Abstract
Steroid hormone receptors function classically in the nucleus as transcription factors. However, recent data indicate that there are also non-nuclear subpopulations of steroid hormone receptors, including estrogen receptors (ERs), that mediate membrane-initiated signaling of unclear basis and significance. Here we have shown that an estrogen-dendrimer conjugate (EDC) that is excluded from the nucleus stimulates endothelial cell proliferation and migration via ERα, direct ERα-Gαi interaction, and endothelial NOS (eNOS) activation. Analysis of mice carrying an estrogen response element luciferase reporter, ER-regulated genes in the mouse uterus, and eNOS enzyme activation further indicated that EDC specifically targets non-nuclear processes in vivo. In mice, estradiol and EDC equally stimulated carotid artery reendothelialization in an ERα- and G protein–dependent manner, and both agents attenuated the development of neointimal hyperplasia following endothelial injury. In contrast, endometrial carcinoma cell growth in vitro and uterine enlargement and MCF-7 cell breast cancer xenograft growth in vivo were stimulated by estradiol but not EDC. Thus, EDC is a non-nuclear selective ER modulator (SERM) in vivo, and in mice, non-nuclear ER signaling promotes cardiovascular protection. These processes potentially could be harnessed to provide vascular benefit without increasing the risk of uterine or breast cancer.
Introduction
Steroid hormone receptors (SHRs) function classically as transcription factors. More recently, it has become apparent that steroid hormones also initiate nongenomic responses via activation of a subpopulation of these receptors that are non-nuclear (1–3). In particular, this has been elucidated regarding estrogen activation of non-nuclear estrogen receptors (ERs), resulting in rapid signaling in cell types in culture as diverse as oocytes, osteoblasts, osteoclasts, breast cancer cells, adipocytes, and endothelial cells (1, 2, 4–6). However, because it is difficult to discriminate between extranuclear-initiated and nuclear-initiated ER actions, it remains unknown whether non-nuclear signaling by ER, or any other SHR, is operative and biologically relevant in vivo.
The mechanisms underlying ER non-nuclear action are exemplified by the identification of a caveolae membrane–associated population of ERα in endothelial cells that activates Src kinases, PI3K/Akt kinase, and ERK to stimulate NO production by eNOS (7). This ERα signaling is mediated by a recently revealed direct interaction between Gαi and amino acids 251–260 of ERα that promotes Gβγ signaling independent of guanine nucleotide exchange (8). NO derived from eNOS has diverse beneficial vascular actions, including the promotion of endothelial cell growth and migration, the attenuation of vascular smooth muscle (VSM) cell growth and migration, and the antagonism of leukocyte adhesion (9), and these mechanisms may be important in the cardiovascular-protective potential of hormone replacement therapy (HRT) with estrogen (10).
To interrogate non-nuclear ER action in cell-based assays, we previously synthesized estrogen-macromolecule conjugates in which estrogen is attached to a large, positively charged nondegradable poly(amido)amine (PAMAM) dendrimer via hydrolytically stable linkages (11). An estrogen-dendrimer conjugate (EDC) with a short tether linking the PAMAM to estradiol (E2) through a 17α-phenylethynyl unit affords optimal ligand access to ER and gives a binding affinity indistinguishable from that of the ligand derivative alone (12). In studies in breast cancer cells, EDC was excluded from the nucleus, and it was highly effective in stimulating non-nuclear signaling but ineffective in stimulating nuclear ER target gene expression (11). Since PAMAM dendrimers have been widely employed for drug delivery in animal models (13, 14), EDC may provide a unique means to interrogate non-nuclear estrogen actions in vivo.
To determine the basis for non-nuclear ER function and its relevance in vivo, we tested the hypothesis that EDC is a selective ER modulator (SERM) capable of exclusive non-nuclear action in mice. In addition, after elucidating the underlying mechanisms in cell culture, we used EDC in mice to test the hypothesis that non-nuclear ERα signaling via Gαi modulates vascular cell phenotype, with significant long-term consequences for cardiovascular protection. Since uterus and breast are major target organs for estrogen, and HRT with estrogen is associated with undesired uterotrophic and cancer-promoting effects (15–17), we also asked: (a) In endometrial carcinoma cells, do non-nuclear ER actions mediate estrogen-induced growth?; (b) Do non-nuclear ER actions underlie the uterotrophic response to estrogen in vivo?; and (c) Do non-nuclear ER actions promote breast tumor growth in vivo? Our findings indicate that EDC is a novel extranuclear SERM in vivo and that non-nuclear ERα signaling mediated by Gαi provides cardiovascular protection without stimulating uterine or breast cancer growth. Thus, non-nuclear ER action is operative in vivo, it provides a means of differential tissue responsiveness to steroid hormones, and it can potentially be targeted to yield selective cardiovascular benefit. Furthermore, the development of EDC represents a new strategy to elucidate the non-nuclear actions of SHR in vivo.
Results
EDC does not activate nuclear ER function in cultured cells.
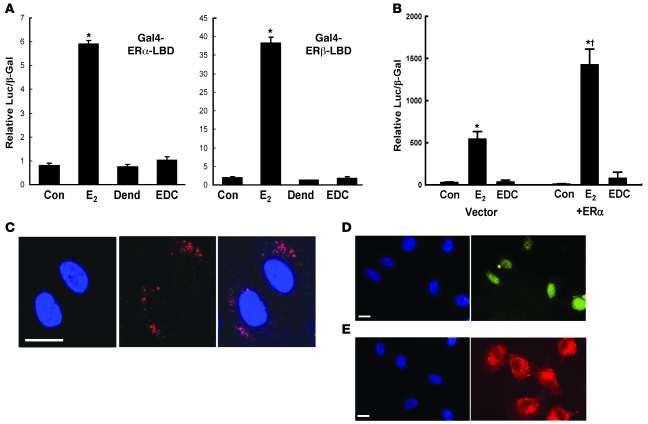
Using Gal4-ERα and -ERβ ligand-binding domain one-hybrid assays, we first confirmed a lack of ER nuclear function induced by EDC in HEK293 cells (Figure 1A). Whereas activation by E2 was apparent, EDC had no effect on ERα- or ERβ-mediated responses. We next determined the impact of EDC on ER-induced transcriptional activation in bovine aortic endothelial cells (BAECs) using an estrogen-responsive reporter plasmid. Cells were cotransfected with vector or human ERα cDNA to amplify potential nuclear ER responses (Figure 1B). In cells expressing endogenous ER alone, E2 activated transcriptional activity and EDC did not. Overexpression of ERα caused a 3-fold increase in transcriptional activation by E2, whereas EDC continued to have no effect.
Figure 1. EDC does not activate nuclear ER function in cultured cells.
(A) HEK293 cells were transfected with either an ERα-based or an ERβ-based Gal4-ER-LBD chimera and the Gal4-responsive luciferase reporter plasmid TKMH100X4-Luc, and reporter activity was determined after 24 hours exposure to vehicle (control [Con]), 10–8 M E2, dendrimer (Dend) alone at a concentration equivalent to EDC, or EDC at a concentration equivalent to 10–8 M E2. (B) BAECs were cotransfected with 3ERE-Luc luciferase reporter plasmid and either empty vector or ERα construct, and reporter activity was determined after exposure to vehicle, E2 or EDC as in A for 48 hours. In A and B, values are mean ± SEM, n = 3–4. *P < 0.05 versus Con; †P < 0.05 versus vector. (C–E) EDC does not enter the endothelial cell nucleus. BAECs were treated with Cy5-labeled EDC (red) or fluorescein-labeled estrogen (yellow) for 24 hours, and subcellular localization was evaluated by laser scanning confocal microscopy (C) or by epifluorescence microscopy (D and E). Nuclei were imaged with DAPI (blue). Scale bars in C–E: 20 μm.
