Summary
The sperm plasma membrane is known to be critical to fertilization and to be highly regionalized into domains of head, mid- and principal pieces. However the molecular composition of the sperm plasma membrane and its alterations during genital tract passage, capacitation and the acrosome reaction remains to be fully dissected. A two dimensional gel- based proteomic study previously identified 98 human sperm proteins which were accessible for surface labelling with both biotin and radioiodine. In this report twelve dually labelled protein spots were excised from stained gels or PDVF membranes and analysed by mass spectrometry (MS) and Edman degradation. Seven members from four different heat shock protein (HSP) families were identified including HYOU1 (ORP150), HSPC1 (HSP86), HSPA5 (Bip), HSPD1 (HSP60), and several isoforms of the two testis-specific HSP70 chaperones HSPA2 and HSPA1L. An antiserum raised against the testis specific HSPA2 chaperone reacted with three 65 kDa HSPA2 isoforms and three high molecular weight surface proteins (78–79 kDa, 84 kDa and 90–93 kDa). These proteins, together with seven 65 kDa HSP70 forms, reacted with human anti-sperm IgG antibodies that blocked in vitro fertilization in humans. Three of these surface biotinylated human sperm antigens were immunoprecipitated with a rabbit antiserum raised against a linear peptide epitope in Chlamydia trachomatis HSP70. The results indicate diverse HSP chaperones are accessible for surface labelling on human sperm. Some of these share epitopes with Chlamydia trachomatis HSP70, suggesting an association between genital tract infection, immunity to HSP70 and reproductive failure.
Keywords: Human Sperm, Proteomics, Surface Proteins, Heat Shock Proteins, Immunoinfertility, Chlamydia trachomatis
Introduction
Mammalian heat shock proteins (HSP) are evolutionarily highly conserved molecular chaperones, which appear to have derived from prokaryotic ancestors that originally evolved to solve problems in protein folding (Mayer et al., 2005). HSPs are induced in cells exposed to elevations in temperature, chemical or physical stress, viral infection, drugs and transforming agents, where they mediate cytoprotective effects through maintenance of protein homeostasis and blockade of caspase dependent apoptosis (Lindquist and Craig, 1988, Beere, 2004). Stress induced HSPs act by aiding the refolding of denatured proteins, thereby preventing adverse metabolic effects arising from accumulation of misfolded proteins and proteotoxic cell death (Beere, 2004, Vos et al., 2008). Physiological events such as cell growth, differentiation, development and aging can also induce the synthesis of HSPs (Calderwood et al., 2006, Bukau et al., 2006).
While some members of the HSP-families are strictly inducible by stress, others are constitutively expressed and less susceptible to heat shock induction. Under normal physiological conditions constitutively expressed HSPs assist in the folding and trafficking of proteins, the assembly of multi-protein structures, and the translocation of proteins across membranes (Bukau et al., 2006). Due to their ability to maintain proteins in an inactive form, thus preventing premature folding and nonproductive interactions between newly synthesized proteins, and their capacity to determine the fate of misfolded client proteins (processing via the refolding/storage or proteasomal degradation pathways), cytosolic HSP chaperones are essential for normal protein quality control and homeostasis within the cell (Vos et al., 2008, Bukau et al., 2006).
Heat shock proteins make up a group of structurally unrelated proteins which are divided in mammals into the HSP100 (HSPH), HSP90 (HSPC), HSP70 (HSPA), HSP60 (HSPD), and HSP27 (HSPB) families. The human genome contains several members of each gene family, some with a high degree of sequence homology, others with a substantial sequence divergence. Part of this redundancy may relate to the intra-compartmental distribution of the diverse family members and their tissue or development specific expression patterns (Vos et al., 2008). The existence of different HSP families or family members within the same compartment indicates a need for specialized chaperone functions and suggests that the chaperones are not merely promiscuous in terms of client proteins (Vos et al., 2008).
HSP chaperones and their cofactors form high molecular weight, multi-protein complexes that bind to and stabilize non-native hydrophobic conformations on protein surfaces, forming relatively stable complexes. While the activity and oligomerization of small HSP (HSPB) is regulated by phosphorylation, the substrate specificity of larger chaperones (such as HSPA, HSPC) is at least in part determined by interacting co-chaperones (Vos et al., 2008, Panaretou et al., 2002) and their ATP/ADP binding. The ATP-dependent HSPA chaperone machine, for example, constantly shuttles between ATP-bound and ADP-bound states, which have different affinities for client proteins (Held et al., 2006).
In addition to the intracellular transcription and translation of HSPs, stress also triggers the release of HSPs into extracellular spaces. Indeed stress proteins such as HSPs 27, 60, 70, 90 and 110, and glucose regulated proteins (GRP) 78, 94, 170 and calreticulin are released from cells in a variety of circumstances, and subsequently interact with adjacent cells or may enter the bloodstream (Calderwood et al., 2007). A variety of cell types secrete stress proteins, including neuronal cells, monocytes, macrophages, B cells and tumor cells of epithelial origin (Robinson et al., 2005, Clayton et al., 2005, Davies et al., 2006).
Membrane bound or extracellularly located HSP60 and HSP70 are thought to act as physiological alarm signals for cell trauma (Calderwood et al., 2007, Multhoff, 2007). Several cancers have been found to express HSP members on the cell surface (Shin et al., 2003, Hastie et al., 2005), and elevated levels of HSPs are frequently detected in serum from cancer patients (Calderwood et al., 2007). Recent studies have revealed that HSPs are key regulators of both the innate and the adaptive immune system (Van Eden et al., 2007), and due to their role as carriers for cell-specific immunogenic peptides, tumor-derived HSPs are regarded as potent stimulators of immune responses against cancer (reviewed in Multhoff, 2007).
Several HSP family members are expressed in the mammalian testis and sperm. Testisspecific HSP70 forms (HSP70-2 and HSC70T) were first described in mice (Allen et al., 1988), where they are regulated developmentally and expressed specifically in spermatogenic cells (Eddy, 1999). Their homologues were later discovered in rat and human testis (Eddy, 1999, Son et al., 1999). HSP70-2 has been shown to be necessary for the progression of meiosis in mouse germ cells (Eddy, 1999). The human homologue is termed HSPA2 and differs from mouse HSP70-2 by four amino acids. HSPA2 additionally contains a six amino acid sequence near the carboxy terminal end not present in mice and rats (Bonnycastle et al., 1994).
Besides HSPA2, two other human heat shock proteins HSPA1L and HSPH3 are also mainly expressed in the testis (Vos et al., 2008). HSPH3 knockout mice show defects in spermatogenesis (Held et al., 2006), suggesting that this chaperone, like HSPA2, has a unique role during germ cell differentiation (Vos et al., 2008). The expression of HSPA1L peaks in spermatides and is not influenced by heat (Ito et al., 1998).
