Day ML, Sargent EE, Keller ET. RANKL inhibition is an effective adjuvant for docetaxel in a prostate cancer bone metastases model. Prostate. 2008;68:820–829. doi: 10.1002/pros.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cicek M, Oursler MJ. Breast cancer bone metastasis and current small therapeutics. Cancer Metastasis Rev. 2006;25:635–644. doi: 10.1007/s10555-006-9035-x. [DOI] [PubMed] [Google Scholar]
- 63.Meir T, Dror R, Yu X, Qian J, Simon I, Pe’er J, Chowers I. Molecular characteristics of liver metastases from uveal melanoma. Invest Ophthalmol Vis Sci. 2007;48:4890–4896. doi: 10.1167/iovs.07-0215. [DOI] [PubMed] [Google Scholar]