The subcellular localization of Cy5-labeled EDC in endothelial cells was then assessed by confocal microscopy. At 24 hours, EDC was distributed in a punctate pattern, which may represent plasma membrane staining or presence in endocytic vesicles (Figure 1C). Most importantly, EDC was excluded from the nucleus. In addition, whereas epifluorescence imaging showed fluorescein-labeled estrogen localized primarily in nucleus (Figure 1D), EDC remained extranuclear (Figure 1E). Thus, EDC does not activate nuclear ER function in endothelial cells due to exclusion from the nucleus.
Non-nuclear ERα signaling via Gαi activates eNOS.
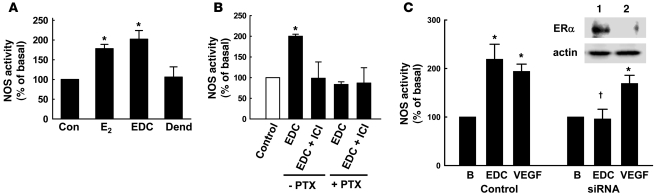
To determine whether non-nuclear ER actions underlie estrogen-induced stimulation of eNOS, enzyme activation was evaluated in BAECs. E2 and EDC caused comparable eNOS activation (Figure 2A), and further experiments with the ER antagonist ICI 182,780 and pertussis toxin demonstrated that this required ER and Gαi (Figure 2B).
Figure 2. Non-nuclear ERα activation stimulates eNOS via Gαi.
(A) eNOS activation was assessed in intact BAECs by measuring 14C-l-arginine to 14C-l-citrulline conversion during 15-minute incubations with vehicle (control), 10–8 M E2, EDC at a concentration equivalent to 10–8 M E2, or dendrimer alone at a concentration equivalent to EDC at 10–8 M. EDC at 10–10 M estrogen equivalents did not activate eNOS (data not shown). (B) Parallel experiments were performed in cells incubated with EDC (10–8 M estrogen equivalents) in the absence or presence of 10–5 M ICI 182,780 or 100 ng/ml pertussis toxin (PTX). In A and B, values are mean ± SEM, n = 4. *P < 0.05 versus control. (C) eNOS activation by EDC and VEGF (100 ng/ml) above basal activity (B) was evaluated following siRNA knockdown of ERα. Inset shows ERα abundance in control siRNA cells (lane 1) and ERα siRNA cells (lane 2). Values in C are mean ± SEM, n = 4. *P < 0.05 versus basal; †P < 0.05 versus control.
To specifically assess the role of ERα, we employed an siRNA strategy to knock down receptor expression in BAECs (Figure 2C, inset). The loss of ERα fully prevented eNOS activation by EDC, whereas stimulation of the enzyme by VEGF was unaffected (Figure 2C). These cumulative findings indicate that signaling by extranuclear ERα coupled to Gαi activates eNOS.
Non-nuclear ER signaling via Gαi promotes endothelial cell growth and migration.
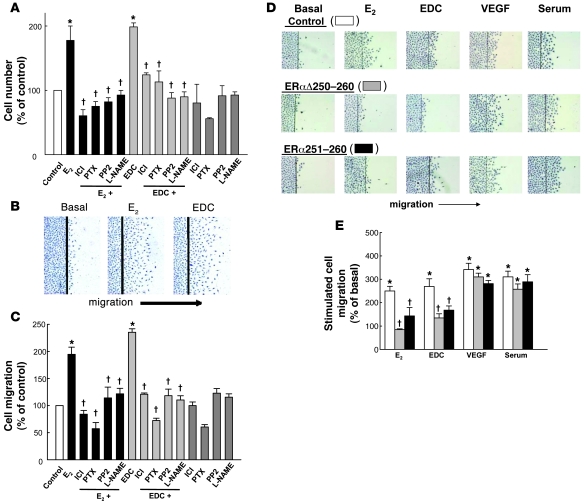
The participation of non-nuclear ER signaling in the promotion of endothelial cell proliferation was also interrogated. Proliferation was stimulated by E2, and this was prevented by ICI 182,780, pertussis toxin, the Src kinase antagonist PP2, or nitro-l-arginine methyl ester (L-NAME) (Figure 3A). EDC caused a comparable increase in proliferation, and it too was dependent on ER, G proteins, Src, and eNOS. Endothelial cell migration was also comparably stimulated by E2 and EDC (Figure 3B), and with both ligands it required ER, G protein, Src, and eNOS activation (Figure 3C).
Figure 3. Non-nuclear ER activation stimulates endothelial cell proliferation and migration via ER, Gαi, Src, and eNOS and through direct interaction of the receptor with Gαi.
(A) Cell number was determined after 48 hours treatment with vehicle or dendrimer controls, or 10–8 M E2 or EDC (10–8 M E2 equivalents), in the absence or presence of 10–5 M ICI 182,780, 100 ng/ml pertussis toxin, 10 μM PP2, or 2 mM L-NAME. (B and C) To evaluate migration, cells were wounded and treated as in A for 24 hours, and the number of cells that had migrated past the wound edge per high-power field was quantified. Images in B are representative optical fields. In A and C, values are expressed as percent of control treatment, mean ± SEM, n = 6. *P < 0.05 versus control; †P < 0.05 versus E2 or EDC alone. (D) Migration was assessed in BAECs transfected with sham plasmid (control), plasmid encoding the dominant-negative mutant receptor ERαΔ250–260, or plasmid that expressed a peptide consisting of amino acids 251–260 of the receptor (ERα251–260). Treatments were vehicle (basal), 10–8 M E2, EDC (10–8 M E2 equivalents), 100 ng/ml VEGF, or 10% serum for 24 hours. Summary data are provided in E. White bars, cells transfected with sham plasmid (control); gray bars, cells transfected with the mutant receptor ERαΔ250–260; black bars, cells expressing ERα251–260 peptide. The approximately 50% attenuation of the migration responses to E2 and EDC caused by the interventions is consistent with the transfection efficiency attained under these conditions. Values are mean ± SEM, n = 6. *P < 0.05 versus basal; †P < 0.05 versus control.
The participation of direct ERα-Gαi interaction in ER activation of endothelial cell migration was then queried. In sham-transfected control cells, comparable migration was observed with E2, EDC, and VEGF, or serum, with the latter two agents employed as positive controls (Figure 3, D and E). In cells expressing a dominant-negative mutant form of ERα that lacks the Gαi-binding domain (ERαΔ250–260) and prevents eNOS activation by endogenous ER (8), migration responses to E2 and EDC were blunted, whereas migration with VEGF or serum was unaffected. Similarly, in cells transfected with a Gαi-binding domain blocking peptide (HA-ERα251–260) that prevents ERα-Gαi interaction (8), there was attenuated migration with E2 or EDC but not with VEGF or serum. These interventions did not affect nuclear ER function in endothelial cells (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI38291DS1). Thus, non-nuclear signaling by ERα, which requires direct interaction of the receptor with Gαi, leads to Src- and eNOS-dependent stimulation of endothelial cell proliferation and migration.