Heat shock proteins have been detected on the surface of mouse, rat, bull, boar, and human sperm, and HSP70 family members appear to be abundant components of the sperm surface (Boulanger et al., 1995, Miller et al., 1992). HSP70 antigens were localized over the entire human sperm surface by immunofluorescence (Miller et al., 1992), and the two HSP70 family members HSPA2 and HSPA1L were among the human sperm membrane antigens recognized by antisperm antibodies from seminal plasma samples of infertile men (Bohring & Krause, 2003). A recent study by Mitchell and colleagues, however, failed to detect HSP chaperones on the human sperm surface by either an immunobead binding assay or FACS analysis (Mitchell et al., 2007), emphasizing a well-known verity, namely that the outcome of an antibody-based sperm surface analysis is significantly influenced by the employed antibodies.
Some HSP family members have been shown to be the subjects of dynamic redistribution as the sperm undergoes capacitation and acrosome reaction. While HSP70 is relocalized from the acrosome to the equatorial segment, post-acrosomal region and midpiece during capacitation and the acrosome reaction in bull sperm (Kamaruddin et al., 2004), the chaperone is both relocalized and translocated from the inner to the outer leaflet of the plasma membrane following acrosome reaction of boar sperm (Spinaci et al., 2005). HSP60 (HSPD1) and GRP94 (HSP90B1) undergo tyrosine phosphorylation and become exposed on the cell surface during the capacitation of mouse sperm (Asquith et al., 2004, Asquith et al., 2005).
The present study was undertaken to identify and characterize proteins expressed on the surface of ejaculated human sperm. Twelve abundant surface protein spots, previously shown to be accessible for labelling with both biotin and radioiodine on the surface of motile sperm (Naaby-Hansen et al., 1997) were identified on stained two dimensional (2-D) gels by computer assisted image analysis. The proteins were carefully excised and analysed by mass spectrometry (MS) and/or Edman degradation yielding seven different HSP family members, and identifying HYOU1 and HSPD1 as new HSP chaperones on the human sperm surface. Serum IgG from an infertile female patient that had been shown to completely inhibit in vitro fertilization in humans (Clarke et al., 1988) interestingly recognized HSPA2 and HSPA1L sperm surface proteins as dominant antigens. Four biotin labelled sperm surface antigens with molecular weights of 90 kDa, 78 kDa, 65 kDa and 60 kDa were immunopurified with a monospecific, polyclonal rabbit antiserum raised against a linear peptide epitope in Chlamydia trachomatis HSP70. Three of these were among the sperm surface antigens recognized by the infertile female patients’ serum. The functional and immunological implications of these findings are discussed.
Materials and Methods
Preparation of Human Spermatozoa
Semen specimens were obtained from normal, healthy young men by masturbation. Individual semen samples were allowed to liquify at room temperature and the mature spermatozoa were separated from seminal plasma, immature germ cells and non-sperm cells by Percoll density gradient centrifugation and/or by the swim-up procedure. All samples were obtained under informed consent using protocols and forms approved by the University of Virginia Human Investigation Committee.
Surface labeling procedures
Sperm surface proteins accessible to radioiodination following Iodo-Bead oxidation and proteins exposing primary amines accessible to the N-hydroxysuccinimide ester of sulfo- NHS-LC-Biotin were labelled as previously described (Naaby-Hansen et al., 1997).
Solubilization Procedures
Purified spermatozoa were solubilized in a lysis buffer containing: 2% (v/v) NP-40; 8.8 M urea; 2% (v/v) ampholines pH 3.5–10; and the protease inhibitors 2 mM PMSF, 5 mM iodoacetamide, 5 mM EDTA, 3 mg/ml TLCK, 1.46 mM Pepstatin A and 2.1 mM Leupeptin. 5 × 108 cells per ml were solubilized by constant shaking at 4°C for 60 min. DTT was added to a final concentration of 100 mM, and insoluble material removed by centrifugation at 10,000 × g for 5 min. The protein concentration in the supernatant was determined by the bicinchoninic acid method, employing bovine serum albumin as a standard.
Protein separation and analysis
Analytical two-dimensional gel electrophoresis (IEF/PAGE) in 16 × 16 cm gel format was performed as previously described (Naaby-Hansen et al., 1997). The following ampholine composition: 20% pH 5–7, 20% pH 7–9 and 60% pH 3.5–10, was used for analytical and preparative two-dimensional gel electrophoresis in large format gels (23 × 23 cm). Proteins separated by preparative 2-D gel electrophoresis were visualized by Coomassie staining. The centre of the spot of interest was carefully excised from the gel trypsinized and analysed by tandem mass spectrometry. Electrotransfer to PVDF membranes (0.2 mm pore size, Pierce) was carried out as described by Henzel et al. (1993) using the transfer buffer composition of Matsudaira (1987) (10mM 3-[cyclohexylamino]-1-propanesulfonic acid, 10% methanol, pH 11). Proteins were visualized by staining in a solution containing 0.1% Coomassie R250, 40% methanol and 0.1% acetic acid for one minute, followed by destaining in a solution of 10% acetic acid and 50% methanol for 3 × 3 minutes. The centre of the Coomassie stained spot was carefully cut from the PVDF membrane and microsequenced by Edman degradation. Mass spectrometry analysis and Edman degradation were performed at the Biomolecular Research Facility, University of Virginia. Lectin blotting was performed as previously described (Naaby-Hansen et al., 1985).
Immunological analysis
Antiserum against the major 65 kDa human sperm HSP70 form was generated as follows: Spot number 4.3, identified as HSPA2 by microsequencing, was excised from 3 Coomassie stained preparative 2-D gels. The gel pieces were washed twice in PBS and minced using a scalpel blade. The resulting fragments were placed in 1 ml of PBS, mixed an equal volume of complete Freunds adjuvant and emulsified. The emulsion was injected subcutaneously into virgin female New Zealand rabbits. The rabbits were boosted with gel purified antigen at 20 and 40 days following the initial injection, with incomplete Freunds adjuvant replacing complete Freunds adjuvant. Blood was drawn 10 days following the second boost and the serum was aliquoted and stored at −20C.
Incubation with antibody was sometimes preceded by Protogold staining of nitro cellulose blotting membranes in order to identify the exact location of an antigen in the complex 2-D protein pattern. Here, antibody binding was visualized employing DAB (diaminobenzidine) as a substrate for HRP-conjugated secondary antibodies and the staining enhanced by the addition of 2 mM NiCl2. In some experiments secondary antibodies was employed alone as a control.
Immuno precipitation from biotin labelled cells
Fresh, swim-up purified sperm were biotinylated, harvested by percoll density gradient centrifugation and washed three times in cell culture media. The labelled cells were solubilized in a PBS buffer containing 1% TX-100, PMSF, Pepstatin A, and Leupeptin. Insoluble material was removed by centrifugation at 10,000 G for 5 min, and the supernatant used for immunoprecipitation. Following separation by SDS-PAGE, immunopurified surface proteins were detected by affinity blotting with HRP-avidin and enhanced chemiluminescence (ECL).
Computer Analysis
2-D images were analyzed using the Bio Image “2-D Analyzer,” version 6.1 (Bio Image, Ann Arbor, MI). The software automates the identification, quantification, and inter-gel comparison of 2-D gel separated protein spots, as described previously (Naaby-Hansen et al., 1997).
Results
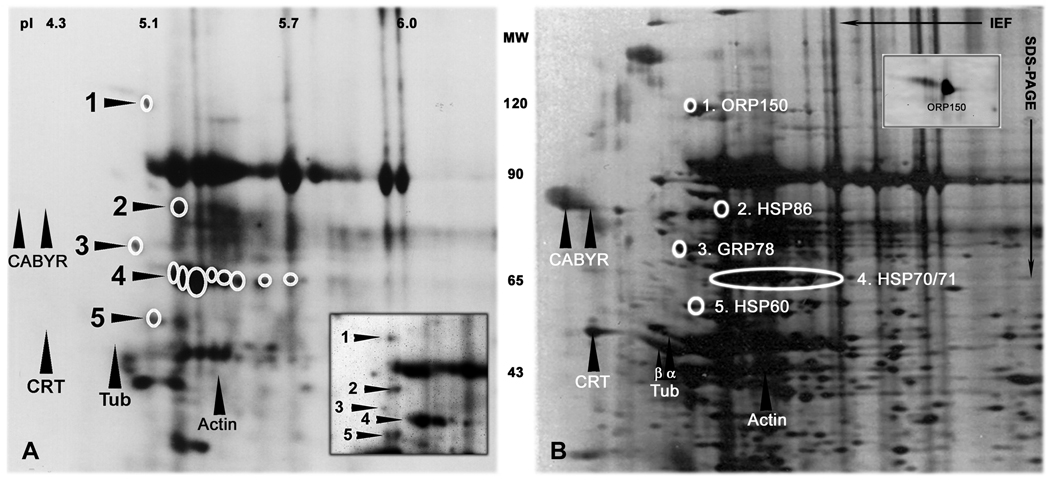
Ninety-eight human sperm surface proteins were previously mapped by a combination of sulfo-N-hydroxysuccinimide biotin and radioiodine labelling, two-dimensional gel electrophoresis and computer assisted image analysis (Naaby-Hansen et al., 1997). Twelve dually labelled, putative cell surface protein spots (designated 1–5 in Figure 1A), including cluster 4 containing eight protein spots, were identified on stained gels or PVDF membranes (Figures 1 and 2), excised and microsequenced by mass spectrometry and/or Edman degradation.
Figure 1.
Two-dimensional gel electrophoretic analysis of high molecular weight, acidic human sperm surface proteins
A: Human sperm surface proteins vectorially labeled with sulfo-NHS-LC-biotin, and visualized using HRP-conjugated avidin and ECL. The positions of the twelve protein spots selected for coring and microsequencing are indicated by circles. Fig. 2 provides an expanded 2-D gel with consecutive numbering of the isoforms within the cluster at position 4. The targeted proteins were also vectorially labeled with radioiodine (insert). Intracellular proteins, such as CABYR, calreticulin (CRT), tubulin (Tub), and actin were not biotinylated (upward arrowheads; compare with silver stain in B). Densitometric analysis revealed that the 12 HSP variants analysed comprise approximately 8% of all dually labelled sperm surface proteins with MW 5–150 kDa, and pI 4–7. The pH gradient of the first-dimensional gels is indicated at the top of the figures. The MW of the proteins is indicated between the images.
B: Area of interest from silver stained 2-D gel of human sperm proteins. The targeted proteins are circled and their identity is given to the right. SDS-PAGE was performed with the anode at the bottom of the gels. Concanavalin A bound strongly to surface protein number 1, suggesting that ORP150 (HYOU1) is expressed on the human sperm surface as a glycoprotein (insert).
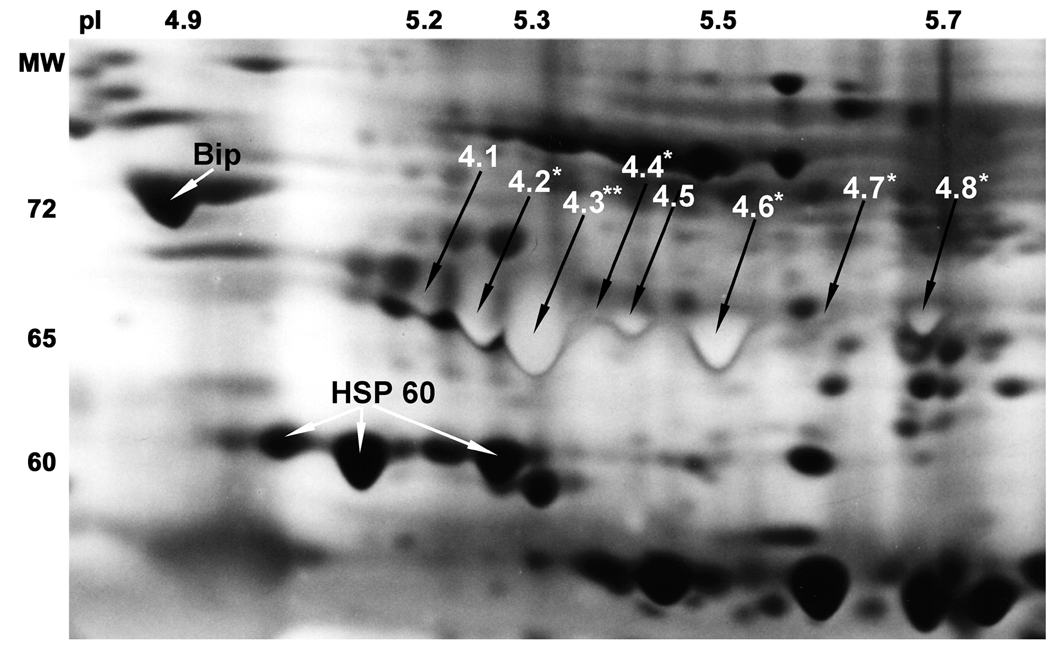
Figure 2.
Expansion of the sperm proteome in the region showing the position of the microsequenced 65 kDa HSP70 proteins.
Enhanced resolution of region of interest was achieved with an ampholine composition of 20% pH 3.5–5, 25% pH 5–7, 10% pH 7–9 and 45% pH 3.5–10 in the first dimension. The unique light staining of the eight 65 kDa surface exposed HSP70 forms was achieved by using four times the normal concentration of ammonium hydroxide in the silver nitrate solution, aiding their identification and excision from silver stained gels. The eight proteins in position 4 are numbered from 4.1 to 4.8. Amino acid substitutions were found in peptides from 6 of the protein spots (stars). The positions of Bip (HSPA5) and HSP60 (HSPD1) are indicated by white arrows. The most acidic HSP60 form was microsequenced, while the two basic forms were identified by immunoblotting.
Eight peptide sequences were obtained from protein spot number 1 (MW115 kDa and pI 5.1), identifying the protein as HYOU1 (oxygen regulated protein 150 [ORP150]), a heat shock protein 70-family member with sequence similarities to the atypical family members HSP110 and GRP170 (Fig. 1). HYOU1 has previously been detected on the surface of human carcinoma cell lines (Sahasrabuddhe et al., 2006), and on the plasma membrane of mature mouse oocytes (Calvert et al., 2003). However, this is the first demonstration of HYOU1 on the surface of mammalian sperm. HYOU1 has been reported to possess a short carbohydrate sidechain of importance for its ability to bind jacalin lectin on the surface of A431 cells (Sahasrabuddhe et al., 2006), and to translocate client proteins from the cytosol to the nucleus in HT29 cells (Yu et al., 2002). Human sperm HYOU1 was found to bind Concanavalin A (ConA) in lectin blotting experiments (insert in Fig. 1B), indicating the human sperm chaperone is similarly glycosylated.