EDC distinguishes non-nuclear from nuclear ER function in vivo.
The impact of EDC on ER-mediated gene transcription was interrogated in vivo in ovariectomized female ERE-Luc reporter mice by whole body imaging (18, 19) (Supplemental Figure 2). Continuous E2 (6 μg/d) administration caused luciferase activation at 24 hours primarily in liver, and signal was decreased at 48 hours. In contrast, EDC (6 μg/d estrogen) did not invoke detectable luciferase activity.
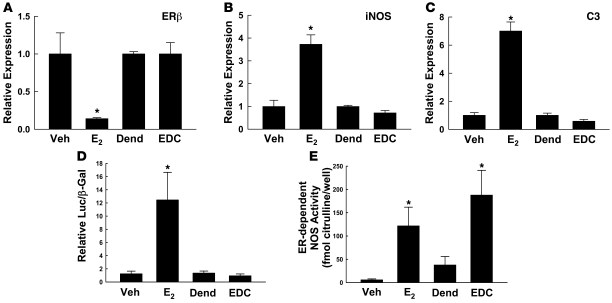
Further studies performed in ovariectomized female C57BL/6 mice compared the actions of E2 and EDC administered continuously by i.p. osmotic minipump for 24 days (6 μg/d estrogen). Serum E2 concentrations achieved ranged from 12 to 49 nM, and as determined using 3H-labeled EDC, serum concentrations of estradiol equivalents provided by EDC were similar and ranged from 14 to 36 nM. To test the impact of EDC on known ER target genes in a highly responsive tissue, we performed quantitative RT-PCR on RNA isolated from uterus. Whereas E2 downregulated ERβ expression and upregulated iNOS and complement C3 expression (Figure 4, A–C), EDC had no effect.
Figure 4. EDC distinguishes non-nuclear from nuclear ER function in vivo.
(A) Quantitative RT-PCR was performed to assess ERβ expression in the uteri of mice treated 24 days with vehicle, E2, dendrimer, or EDC. iNOS (B) and C3 expression (C) were similarly evaluated. In A–C, values are mean ± SEM, n = 4. *P < 0.05 versus vehicle. (D) Using HEK293 cells cotransfected with 3ERE-Luc luciferase reporter plasmid and ERα cDNA, ER-dependent gene transcription was evaluated over 48 hours in response to serum from mice treated 24 days with vehicle, E2, dendrimer, or EDC. Values are mean ± SEM, n = 5–8. *P < 0.05 versus vehicle. (E) ER-dependent, acute NOS activation was assessed over 15 minutes in BAECs by measuring 14C-l-arginine to 14C-l-citrulline conversion in the absence versus presence of 10–5 M ICI 182,780. Responses to 10% serum from vehicle-, E2-, dendrimer-, or EDC-treated animals were compared. Values are mean ± SEM, n = 8. *P < 0.05 versus vehicle or dendrimer.
The tissue distribution of a single dose of i.p. administered EDC was evaluated in a preliminary experiment (Supplemental Figure 3). Similar to previously studied comparable PAMAM-based molecules (20), 2 hours after systemic administration EDC was detected in numerous tissues including the uterus, liver, and spleen, and abundance declined in all tissues but the uterus over the ensuing 72 hours. The half-life of EDC in the serum was 28 hours.
To assess the stability and activity of circulating EDC when it is provided long-term, serum was obtained at 24 days from mice receiving E2 or EDC, or the vehicle for E2 or dendrimer alone as control treatments, continuously by i.p. osmotic minipump, and 3ERE-Luc activation in response to 10% serum was evaluated in ERα-expressing HEK293 cells (Figure 4D). Whereas serum from E2-treated mice caused a 10-fold increase in transcriptional activation versus serum from vehicle-treated mice, serum from EDC-treated mice did not activate the reporter. Nongenomic ER activation was tested by measuring ER-dependent, ICI 182,780–inhibited eNOS activation in BAECs in response to 10% serum (Figure 4E). Serum from E2- and EDC-treated animals caused similar ER-dependent eNOS activation. ER-dependent rapid signaling was not measurable above baseline in serum from vehicle- or dendrimer-treated mice. Thus, EDC has a wide tissue distribution when administered to mice, and it remains intact and biologically active to invoke non-nuclear ER signaling without causing ER-mediated gene transcription. As such, EDC is a novel SERM that elicits non-nuclear ER function in vivo.
Non-nuclear ERα signaling promotes endothelial monolayer integrity.
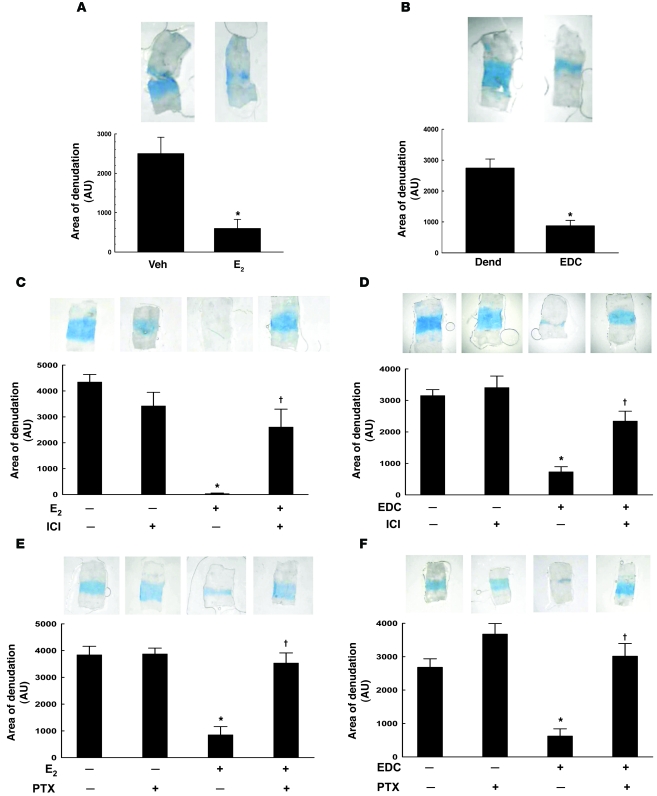
Maintenance of the integrity of the endothelial monolayer is critical to cardiovascular health. Even minimal disruption, such as gap formation between endothelial cells caused by disturbed shear stress, promotes vascular disease (21–23). We therefore assessed carotid artery reendothelialization after perivascular electric injury to reveal whether non-nuclear ER signaling promotes endothelial monolayer integrity in vivo. Figure 5A shows the area of remaining endothelial denudation incorporating Evans blue dye in arteries from vehicle- versus E2-treated mice 3 days after injury. As shown previously, E2 caused reendothelialization (24), with the remaining area of denudation decreased by 77%. In identical fashion, EDC promoted reendothelialization (Figure 5B). Both E2- and EDC-induced reendothelialization were prevented by ICI 182,780 (Figure 5, C and D), and in contrast to findings in wild-type ERα+/+ mice, negligible reendothelialization occurred with EDC in ERα–/– mice (Supplemental Figure 4A). These findings are consistent with the observation that ERα-deleted cultured endothelial cells are incapable of non-nuclear signaling in response to E2 (Figure 2C), implicating ERα as the mediator and suggesting that other estrogen-binding proteins such as GPR30 are unlikely to be involved in non-nuclear signaling in endothelium (25).