Eleven N-terminal amino acids were obtained from protein number 2 (MW of 84 kDa and pI of 5.2) by Edman degradation, identifying the protein as HSPC1 (HSP 90-ALPHA or HSP86). HSP90 antigens have previously been localized to the neck and tail of unfixed human sperm by immunohistochemistry (Miller et al., 1992), and HSP86 becomes tyrosine phosphorylated during in vitro capacitation of mouse, rat and human sperm (Ecroyd et al., 2003).
Eight peptides from the tryptic digest of protein number 3 (MW of 72 kDa and pI of 5) were sequenced by CAD-MS, identifying the protein as HSPA5, also known as glucose regulated protein 78 or BiP, a member of the HSP70 family (Fig. 1).
Eight dually labelled protein spots with molecular weights ranging from 64 to 67 kDa (position 4 in Fig. 1) were identified on stained gels by computer-aided triangulation. Silver stained gels showed a unique, bright yellow staining pattern of the 8 surface labelled protein spots (numbered 4.1–4.8 in Fig. 2), which were excised and subjected to in-gel-trypsination. Mass spectrometry analysis revealed that all eight surface labelled 2-D spots contained proteins with homology to HSP70.
Amino acid sequences from four peptides were obtained from spot number 4.1, identifying the protein as HSPA8 a constitutively synthesized, ubiquitously expressed member of the HSP70 family. Peptide sequences from spot number 4.2 revealed three peptides from HSPA1L or HSPA1 A/B proteins, and three peptides from HSPA2 including one with the threonine to serine substitution at position 611 also found in the testis-specific mouse and rat homologues. Eight peptide sequences were obtained from spot 4.3, which along with 4 unique amino acid substitutions in two of the peptides identified the most abundant of the protein spots in group 4 as HSPA2. Seven peptide sequences were obtained from spot number 4.4. They were identical to those found in spot 4.3 including the alanine to threonine substitution at position 166, which is characteristic for HSPA2. Spots 4.5–4.8 were identified as four isoforms of another testis-specific HSP70 member, HSPA1L.
Sixteen N-terminal amino acids were sequenced from protein number 5 (MW of 61 and pI of 5.1) by Edman degradation, identifying the protein as HSPD1 (HSP60). While HSP60 chaperones mainly reside and function within the mitochondrion, they are also found on cell surfaces and in extra cellular fluids (Calderwood et al., 2007). HSPD1 becomes exposed on the surface of mouse sperm during capacitation (Asquith et al., 2004). The HSPD1 protein detected on the surface of human sperm lacked the mitochondrion localization peptide, which is normally situated at the N-terminus (aa 1–26) of the protein. This is the first demonstration of HSPD1 on the surface of human sperm.
Immunogenecity of human sperm heat shock proteins
In a benchmark study, Clarke and colleagues demonstrated that preincubation of donor sperm with female sera containing high titers of anti-sperm antibodies caused significant inhibition of in vitro fertilization in humans (Clarke et al., 1988). One of the six anti-sperm positive sera examined in the study was found to completely block IVF. Absorption with protein A abolished the serum’s ability to inhibit sperm-zona binding and block IVF. The female serum (# 629) was later shown to react with a prominent 65 kDa band in western blots of SDS- PAGE separated human sperm proteins (Clarke et al., 1995).
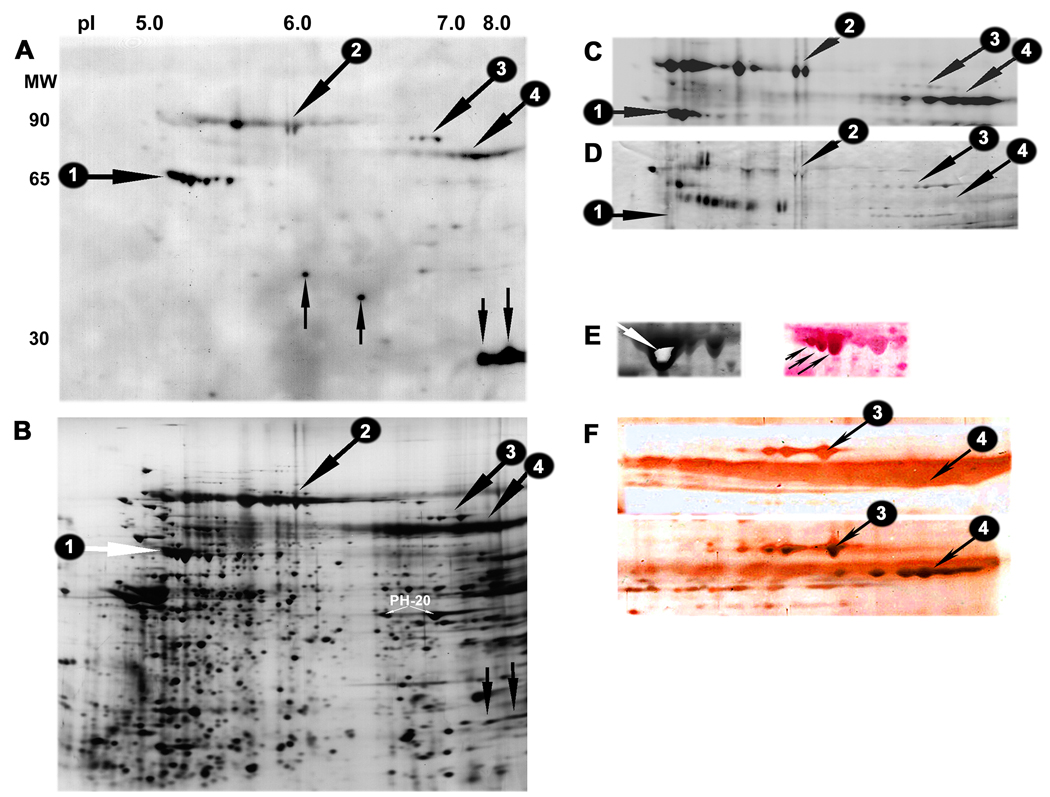
When serum 629 was used to stain 2-D blots of human sperm proteins, the prominent 65 kDa surface antigen detected by the IgG antibodies was found to consist of the testis-specific HSP70 family members, HSPA2 and HSPA1L (protein antigen 1 indicated by horizontal arrows in Fig. 3A–D). Six additional sperm antigens or charge-trains of similar sized antigens were detected by serum 629. Three of these groups of sperm antigens with MW of 90–93 kDa, 84 kDa and 78–79 kDa, were also accessible for vectorial labelling with biotin (antigens number 2 to 4, indicated by oblique downward arrows in Fig. 3A–D). The sperm surface antigens in group 3 were stained with HRP-ConA in affinity blotting experiments (Fig. 3D), indicating that they are modified by glycosylation.
Figure 3.
Immunogenecity of human sperm HSP
A: Two dimensional western blot demonstrating anti-sperm IgG antibody reactivity in patient serum number 629 towards seven of the eight HSP70 forms detected on the surface of human sperm (group #1 indicated by a horizontal arrow), as well as against two slightly basic proteins migrating at MW 27 kDa (downward vertical arrows). The serum also reacted with two sperm antigens of MW 34 kDa and 37 kDa, and three groups of proteins with MW 90–93 kDa, 84 kDa and 78–79 kDa, (respectively numbered 2–4 and indicated by oblique downward arrows).
B: Companion silver stained IEF/PAGE gel showing the position of the major antigens or groups of antigens detected by serum 629 in blot A.
C: Biotin label demonstrating surface exposure of the high molecular weight sperm antigen groups 1–4. The remaining major sperm antigens recognized by the serum were not accessible for surface labelling with neither biotin nor radioiodine (data not shown).
D: Two dimensional affinity blotting with HRP-conjugated Concanavalin A (ConA) demonstrating relatively strong staining of group 3 human sperm surface antigens. The HSP70 surface antigens did not bind ConA, while weak staining of the antigens in group 2 & 4 was observed.
E: Coomassie stained HSP70 forms from large preparative gel (left). The center of the most abundant HSPA2 isoform (spot 4.3 in Figure 2) was excised (oblique white arrow) and used as immunogen in female rabbits. The right image shows the same gel area on a gold stained NC-membrane. The membrane was subsequently stained with the antiserum raised against the excised HSPA2 form. Note the immunostaining of several HSP70 isoforms with approximate MW 65 kDa, including the three strongly stained species indicated by oblique black arrows.
F: High MW area of gold stained membranes incubated with the preimmune rabbit serum (top) and the antiserum against testis-specific HSP70 (bottom). Note the strong staining of sperm surface antigen groups 3 and 4 with the HSP70 antiserum.
The 34 kDa and 37 kDa sperm antigens recognized by serum 629 (upward vertical arrows in Fig. 3A) were neither labelled with biotin, radioiodine, nor lectin. Two strongly reactive antigens (downwards vertical arrows in Fig. 3A & 3B) were identified as outer dense fiber protein 1 (ODF1) isoforms. ODF1 is located on the outside of the axoneme and is known to be an immunodominant autoantigen in sperm (Flickinger et al., 1999 & 2001). However, none of these sperm antigens are likely to implement the IVF blocking effect of serum 629 as they are localized intracellularly.
Interestingly, the three high MW groups of surface antigens detected by serum 629 (proteins number 2–4 in Fig. 3) were also recognised by a polyclonal rabbit antiserum raised against the most abundant form of the testis-specific HSPA2 antigen (Fig. 3E & 3F and Supplementary Fig. 1). This suggests that all the serum 629 reactive human sperm surface isoantigens (proteins number 1 to 4 in Fig. 3) either belong to or are closely related to the HSP70 family of chaperones.
This observation instigated us to look for mechanisms by which such anti-HSP immunity might be induced. HSPs are immunodominant antigens in numerous microbial pathogens (Neuer et al., 2000) and both humoral and cellular immunity against microbial HSPs have been shown to cross-react with human HSPs (Van Eden et al., 2007). There is a high prevalence of asymptomatic persistent genital tract infections among infertile couples (Idahl et al., 2004), and Chlamydia trachomatis serum antibodies in men correlate with reduced chances of achieving pregnancy (Idahl et al., 2007). The authors found no correlations between serum IgG against ctHSP60 in the male partners and pregnancy rates.
To ascertain whether HSP70 family members might be involved in microbial-induced immune-mediated infertility, the molecular cross-reactivity between Chlamydia trachomatis HSP70 protein (ctHSP70) and antigens on the human sperm surface was investigated next.
Swim-up harvested sperm were labelled with sulfo-NHS-lc-biotin, solubilized and subjected to immunoprecipitation with pre-immune serum or a monospecific, polyclonal peptide rabbit antiserum raised against amino acid residues 341 to 368 of the C. trachomatis serovar E HSP70 protein. The antiserum is targeted against part of the hypothetical peptide interaction domain of the sea urchin sperm receptor, which comprises a linear epitope in Chlamydia HSP70 (J. Raulston, personal communication).
While a 65 kDa biotinylated sperm protein was immunoprecipitated with the preimmune rabbit serum, the rabbit antiserum isolated additional surface proteins with MW of approximately 80–90 kDa and 60 kDa (Fig. 4). Two dimensional gel analysis identified the biotinylated surface proteins that were immunopurified with the rabbit antiserum as HSPA2, HSPA1L, and the two groups of HSPA2 cross-reactive antigens with molecular weights of 78 kDa and 90 kDa, respectively, which were also recognized by serum 629 IgG antibodies (Supplementary Fig. 2). This suggests that an interaction domain on C. trachomatis HSP70 is immunological cross-reactive with several abundant antigens on the human sperm surface, and provides a putative mechanism that links genital tract infection, immunity to HSP70 and reproductive failure.
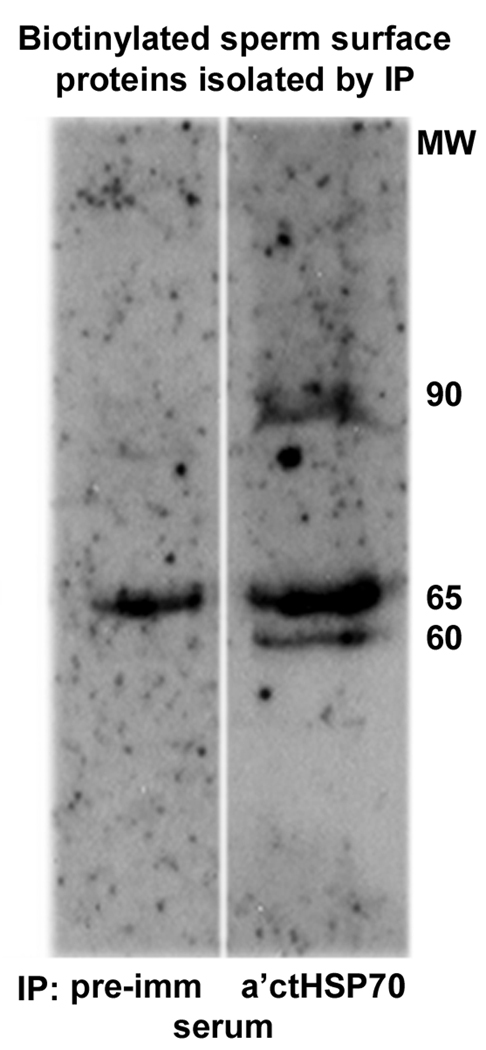
Figure 4.
Immuno precipitation of biotinylated human sperm proteins with rabbit antiserum against the HSP70-like membrane protein of Chlamydia trachomatis (ctHSP70).
Biotin-labelled sperm proteins precipitated with preimmune rabbit serum (left) and antiserum (right) against a putative peptide interaction domain of sea urchin sperm receptor (represented by aa 351–368 of ctHSP70). Immuno precipitated surface biotinylated proteins were detected by affinity staining with HRP-avidin. While abundant ~60 kDa and ~80–90 kDa sperm surface antigens were specifically purified with the anti-ctHSP70 antiserum, a common 65 kDa surface antigen was immune-precipitated with both the preimmune serum and the antiserum. However, computer densitometry analysis revealed that approximately four times more biotin labelled 65 kDa protein was isolated with the anti-ctHSP70 antiserum, compared to that precipitated with similar amounts of preimmune serum.