Figure 5. Non-nuclear ER signaling via Gαi promotes endothelial monolayer integrity in vivo.
(A) Carotid artery reendothelialization was evaluated following perivascular electric injury in ovariectomized female mice. The intimal surface of Evans blue–stained arteries from vehicle- versus E2-treated mice 3 days after injury is shown (upper panels). Area of denudation that incorporated dye was quantified and expressed in arbitrary units (lower panel). (B) Parallel experiments were performed in dendrimer- (Dend) versus EDC-treated mice. In A and B, values are mean ± SEM, n = 10–12. *P < 0.05 versus vehicle or dendrimer. In addition, the impact of ER antagonism with ICI 182,780 on reendothelialization was determined in E2- (C) or EDC-treated mice (D). In C and D, values are mean ± SEM, n = 8–9. *P < 0.05 versus vehicle or dendrimer; †P < 0.05 versus no ICI 182,780. Furthermore, the requirement for G proteins was assessed in E2- (E) or EDC-treated mice (F) also treated with B-oligomer (control) or pertussis toxin (PTX). In E and F, values are mean ± SEM, n = 8–9. *P < 0.05 versus vehicle or dendrimer; †P < 0.05 versus no PTX.
The domains of ERα required for in vivo non-nuclear action in endothelium were then interrogated by evaluating reendothelialization in ERα-Neo knockout mice expressing a chimeric ERα protein of 55 kDa (ERα55) in which 64 amino acids have been deleted from the N-terminal A/B regions of the receptor and replaced by 7 amino acids encoded by a small portion of the Neo insert (26, 27). Paralleling the responses in mice with intact ERα (Supplemental Figure 4B), E2 and EDC caused comparable reendothelialization in ERα-Neo mice (Supplemental Figure 4C). Thus, the components of the amino terminus lacking in the 55-kDa form of ERα are dispensable for its non-nuclear function in endothelium in vivo.
To query the requirement for Gαi in non-nuclear ERα function in endothelium in vivo, we compared E2-induced reendothelialization following treatment with pertussis toxin or its inert B-oligomer (Figure 5E). Whereas pertussis toxin did not alter reendothelialization in control mice, it prevented reendothelialization caused by E2. In parallel, EDC-induced reendothelialization was negated by pertussis toxin (Figure 5F). These collective findings indicate that in the context of all other factors regulating endothelial cell phenotype in vivo, non-nuclear ERα signaling via Gαi is a major promoter of endothelial monolayer integrity.
Non-nuclear ER signaling prevents neointima formation.
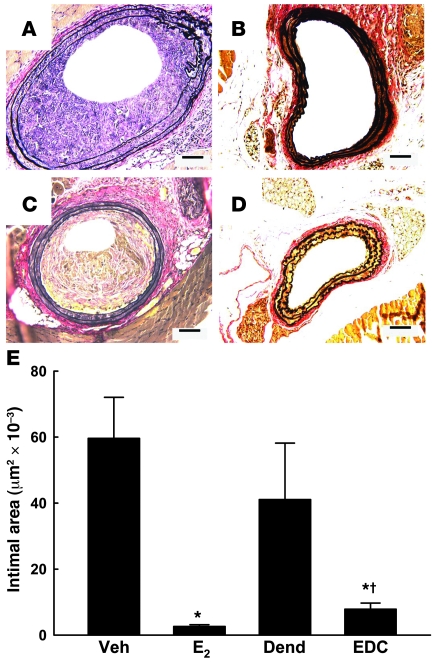
To determine the impact of non-nuclear ER signaling on vascular disease, we evaluated neointima formation in the carotid arteries of ovariectomized female Apoe–/– mice 2 weeks following endothelial denudation with an Epon resin probe. In these studies, serum cholesterol and triglyceride levels were unaffected by either E2 or EDC administration (data not shown). Figure 6A shows the marked degree of neointima formation observed in vehicle-treated mice; in contrast, E2 administration blunted neointima formation (Figure 6B). Whereas dendrimer-treated mice developed abundant neointima (Figure 6C) similar to that found in vehicle-treated mice, neointima formation was prevented by EDC (Figure 6D). Summary findings indicate that E2 and EDC both prevented neointima formation (Figure 6E). They further reveal that the vast majority of estrogen-related protection is derived from non-nuclear ER action, with a modest degree of additional protection provided by nuclear ER activation.
Figure 6. Non-nuclear ER signaling prevents neointima formation.
Neointima formation in the carotid artery was evaluated in ovariectomized female Apoe–/– mice 2 weeks following endothelial denudation. Representative arteries are shown for mice treated with (A) vehicle, (B) E2, (C) empty dendrimer, or (D) an estrogen equivalent amount of EDC. Scale bars in A–D: 100 μm. Summary data for intimal area are shown in E. Values are mean ± SEM, n = 14–17. *P < 0.05 versus vehicle or dendrimer; †P < 0.05 versus E2.
Non-nuclear ER signaling does not induce endometrial cell growth or a uterotrophic response.
Estrogen stimulates the proliferation of normal and malignant endometrial cells expressing ER (28). To determine the role of non-nuclear ER signaling in this process, we investigated ERK phosphorylation and growth responses in human endometrial Ishikawa cells. E2 and EDC caused comparable ERK phosphorylation (Supplemental Figure 5, A and B). However, whereas Ishikawa cell growth was stimulated by E2, EDC did not activate cell growth (Supplemental Figure 5C).
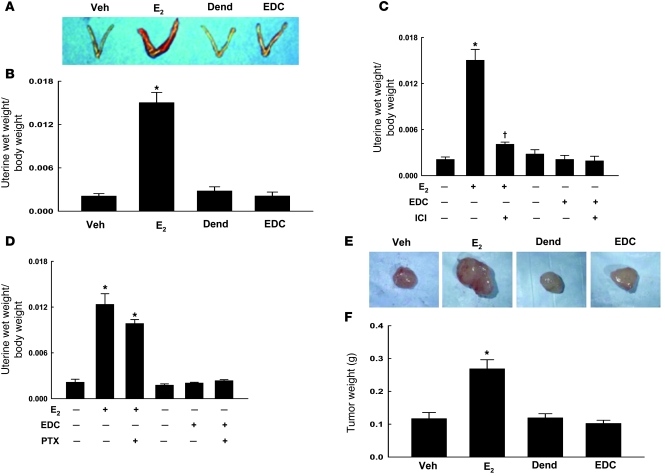
To determine the role of non-nuclear ER signaling in the uterotrophic response to estrogen, we compared uterine wet weights in ovariectomized female mice given vehicle, E2, dendrimer, or EDC for 24 days. Whereas E2 caused a 7-fold increase in uterine size, EDC had no effect (Figure 7, A and B). ICI 182,780 treatment reversed the uterotrophic response to E2 by 85% (Figure 7C). Furthermore, whereas treatment with pertussis toxin prevented reendothelialization stimulated by E2 (Figure 5E), it did not modify the increase in uterine size (Figure 7D). In additional studies performed over 72 hours, the same daily dose of E2 caused a 2.3 ± 0.19–fold increase in uterine weight (P < 0.05), and a 40-fold-higher daily dose of E2 caused a 6.1 ± 2.2–fold increase (P < 0.05). In contrast, 72-hour treatment with EDC at either the standard daily dose or a 40-fold-greater dose had no effect. Thus, non-nuclear ER signaling does not promote the growth of endometrial cells, and it does not induce a uterotrophic response.
Figure 7. Non-nuclear ER signaling does not induce a uterotrophic response or promote breast cancer tumor growth in vivo.
(A) Uteri from mice treated 24 days with vehicle, E2, dendrimer, or EDC. (B) Uterine wet weight/body weight ratio. In additional experiments, uteri were obtained from mice that were also treated with either ICI 182,780 (C) or pertussis toxin (D). In B–D, values are mean ± SEM, n = 8–9. *P < 0.05 versus vehicle; †P < 0.05 versus no ICI 182,780. (E) MCF-7 cell tumor xenografts were established with E2 treatment for 28 days in SCID mice, and treatment was then randomized to continue with E2 for 21 days or switched to vehicle, dendrimer, or EDC for 21 days. Representative tumors are shown in E. (F) Tumor weight at end of study. In F, values are mean ± SEM, n = 11–18. *P < 0.05 versus vehicle.
Non-nuclear ER signaling does not promote breast cancer tumor growth in vivo.
To determine whether non-nuclear actions of ER stimulate breast cancer tumor growth in vivo, we studied MCF-7 cell tumor xenografts established with E2 treatment for 28 days in SCID mice. Whereas the continuation of E2 for 21 days following tumor establishment caused more than a doubling in tumor size compared with that in controls, EDC did not promote tumor growth (Figure 7, E and F). Thus, mirroring what has been shown in MCF-7 cells in culture (11), non-nuclear ER signaling does not cause breast cancer tumor growth in vivo.
Discussion
In this study, we used EDC to demonstrate that non-nuclear ER activation causes potent eNOS activation and eNOS-dependent stimulation of endothelial cell growth and migration, which are highly relevant to estrogen-related cardiovascular protection. In addition, using blocking peptide and other strategies, we show that ER-dependent endothelial cell migration requires the unique protein-protein interaction between ERα and Gαi that mediates a guanine nucleotide exchange–independent mechanism of Gβγ signaling (8). Furthermore, we demonstrate that EDC is a novel SERM that selectively activates non-nuclear ER in vivo. Moreover, using EDC, we show that non-nuclear ER signaling is operative in mice to preserve the integrity of the endothelial monolayer and to prevent neointima formation, thereby providing the first in vivo evidence of non-nuclear ER actions of long-term consequence.
In the past, estrogen-protein conjugates such as E2 conjugated to BSA (E2-BSA) were used to query non-nuclear ER actions in diverse cell types including endothelial cells (29, 30). However, freshly prepared solutions of E2-BSA contain an amount of free immunoassayable E2, E2-BSA binds to ER only poorly because the E2 is linked to BSA through groups that are important for ER binding, and certain E2-BSA preparations are of very high molecular weight, suggesting extreme protein crosslinking (31, 32). As important, E2-BSA is biodegradable, making long-term experiments problematic and in vivo studies unapproachable. In contrast, the specific structural features of EDC provide stable linkage of estradiol to the PAMAM dendrimer, they optimize the access of the ligand to ER, and they prevent entry of the molecule into the nucleus (11, 12). Since PAMAM dendrimers provide a highly effective means for agent delivery in animal models (13, 14), we administered EDC to mice and employed multiple strategies to determine whether EDC has selective actions on non-nuclear ER in vivo. In the ERE-Luc reporter mouse, we found that in contrast to E2, EDC does not invoke reporter activity. We also demonstrated that whereas E2 predictably modulates known ER target genes in a highly sensitive tissue such as the uterus, EDC does not. In addition, we found that EDC had wide tissue distribution in vivo, and sensitive bioassays revealed that there is no leak of free estrogen from the conjugate and that it retains its non-nuclear activity following in vivo administration. Thus, EDC is the archetypal agent for a new pharmacologic strategy to elucidate and specifically harness the non-nuclear actions of steroid hormones in vivo. The potentially critical extranuclear functions of glucocorticoids, progesterone, androgens, mineralocorticoids, thyroid hormone, and vitamin D are all now conceivably approachable in animal models with comparable hormone-dendrimer conjugates (3).
The effectiveness of EDC as the first non-nuclear SERM allowed us to begin to delineate the domains of ERα that mediate its non-nuclear actions in vivo in endothelium. Since EDC-induced reendothelialization occurred in ERα-Neo knockout mice expressing a 55-kDa ERα deleted of 64 residues in the A/B domain (26, 27), the residues within the deleted region are dispensable for in vivo non-nuclear action. The in vivo findings for 55-kDa ERα are consistent with the capacity for rapid signaling by an N-terminal–truncated 46-kDa variant of the receptor that has been found in certain human endothelial cell lines (33). Interestingly, the 55-kDa form of ERα retains the binding domains for Gαi (aa 251–260 in intact ERα), Gβγ (aa 271–595), Src (Y537), and striatin (aa 183–253), which are interacting molecules pertinent to non-nuclear ERα function in cultured cells. 55-kDa ERα also retains a putative region in which palmitoylation promotes membrane association (aa 449–457) and a newly described arginine (R260) whose methylation also participates in non-nuclear ER action in tissue culture (8, 34–36). Experiments should now be feasible with EDC in ERα knock-in mice to definitively identify the roles of these and other domains in non-nuclear receptor function in vivo.
With EDC we were further able to demonstrate that although non-nuclear ER activation stimulates ERK phosphorylation in endometrial cells, it does not underlie the stimulation of endometrial cell growth by estrogen or the uterotrophic response to the hormone that results in increased tissue weight and epithelial cell proliferation in vivo, which are all highly pertinent to uterine carcinogenesis (17, 37). As important, in MCF-7 breast cancer cell xenografts, which readily respond to E2, we show that non-nuclear ER signaling does not promote breast cancer tumor growth in vivo. PAMAM dendrimer conjugates are readily taken up by breast tumor xenografts (38), as well as by other tumors in animals (13, 14). Their uptake is facilitated by the enhanced permeability retention effect, which is a passive uptake mechanism due to the high vascular permeability and poor lymphatic drainage of tumors (39). In addition, PAMAMs readily undergo endocytosis in tumor cells in xenografts (40). Our in vivo findings for the tumors are entirely consistent with the growth that occurs with E2 but not with EDC treatment of MCF-7 cells in culture (11). Thus, although there is considerable in vitro evidence that signaling by non-nuclear ER positively influences the properties of breast cancer cells through diverse mechanisms including the modification of gene expression (11, 41, 42), non-nuclear ER signaling alone is not sufficient to stimulate the growth of breast cancer cells in culture or in vivo.