Discussion
Seven members from four different HSP families were found to be accessible for vectorial labelling with biotin and radioiodine on the surface of fresh motile human sperm. Known intracellular proteins such as CABYR, calreticulin, tubulin and actin remained unlabelled, validating the surface specificity of the employed procedures.
Chaperones of the HSP90 and HSP70 families have previously been associated with the human sperm surface. HSP90 antigens have been localized to the neck, midpiece and tail regions of acrosome intact, unfixed human sperm by immunofluorescence (Miller et al., 1992). HSP70 antigens have been detected over the whole human sperm surface (Miller et al., 1992, Boulanger et al., 1995), and the testis-specific family member HSPA2 was localized to the plasma membrane over the entire tail region by immunofluorescence (Huszar et al., 2000). More notably, in a recent proteomic study enriched human sperm membrane fractions were separated by two dimensional gel electrophoresis and membrane antigens were identified by immunoblotting with antisperm antibodies from seminal plasma samples of infertile men. Two of the six membrane autoantigens identified by mass spectrometry, were HSPA2 and HSPA1L (Bohring & Krause, 2003).
In one experiment, intact motile sperm were subjected to gentle surface trypsination and the released peptides isolated from the media by HPLC. Tandem MS analysis revealed four peptides from HSPA2 and four peptides from HSPC1 among the enzymatic generated products (R.E. Christian and S. Naaby-Hansen, unpublished data), further supporting the notion that these chaperones are exposed on the surface of the human sperm.
This is the first time ORP150 (HYOU1) has been detected on the surface of a mammalian sperm. ORP150 was recently shown to suppress hypoxia-induced apoptosis in human embryonic kidney cells (Ozawa et al., 1999) and to facilitate protein transport/maturation in an environment where less ATP is accessible (Bando et al., 2000). Based on these functional characteristics it is tempting to speculate that ORP150 participates in the protection of sperm membrane protein homeostasis and function during hypoxia-induced cellular perturbations, which are likely to occur as the gamete pass from the testis to the oviduct.
Although the testis-specific HSP forms HSPA2 and HSPA1L most likely become associated with the human sperm plasma membrane during spermatogenesis, the origin of surface bound HYOU1, HSPC1, HSPA5, HSPA8 and HSPD1 molecules remains to be determined. Recombinant HSPA5 and HSPD1 proteins have previously been shown to bind to human sperm (Lachance et al., 2007), and as the chaperones are present in seminal plasma (Miller et al., 1992, Pilch & Mann, 2006) they may simply be adsorbed onto the cell surface following ejaculation and semen liquidifaction. In this respect it is notable that the ATPase domain of HSP70 has been shown to bind 3’sulfogalactosylglycerolipid (SGG), the major glycolipid of mammalian sperm (Mamelak & Lingwood, 2001).
In the present study immunoprecipitation and blotting experiments demonstrated molecular mimicry between Chlamydia trachomatis HSP70 and the human sperm chaperones HSPD1, HSPA2 and HSPA1L. Molecular cross-reactivity was noted between HSPA2 and abundant high molecular weight human sperm surface isoantigens that were stained with IgG antibodies from serum 629, which blocks sperm-zona pellucida (ZP) binding and inhibits IVF in humans (Clarke et al., 1988 & 1995). Two of these high MW surface antigens with MW 78 kDa and 90 kDa were also recognised by a rabbit antiserum raised against ctHSP70 (Supplementary Fig. 2). Taken together these findings suggest an association between Chlamydia trachomatis infection, induction of humoral immunity against one or more epitopes shared by the Chlamydia and human HSP70-like antigens, and reproductive failure.
Humoral immunity to Chlamydia trachomatis has previously been correlated with the development of an autoimmune response to sperm (Witkin et al., 1995), and the appearance of antisperm antibodies in semen (Munoz et al., 1996). The presence of IgG antibodies against Chlamydia trachomatis in men from infertile couples has been correlated with decreased pregnancy rates (Idahl et al., 2004). IgG antibodies to ctHSP60 in men has been correlated with reduced sperm motility (Idahl et al., 2007). Positive Chlamydia serology (Eggert-Kruse et al. 1997, Idahl et al., 2004) and the presence of IgG antibodies to Chlamydia trachomatis HSP60 have been related to tubal factor infertility in woman (Dieterle and Wollenhaupt, 1996, Idahl et al., 2007). The presence of IgA antibodies against Chlamydia trachomatis HSPs in the cervix correlates with adverse IVF treatment outcome (Spandorfer et al., 1999). While several studies have shown how molecular mimicry between microbial and human HSP60 can affect fertilization through obstruction of gamete traffic, the present study suggests that molecular cross-reactivity between HSP70-like antigens from Chlamydia trachomatis and human sperm might also lead to antibody-mediated blockade of gamete interaction.
Antibodies against HSP70 have previously been shown to inhibit sperm-ZP interaction in boar and bovine studies (Spinaci et al., 2005, Matwee et al., 2001), and it has been proposed that sperm surface HSPs can function as adhesins, which mediate sulfoglycolipid recognition during germ cell binding (Boulanger et al., 1995, Mamelak & Lingwood, 2001). The identification of the human sperm surface antigens recognized by serum 629 IgG antibodies (Fig. 3) indicates that humoral immunity towards HSP70 epitopes can influence gamete interaction and fertilization in humans. However, we have previously detected IgG antibodies directed against human sperm HSP70 antigens in serum from fertile couples (Shetty et al., 1999, Shibahara et al., 2002). This suggests that the reproductive consequences of anti- HSP70 antibodies depends on factors such as antibody affinity and avidity, and/or the nature of the HSP70 epitope(s) involved, and suggests that a sperm-ZP assay may be beneficial for appropriate decision making in suspected immune-mediated infertility (Shibahara et al., 2003).
In conclusion, this study demonstrates that the constituency of HSP chaperones on the human sperm surface is far more diverse, abundant and immunogenically cross-reactive than previously recognized, and suggests that plasma membrane-associated chaperones serve multiple functions in human sperm, some of which appear to be critical for sperm-ZP interaction and fertilization. However, the biological role of the individual human sperm HSPs, their regulation and involvement in microbial-induced immune-mediated infertility remains to be defined in future studies.
Supplementary Material
Supplementary Figure 1. Enlarged high MW basic area of goldstained NC-membranes double stained with antiserum against human sperm HSPA2.
Human sperm extracts were separated on large-size (23 × 23 cm) IEF/SDS-PAGE gels, transferred to nitrocellulose membranes and stained with colloidal gold. The blots were subsequently used for western blotting with preimmune rabbit serum (top) and antiserum against HSPA2 (bottom). This antiserum reacted with the same three high MW groups of sperm surface antigens as were detected in 2-D western blot with infertile patient serum 629 (Figure 3). The immune-staining of the group 3 antigens is more pronounced in the image with artificial enhanced contrast (insert).