Recent genome-wide cDNA microarray analysis of MCF-7 cells treated with E2 versus EDC has revealed that E2 uniquely influences the expression of genes involved in transcription and cell cycle regulation (42). Whereas in the endothelium non-nuclear ER activation stimulates growth and migration in response to E2 independent of nuclear ER activation, the modulation of nuclear ER targets is required for the promotion of breast cancer cell growth. We now provide evidence that this is also likely the case in uterine endometrial cells. Since studies of gene expression in MCF-7 cells have revealed changes in gene expression caused by EDC that depend on ER action through extranuclear-initiated, kinase-mediated pathways (42), it is likely that this unique SERM also modifies gene expression in endothelial cells and that these target genes of non-nuclear ER signaling in endothelium, which are yet to be identified, participate in the promotion of their growth and migration. The clear distinctions we observed between responses in endothelium, uterus, and breast cancer in mice indicate that non-nuclear ER signaling is indeed a means of differential tissue responsiveness to estrogen in vivo, as has been suggested in previous in vivo studies of the impact on bone mineralization of ER ligands with reduced nuclear activity in vitro (43).
Multiple observational studies, voluminous basic research, and experimentation in animal models have indicated that estrogen has the capacity to afford cardiovascular protection. Recent analyses of the Women’s Health Initiative and other related studies have provided support of this concept if the HRT is initiated prior to the development of advanced atherosclerosis (44, 45). However, HRT is associated with uterotrophic and cancer-promoting effects that adversely impact the risk/benefit ratio of the therapy (15–17). The present work indicates that via coupling with Gαi, non-nuclear ER action effectively promotes the integrity of the endothelial monolayer, and it potently prevents neointima formation, whereas it does not cause a uterotrophic response or promote breast cancer growth. Thus, non-nuclear ER signaling occurs in vivo with important long-term consequences, and these processes may potentially be harnessed to provide cardiovascular protection without increasing cancer risk.
Methods
EDC, cell culture, transfection, and evaluation of transcription.
EDC, derivatized with estrogen in a 20:1 ligand/dendrimer stoichiometry, was prepared as previously described (11, 12) and stored in methanol at –20°C and used within 3 months of preparation. HEK293 cells (ATCC) were grown in DMEM supplemented with 10% FBS. In a Gal4-receptor ligand-binding domain (LBD) one-hybrid assay, the cells were transfected with either an ERα-based or an ERβ-based Gal4-ER-LBD chimera, the Gal4-responsive reporter plasmid TKMH100X4-Luc with 4 copies of the Gal4-binding motif MH100 coupled to luciferase, and β-galactosidase to normalize for transfection efficiency, and responses to E2 (10–8 M), EDC (equivalent to 10–8 M E2), or dendrimer alone (equivalent to EDC) were compared over 24 hours (24). For studies of ER-dependent gene transcription in response to serum obtained from treated mice, HEK293 cells were plated in phenol red–free DMEM with 5% charcoal-stripped serum and transfected with a luciferase reporter plasmid that contained 3 copies of the Xenopus vitellogenin ERE, designated 3ERE-Luc, as previously reported (46). Cells were cotransfected with β-galactosidase to normalize for transfection efficiency, which as assessed by lacZ staining was 40%–50% under the conditions used. After transfection, cells were placed in phenol red–free DMEM to which 10% serum was added for 24 hours, and reporter activity was measured. The reporter activity observed in the presence of serum from vehicle-treated ovariectomized mice was similar to that found in the absence of added serum. Primary BAECs were cultured and maintained as previously described and used within 7 passages (47). For studies of EDC nuclear action in endothelium, BAECs were cotransfected with 3ERE-Luc, β-galactosidase, and with either empty vector or cDNA encoding wild-type human ERα to enhance potential nuclear estrogen action. Under the conditions used, transfection efficiency as assessed by lacZ staining was 40%–50%. Reporter activity was measured in response to vehicle, 10–8 M E2, or a concentration of EDC providing an equimolar amount of estrogen equivalents over 48 hours. To specifically evaluate the role of ERα in EDC responses in BAECs, receptor expression was knocked down by siRNA. dsRNA, with sequence 5′-CCAGUGCACGAUUGAUAAA-3′, was designed to target the open reading frame of bovine ERα. A dsRNA with sequence 5′-AGUUAGACCAGACCGAGGATT-3′ served as control. BAECs were transfected with 40 nM RNA as described previously (47), and eNOS activation was evaluated 48 hours later. ERα abundance was evaluated by immunoblotting of whole cell lysates with ERα antibody F10 (Santa Cruz Biotechnology Inc.). Human endometrial Ishikawa cells (provided by Hugh Taylor, Yale University School of Medicine, New Haven, Connecticut, USA) were maintained in DMEM supplemented with 10% FBS. In all cell culture studies, findings were confirmed in 3 independent experiments.
EDC subcellular localization.
BAECs were grown in EGM2 (Clonetics) overnight, followed by serum-free IMEM for 5 hours. Cy5-labeled EDC at 10–8 M estrogen equivalents was added for 24 hours, and cells were fixed in 3% paraformaldehyde and mounted with Vectashield mounting medium (Vector Laboratories) containing DAPI. Serial optical sections were obtained with a Zeiss LSM 510 META laser scanning confocal microscope with Chameleon XR NIR (Coherent) laser using oil immersion and a ×63 objective lens. For Cy5 images the samples were excited with a HeNe laser at 633 nm, and for DAPI images the samples were excited with a Chameleon laser at 799 nm. In additional studies, cells were exposed to Cy5-EDC or 10–9 M fluorescein-labeled estrogen (Invitrogen) for 24 hours, and images were obtained with a Zeiss Axio Observer wide-field epifluorescence microscope using oil immersion and a ×63 objective lens.
NOS activation.
NOS activation was assessed in intact BAECs by measuring 14C-l-arginine conversion to 14C-l-citrulline over 15 minutes using previously reported methods (48). Cells were treated with 10–8 M E2, EDC at concentrations providing 10–8 M estrogen equivalents, or empty dendrimer at the concentration that matches that in EDC which provides 10–8 M estrogen equivalents. Additional experiments were done with EDC in the absence or presence of 10–5 M ICI 182,780, or in the absence or presence of 100 ng/ml pertussis toxin (49). NOS activation was also measured over 15 minutes, in the absence or presence of ICI 182,780, in response to cell treatment with 10% serum from vehicle-, E2-, dendrimer, or EDC-treated ovariectomized animals. Similar to buffer controls, serum from ovariectomized, vehicle-treated mice did not yield eNOS activation that was inhibited by ICI 182,780. In selected studies, VEGF (100 ng/ml) was also used as an eNOS agonist (47).
Cell proliferation and migration.
BAECs were plated in 96-well plates at a density of 4,000 cells per well and treated with 10–8 M E2 or a concentration of EDC providing an equimolar amount of estrogen equivalents, in the absence or presence of 10–5 M ICI 182,780, 100 ng/ml pertussis toxin, 10 μM PP2, or 2 mM L-NAME. After 48 hours, cell number was quantified using the CellTiter 96 AQ Non-Radioactive Cell Proliferation Assay (Promega). The assay was also used to evaluate Ishikawa cell growth. Cells were first starved in DMEM/F12 without phenol red (Sigma-Aldrich) supplemented with 10% charcoal-stripped FBS for 48 hours and then trypsinized and seeded in DMEM/F12 without phenol red supplemented with 2% charcoal-stripped FBS. Twenty-four hours later, the medium was changed to DMEM/F12 without phenol red lacking serum supplementation, and after 48 hours the medium was replaced and supplemented with DMSO (vehicle for E2, at a concentration equal to that achieved with E2), 10–8 M E2, dendrimer (control for EDC, at a concentration equal to that achieved with EDC), EDC equimolar to E2, or 1% FBS serving as positive control. The medium and additives were changed, and cell number was quantified every 24 hours. In Ishikawa cells, acute ERK phosphorylation in response to DMSO, 10–8 M E2, dendrimer, or EDC equimolar to E2 was also evaluated at 0–15 minutes by immunoblot analyses of whole cell lysates for phospho-ERK and total ERK (8).
For studies of endothelial cell migration, BAECs were grown to near confluence, and a defined region of cells was removed with a razor blade. Cells were treated for 24 hours with the above-noted reagents and fixed, and the number of cells that had migrated past the wound edge was quantified. PP3 (10 μM), the control compound for PP2, had no effect. In additional experiments, the migration response to E2, EDC, 100 ng/ml VEGF, or 10% fetal calf serum was evaluated in BAECs transfected with empty vector or ERαΔ250–260 or pLP-CMV-HA(ERα251–260), an HA-tagged peptide comprising the Gαi-binding domain of ERα (aa 251–260). The sequences of all constructs were verified, and expression was confirmed by immunoblot analyses (10).
Bioluminescent luciferase reporter imaging.
ER-induced gene transcription was evaluated in vivo in ERE-Luc reporter mice as previously described (18). Female mice at 10–13 weeks were ovariectomized and received intraperitoneal osmotic minipumps (Alzet) prepared to deliver 6 μg/d E2, an estrogen equivalent amount of EDC, or empty dendrimer (control). Twenty-four hours later, the mice were anesthetized, given 30 μl of a 250-mM aqueous solution of luciferin (D-luciferin Na salt; Molecular Probes, Invitrogen) injected subcutaneously, and photon recording with a light emission tomography (LET) system with a CCD camera (50) was begun 10 minutes later. For this, the mice were placed in the light-tight chamber, and a gray-scale image of the animal was first recorded with dimmed light. Photon emission was then integrated over a period of 20 minutes and recorded as pseudo-color images. The images were combined and analyzed by Igor Pro 4.09A software (WaveMetrics Inc.). A second image analysis was performed 48 hours following treatment initiation. In these mice, maximal in vivo luciferase signal in response to E2 occurs within 48 hours (51). Before and during all in vivo studies, the mice received a low phytoestrogen diet (Research Diets, D10001). These and all other animal experiments were performed under protocols approved by the IACUC of UT Southwestern Medical Center.
Real-time PCR analysis.
mRNA abundance was evaluated in triplicate assays by real-time quantitative PCR on an Applied Biosystems Prism 7900HT Sequence Detection System using the comparative cycle time method as previously described (52).
Carotid artery reendothelialization.
Carotid artery reendothelialization was studied following perivascular electric injury in mice by assessing Evans blue dye uptake 3 days after injury (44). Areas of initial denudation were similar in all comparison groups. Endothelial denudation and recovery after injury in this model have been confirmed by immunohistochemistry for von Willebrand factor (47). Study groups included wild-type ERα+/+ mice, ERα–/– mice entirely lacking the receptor, and ERα-Neo knockout mice expressing a chimeric N-terminally truncated 55-kDa ERα protein (26, 27). To create the ERα–/– mice employed in the current work, ERα with exon 3 flanked by loxP sites was generated by Xenogen Inc. (Caliper Life Sciences) using a strategy similar to that previously described (53). ERα was then deleted by crossing mice carrying floxed exon 3 with a global Sox2-cre mouse (Tg[Sox2-cre]1Amc/J, The Jackson Laboratory), and genotyping was performed using the P1 and P3 primers described in ref. 53. These mice, designated as Ex3αERKO, lack ERα in all tissues, and they display the multiple reproductive phenotypes previously found in other global ERα-null mice (S.C. Hewitt, unpublished observations) (53). All comparisons were made between littermates. At the time of ovariectomy at 8–9 weeks of age, female mice received intraperitoneal osmotic minipumps prepared to deliver 6 μg/d E2, an estrogen equivalent amount of EDC, the vehicle for E2, or empty dendrimer at a rate identical to that delivered with EDC. Serum E2 levels achieved were determined by standard procedures (24), and the serum concentration of EDC achieved was determined in parallel studies employing 3H-labeled EDC (specific activity, 0.019 Ci/mmol). In a preliminary study, the tissue distribution of 3H-labeled EDC at 2, 24, and 72 hours was evaluated following a single i.p. injection of 6 μg estrogen equivalents. Tissues were dissolved in Solvable Solution (PerkinElmer) and decolorized with hydrogen peroxide, and liquid scintillation counting was performed. Dried and wet samples yielded similar quantities of 3H-EDC, indicating that the measured activity was not contained in water (20).
During the studies of EDC actions in vivo, additional treatments included subcutaneous injections of vehicle or ICI 182,780 (360 μg/mouse) administered 3 days prior to carotid injury and on the day of injury or an i.p. injection of either B-oligomer or intact pertussis toxin (5 μg/mouse) administered on the day of injury. At the end of study, uteri were also harvested and weighed. In further studies limited to the evaluation of the uterotrophic response, mice received the above-noted doses of E2 or EDC for 72 hours by osmotic minipump, 40-fold-greater doses of E2 or EDC, or comparable amounts of vehicle or empty dendrimer.
Carotid artery neointima formation.
Neointima formation was studied in female C57BL/6 Apoe–/– mice (The Jackson Laboratory). When mice were 6 weeks of age, an ovariectomy was performed, and i.p. osmotic minipumps were placed to deliver 6 μg/d E2, an estrogen equivalent amount of EDC, the vehicle for E2, or empty dendrimer at a rate identical to that delivered with EDC. One week later, endothelial denudation was performed in the right carotid artery with an Epon resin probe using a procedure described previously (54). Animals were euthanized for analysis 2 weeks after denudation. They were perfused with 10% buffered formalin for 20 minutes at a constant pressure of 100 mmHg, and the neck was dissected and fixed in 10% buffered formalin for 48 hours and then decalcified for 48 hours prior to being embedded in paraffin. Whole neck cross sections of 5 μm were prepared beginning from the distal bifurcation of the carotid artery to the point of the Epon resin probe insertion, with 4 levels of serial sections obtained at 500-μm intervals. Parallel sections were stained with hematoxylin and eosin and for Verhoeff–Van Gieson staining of the elastic lamina, and morphometric analysis was performed on elastin-stained sections. Four cross sections with both injured and uninjured carotid arteries were measured at each level, and data were averaged for each animal. For each section, medial area was calculated as the area encircled by external elastic lamina minus the area inside the internal elastic lamina, and intimal area was calculated as the area inside the internal elastic lamina minus the luminal area. To calculate medial thickness for each vessel cross section, the linear distance between the internal elastic lamina and the external elastic lamina was measured independently in 4 locations separated by 90° and then averaged. In the current studies, medial thickness was similar in all groups.
Carotid artery vascular conductance.
To establish a strategy for effective administration of pertussis toxin in vivo, we measured acetylcholine-induced (Ach-induced) changes in carotid artery vascular conductance by previously described methods 3 days following the administration of either the inert B-oligomer of the toxin or the intact toxin to female mice that were previously ovariectomized at 8–9 weeks of age and treated with E2 for 21 days (48). Ach-induced increases in carotid artery vascular conductance are due to eNOS activation via muscarinic, Gαi-coupled receptors (55). In mice treated with the inert B-oligomer (controls), Ach caused a dose-dependent increase in carotid vascular conductance similar in degree to responses we have previously observed (Supplemental Figure 6) (48). In contrast, following treatment with intact pertussis toxin, the Ach response was blunted by 50%, confirming effective pertussis toxin inhibition of Gαi in vivo.