Supplementary Figure 2. Immunoprecipitation of biotinylated human sperm surface proteins with anti-Chlamydia trachomatis HSP70 (ctHSP70) antiserum.
Two dimensional gel analysis of biotin labelled human sperm surface proteins, isolated by immune-precipitation with antiserum raised against a putative peptide interaction domain in ctHSP70. Surface labelled proteins were detected by affinity blotting with HRP-avidin following 2-D gel electrophoretic separation. The numbered biotin labelled proteins indicated by black arrows corresponds to the similar numbered sperm antigens recognized by the infertile female serum 629 in Fig. 3. Two additional sperm surface proteins of MW 60 kDa and 90 kDa were also immunoprecipitated with the antiserum (white arrows).
Acknowledgement
We thank Dr. E. Goldberg for providing us with a sample of serum 629 and Dr. J.E. Raulston for the generous gift of rabbit pre-immune serum and antiserum against Chlamydia trachomatis HSP70. This study was supported by U54 NIH HD29099 to JCH, and by a grant from Karen Elise Jensens Foundation to SNH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RL, O'Brien DA, Jones CC, Rockett DL, Eddy EM. Expression of heat shock proteins by isolated mouse spermatogenic cells. Mol Cell Biol. 1988;8:3260–3266. doi: 10.1128/mcb.8.8.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci. 2004;117:3645–3657. doi: 10.1242/jcs.01214. [DOI] [PubMed] [Google Scholar]
- Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72:328–337. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- Bando Y, Ogawa S, Yamauchi A, Kuwabara K, Ozawa K, Hori O, Yanagi H, Tamatani M, Tohyama M. 150-kDa oxygen-regulated protein (ORP150) functions as a novel molecular chaperone in MDCK cells. Am J Physiol Cell Physiol. 2000;278:C1172–C1182. doi: 10.1152/ajpcell.2000.278.6.C1172. [DOI] [PubMed] [Google Scholar]
- Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- Bohring C, Krause W. Characterization of spermatozoa surface antigens by antisperm antibodies and its influence on acrosomal exocytosis. Am J Reprod Immunol. 2003;50:411–419. doi: 10.1034/j.1600-0897.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, Weber JL, Patterson D, Schellenberg GD. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23:85–93. doi: 10.1006/geno.1994.1462. [DOI] [PubMed] [Google Scholar]
- Boulanger J, Faulds D, Eddy EM, Lingwood CA. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility. J Cell Physiol. 1995;165:7–17. doi: 10.1002/jcp.1041650103. [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–94. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Calvert ME, Digilio LC, Herr JC, Coonrod SA. Oolemmal proteomics--identification of highly abundant heat shock proteins and molecular chaperones in the mature mouse egg and their localization on the plasma membrane. Reprod Biol Endocrinol. 2003;1:27. doi: 10.1186/1477-7827-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GN, Hyne RV, du Plessis Y, Johnston WI. Sperm antibodies and human in vitro fertilization. Fertil Steril. 1988;49:1018–1025. doi: 10.1016/s0015-0282(16)59954-3. [DOI] [PubMed] [Google Scholar]
- Clarke GN, Liu DY, Baker HW. Immunoinfertility: a case study with implications for immunocontraception. Arch Androl. 1995;35:21–27. doi: 10.3109/01485019508987849. [DOI] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- Davies EL, Bacelar MM, Marshall MJ, Johnson E, Wardle TD, Andrew SM, Williams JH. Heat shock proteins form part of a danger signal cascade in response to lipopolysaccharide and GroEL. Clin Exp Immunol. 2006;145:183–9. doi: 10.1111/j.1365-2249.2006.03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle S, Wollenhaupt J. Humoral immune response to the chlamydial heat shock proteins hsp60 and hsp70 in Chlamydia-associated chronic salpingitis with tubal occlusion. Hum Reprod. 1996;11:1352–1356. doi: 10.1093/oxfordjournals.humrep.a019387. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Jones RC, Aitken RJ. Tyrosine phosphorylation of HSP-90 during mammalian sperm capacitation. Biol Reprod. 2003;69:1801–1807. doi: 10.1095/biolreprod.103.017350. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Role of heat shock protein HSP70-2 in spermatogenesis. Rev. Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023. [DOI] [PubMed] [Google Scholar]
- Eggert-Kruse W, Rohr G, Demirakca T, Rusu R, Näher H, Petzoldt D, Runnebaum B. Chlamydial serology in 1303 asymptomatic subfertile couples. Hum Reprod. 1997;12:1464–1475. doi: 10.1093/humrep/12.7.1464. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Bush LA, Williams MV, Naaby-Hansen S, Howards SS, Herr JC. Post-obstruction rat sperm autoantigens identified by two-dimensional gel electrophoresis and western blotting. J Reprod Immunol. 1999;43:35–53. doi: 10.1016/s0165-0378(98)00090-4. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Rao J, Bush LA, Sherman NE, Oko RJ, Jayes FC, Herr JC. Outer dense fiber proteins are dominant postobstruction autoantigens in adult Lewis rats. Biol Reprod. 2001;64:1451–1459. doi: 10.1095/biolreprod64.5.1451. [DOI] [PubMed] [Google Scholar]
- Hastie C, Saxton M, Akpan A, Cramer R, Masters JR, Naaby-Hansen S. Combined affinity labelling and mass spectrometry analysis of differential cell surface protein expression in normal and prostate cancer cells. Oncogene. 2005;24:5905–5913. doi: 10.1038/sj.onc.1208747. [DOI] [PubMed] [Google Scholar]
- Held T, Paprotta I, Khulan J, Hemmerlein B, Binder L, Wolf S, Schubert S, Meinhardt A, Engel W, Adham IM. Hspa4l-deficient mice display increased incidence of male infertility and hydronephrosis development. Mol Cell Biol. 2006;26:8099–8108. doi: 10.1128/MCB.01332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzel WJ, Billeci TM, Stults JT, Wong SC, Grimley C, Watanabe C. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc Natl Acad Sci. 1993;90:5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar G, Stone K, Dix D, Vigue L. Putative creatine kinase M-isoform in human sperm is identifiedas the 70-kilodalton heat shock protein HspA2. Biol Reprod. 2000;63:925–932. doi: 10.1095/biolreprod63.3.925. [DOI] [PubMed] [Google Scholar]
- Idahl A, Boman J, Kumlin U, Olofsson JI. Demonstration of Chlamydia trachomatis IgG antibodies in the male partner of the infertile couple is correlated with a reduced likelihood of achieving pregnancy. Hum Reprod. 2004;19:1121–1126. doi: 10.1093/humrep/deh155. [DOI] [PubMed] [Google Scholar]
- Idahl A, Abramsson L, Kumlin U, Liljeqvist JA, Olofsson JI. Male serum Chlamydia trachomatis IgA and IgG, but not heat shock protein 60 IgG, correlates with negatively affected semen characteristics and lower pregnancy rates in the infertile couple. Int J Androl. 2007;30:99–107. doi: 10.1111/j.1365-2605.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Ando A, Ando H, Ando J, Saijoh Y, Inoko H, Fujimoto H. Genomic structure of the spermatid-specific hsp70 homolog gene located in the class III region of the major histocompatibility complex of mouse and man. J Biochem. 1998;124:347–353. doi: 10.1093/oxfordjournals.jbchem.a022118. [DOI] [PubMed] [Google Scholar]