Orthotopic breast tumor xenograft model.
At the time of ovariectomy of female SCID mice at 8 weeks of age, 5 × 106 MCF-7 cells were implanted into the right mammary fat pad, and an osmotic minipump was placed in the intraperitoneal cavity to deliver 6 μg/d E2. Tumor volume was measured intermittently with calipers, and at 28 days the minipumps were exchanged to provide vehicle, E2, dendrimer, or EDC at equimolar amounts of estrogen. The mice were euthanized 21 days later, and the tumors and uteri were harvested and weighed.
Statistics.
Comparisons were made between multiple groups by ANOVA with Neuman-Keuls post-hoc testing or by ANOVA using the Holm-Sidak method (neointima studies). When indicated, nonparametric ANOVA (Kruskal-Wallace) and post-hoc Dunn testing was performed. A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by NIH grants HD30276 (to P.W. Shaul), DK15556 (to J.A. Katzenellenbogen), CA18119 (to B.S. Katzenellenbogen), and DK56930 (to D.Y. Hui), by an American Heart Association (Texas Affiliate) Postdoctoral Fellowship (0325033Y to G.D. Thomas), by the Lowe Foundation (to P.W. Shaul), by the Crystal Charity Ball Center for Pediatric Critical Care Research (to P.W. Shaul), and by the Division of Intramural Research of the National Institute of Environmental Health Sciences (to K.S. Korach). We thank M. Dixon for preparing the manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(7):2319–2330. doi:10.1172/JCI38291.
See the related Commentary beginning on page 2277.
References
- 1.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3(1):27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 2.Cheskis BJ. Regulation of cell signalling cascades by steroid hormones. J Cell Biochem. 2004;93(1):20–27. doi: 10.1002/jcb.20180. [DOI] [PubMed] [Google Scholar]
- 3.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23(8):1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Levin ER. Integration of the extra-nuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12(4):152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 7.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, et al. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21(6):1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 9.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 10.Mendelsohn ME. Nongenomic, ER-mediated activation of endothelial nitric oxide synthase: how does it work? What does it mean? Circ Res. 2000;87(11):956–960. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- 11.Harrington WR, et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, non-genomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Katzenellenbogen JA. Hormone-PAMAM dendrimer conjugates: polymer dynamics and tether structure affect ligand access to receptors. Angew Chem Int Ed Engl. 2006;45(43):7243–7248. doi: 10.1002/anie.200601923. [DOI] [PubMed] [Google Scholar]
- 13.Kukowska-Latallo JF, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 14.Wu G, Barth RF, Yang W, Kawabata S, Zhang L, Green-Church K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol Cancer Ther. 2006;5(1):52–59. doi: 10.1158/1535-7163.MCT-05-0325. [DOI] [PubMed] [Google Scholar]
- 15.Harman SM. Estrogen replacement in menopausal women: recent and current prospective studies, the WHI and the KEEPS. Gend Med. 2006;3(4):254–269. doi: 10.1016/S1550-8579(06)80214-7. [DOI] [PubMed] [Google Scholar]
- 16.Conner P. Breast response to menopausal hormone therapy--aspects on proliferation, apoptosis and mammographic density. Ann Med. 2007;39(1):28–41. doi: 10.1080/07853890601039842. [DOI] [PubMed] [Google Scholar]
- 17.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85(2):304–313. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 18.Ciana P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9(1):82–86. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- 19.Ciana P, et al. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol. 2001;15(7):1104–1113. doi: 10.1210/me.15.7.1104. [DOI] [PubMed] [Google Scholar]
- 20.Nigavekar SS, et al. 3H–dendrimer nanoparticle organ/tumor distribution. Pharm Res. 2004;21(3):476–483. doi: 10.1023/B:PHAM.0000019302.26097.cc. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1111/1523-1747.ep12274489. [DOI] [PubMed] [Google Scholar]
- 22.Rossig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96(1):11–22. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 23.Werner N, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93(2):e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 24.Umetani M, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13(10):1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 25.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 26.Couse JF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9(11):1441–1454. doi: 10.1210/me.9.11.1441. [DOI] [PubMed] [Google Scholar]
- 27.Pendaries C, et al. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci U S A. 2002;99(4):2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito K, Utsunomiya H, Yaegashi H, Sasano H. Biololgical roles of estrogen and progesterone in human endometrial carcinoma- new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54(5):667–679. doi: 10.1507/endocrj.KR-114. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100(8):4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J Biol Chem. 2000;275(49):38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- 31.Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140(11):5455–5458. doi: 10.1210/en.140.11.5455. [DOI] [PubMed] [Google Scholar]
- 32.Temple JL, Wray S. Bovine serum albumin-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146(2):558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- 33.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147(12):5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor alpha. Proc Natl Acad Sci U S A. 2004;101(49):17126–17131. doi: 10.1073/pnas.0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18(5):1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 36.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 37.Hewitt SC, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17(10):2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 38.Xu R, Wang Y, Wang X, Jeong EK, Parker DL, Lu ZR. In vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp Biol Med (Maywood). 2007;232(8):1081–1089. doi: 10.3181/0702-RM-33. [DOI] [PubMed] [Google Scholar]
- 39.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 40.Shukla R, et al. HER2 specific tumor targeting with dendrimer conjugated anti-HER2 mAb. Bioconjug Chem. 2006;17(5):1109–1115. doi: 10.1021/bc050348p. [DOI] [PubMed] [Google Scholar]
- 41.Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108(3):351–361. doi: 10.1007/s10549-007-9618-4. [DOI] [PubMed] [Google Scholar]
- 42.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22(9):2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otto C, et al. In vivo characterization of estrogen receptor modulators with reduced genomic versus nongenomic activity in vitro. J Steroid Biochem Mol Biol. 2008;111(1-2):95–100. doi: 10.1016/j.jsbmb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Hsia J, et al. Conjugated equine estrogens and coronary heard disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–365. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 45.Manson JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 46.Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of ERalpha domains with known nuclear functions. Mol Endocrinol. 2005;19(2):277–289. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- 47.Seetharam D, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98(1):63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 48.Mineo C, et al. FcgammaRIIB mediates C-reactive protein inhibition of endothelial NO synthase. Circ Res. 2005;97(11):1124–1131. doi: 10.1161/01.RES.0000194323.77203.fe. [DOI] [PubMed] [Google Scholar]
- 49.Wyckoff MH, et al. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i). J Biol Chem. 2001;276(29):27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 50.Zhao D, Richer E, Antich PP, Mason RP. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging and confirmed by MRI. FASEB J. 2008;22(7):2445–2451. doi: 10.1096/fj.07-103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciana P, et al. The dynamics of estrogen receptor activity. Maturitas. 2006;54(4):315–320. doi: 10.1016/j.maturitas.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha and beta on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 54.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9(3):251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorin E. Functional cross-talk between endothelial muscarinic and alpha2-adrenergic receptors in rabbit cerebral arteries. Br J Pharmacol. 1998;125(6):1188–1193. doi: 10.1038/sj.bjp.0702199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.