- Kamaruddin M, Kroetsch T, Basrur PK, Hansen PJ, King WA. Immunolocalization of heat shock protein 70 in bovine spermatozoa. Andrologia. 2004;36:327–334. doi: 10.1111/j.1439-0272.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- Lachance C, Bailey JL, Leclerc P. Expression of Hsp60 and Grp78 in the human endometrium and oviduct, and their effect on sperm functions. Hum Reprod. 2007;22:2606–2614. doi: 10.1093/humrep/dem242. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Mamelak D, Lingwood C. The ATPase domain of hsp70 possesses a unique binding specificity for 3'-sulfogalactolipids. J Biol Chem. 2001;276:449–456. doi: 10.1074/jbc.M006732200. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–837. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Brough S, al-Harbi O. Characterization and cellular distribution of human spermatozoal heat shock proteins. Hum Reprod. 1992;7:637–645. doi: 10.1093/oxfordjournals.humrep.a137711. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Aitken RJ. Analysis of chaperone proteins associated with human spermatozoa during capacitation. Mol Hum Reprod. 2007;13:605–613. doi: 10.1093/molehr/gam043. [DOI] [PubMed] [Google Scholar]
- Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods. 2007;43:229–237. doi: 10.1016/j.ymeth.2007.06.006. Review. [DOI] [PubMed] [Google Scholar]
- Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- Munoz MG, Jeremias J, Witkin SS. The 60 kDa heat shock protein in human semen: relationship with antibodies to spermatozoa and Chlamydia trachomatis. Hum Reprod. 1996;11:2600–2603. doi: 10.1093/oxfordjournals.humrep.a019177. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen S, Flickinger CJ, Herr JC. Two-dimensional gel electrophoretic analysis of vectorially labeled surface proteins of human spermatozoa. Biol Reprod. 1997;56:771–787. doi: 10.1095/biolreprod56.3.771. [DOI] [PubMed] [Google Scholar]
- Naaby-Hansen S, Lihme AOF, Bog-Hansen TC, Bjerrum OJ. Lectins. Vol. 4. Berlin: Walter de Gruyter and Co.; 1985. Lectinblotting of normal and derivatized membrane proteins; pp. 241–252. [Google Scholar]
- Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6:149–159. doi: 10.1093/humupd/6.2.149. Review. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Kuwabara K, Tamatani M, Takatsuji K, Tsukamoto Y, Kaneda S, Yanagi H, Stern DM, Eguchi Y, Tsujimoto Y, Ogawa S, Tohyama M. 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J Biol Chem. 1999;274:6397–6404. doi: 10.1074/jbc.274.10.6397. [DOI] [PubMed] [Google Scholar]
- Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, Prodromou C. Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol Cell. 2002;10:1307–1318. doi: 10.1016/s1097-2765(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: a critical component for motoneuron survival. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabuddhe AA, Ahmed N, Krishnasastry MV. Stress-induced phosphorylation of caveolin-1 and p38, and down-regulation of EGFr and ERK by the dietary lectin jacalin in two human carcinoma cell lines. Cell Stress Chaperones. 2006;11:135–147. doi: 10.1379/CSC-160R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty J, Naaby-Hansen S, Shibahara H, Bronson R, Flickinger CJ, Herr JC. Human sperm proteome: immunodominant sperm surface antigens identified with sera from infertile men and women. Biol Reprod. 1999;61:61–69. doi: 10.1095/biolreprod61.1.61. [DOI] [PubMed] [Google Scholar]
- Shibahara H, Sato I, Shetty J, Naaby-Hansen S, Herr JC, Wakimoto E, Koyama K. Two-dimensional electrophoretic analysis of sperm antigens recognized by sperm immobilizing antibodies detected in infertile women. J Reprod Immunol. 2002;53:1–12. doi: 10.1016/s0165-0378(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Son WY, Hwang SH, Han CT, Lee JH, Kim S, Kim YC. Specific expression of heat shock protein HspA2 in human male germ cells. Mol Hum Reprod. 1999;5:1122–1126. doi: 10.1093/molehr/5.12.1122. [DOI] [PubMed] [Google Scholar]
- Spandorfer SD, Neuer A, LaVerda D, Byrne G, Liu HC, Rosenwaks Z, Witkin SS. Previously undetected Chlamydia trachomatis infection, immunity to heat shock proteins and tubal occlusion in women undergoing in-vitro fertilization. Hum Reprod. 1999;14:60–64. doi: 10.1093/humrep/14.1.60. [DOI] [PubMed] [Google Scholar]
- Spinaci M, Volpe S, Bernardini C, De Ambrogi M, Tamanini C, Seren E, Galeati G. Immunolocalization of heat shock protein 70 (Hsp 70) in boar spermatozoa and its role during fertilization. Mol Reprod Dev. 2005;72:534–541. doi: 10.1002/mrd.20367. [DOI] [PubMed] [Google Scholar]
- Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. Review. [DOI] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- Witkin SS, Kligman I, Bongiovanni AM. Relationship between an asymptomatic male genital tract exposure to Chlamydia trachomatis and an autoimmune response to spermatozoa. Hum Reprod. 1995;10:2952–2955. doi: 10.1093/oxfordjournals.humrep.a135827. [DOI] [PubMed] [Google Scholar]
- Yu LG, Andrews N, Weldon M, Gerasimenko OV, Campbell BJ, Singh R, Grierson I, Petersen OH, Rhodes JM. An N-terminal truncated form of Orp150 is a cytoplasmic ligand for the anti-proliferative mushroom Agaricus bisporus lectin and is required for nuclear localization sequence-dependent nuclear protein import. J Biol Chem. 2002;277:24538–24545. doi: 10.1074/jbc.M203550200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Enlarged high MW basic area of goldstained NC-membranes double stained with antiserum against human sperm HSPA2.
Human sperm extracts were separated on large-size (23 × 23 cm) IEF/SDS-PAGE gels, transferred to nitrocellulose membranes and stained with colloidal gold. The blots were subsequently used for western blotting with preimmune rabbit serum (top) and antiserum against HSPA2 (bottom). This antiserum reacted with the same three high MW groups of sperm surface antigens as were detected in 2-D western blot with infertile patient serum 629 (Figure 3). The immune-staining of the group 3 antigens is more pronounced in the image with artificial enhanced contrast (insert).
Supplementary Figure 2. Immunoprecipitation of biotinylated human sperm surface proteins with anti-Chlamydia trachomatis HSP70 (ctHSP70) antiserum.
Two dimensional gel analysis of biotin labelled human sperm surface proteins, isolated by immune-precipitation with antiserum raised against a putative peptide interaction domain in ctHSP70. Surface labelled proteins were detected by affinity blotting with HRP-avidin following 2-D gel electrophoretic separation. The numbered biotin labelled proteins indicated by black arrows corresponds to the similar numbered sperm antigens recognized by the infertile female serum 629 in Fig. 3. Two additional sperm surface proteins of MW 60 kDa and 90 kDa were also immunoprecipitated with the antiserum (white arrows).