Abstract
Background:
The aim of this prospective study was to assess whether the presence of septic shock could influence the dose response to inhaled nitric oxide (NO) in NO-responding patients with adult respiratory distress syndrome (ARDS).
Results:
Eight patients with ARDS and without septic shock (PaO2 = 95 ± 16 mmHg, PEEP = 0, FiO2 = 1.0), and eight patients with ARDS and septic shock (PaO2 = 88 ± 11 mmHg, PEEP = 0, FiO2 = 1.0) receiving exclusively norepinephrine were studied. All responded to 15 ppm inhaled NO with an increase in PaO2 of at least 40 mmHg, at FiO2 1.0 and PEEP 10 cmH2O. Inspiratory intratracheal NO concentrations were recorded continuously using a fast response time chemiluminescence apparatus. Seven inspiratory NO concentrations were randomly administered: 0.15, 0.45, 1.5, 4.5, 15, 45 and 150 ppm. In both groups, NO induced a dose-dependent decrease in mean pulmonary artery pressure (MPAP), pulmonary vascular resistance index (PVRI), and venous admixture (QVA/QT), and a dose-dependent increase in PaO2/FiO2 (P ≤ 0.012). Dose-response of MPAP and PVRI were similar in both groups with a plateau effect at 4.5 ppm. Dose-response of PaO2/FiO2 was influenced by the presence of septic shock. No plateau effect was observed in patients with septic shock and PaO2/FiO2 increased by 173 ± 37% at 150 ppm. In patients without septic shock, an 82 ± 26% increase in PaO2/FiO2 was observed with a plateau effect obtained at 15 ppm. In both groups, dose-response curves demonstrated a marked interindividual variability and in five patients pulmonary vascular effect and improvement in arterial oxygenation were dissociated.
Conclusion:
For similar NOinduced decreases in MPAP and PVRI in both groups, the increase in arterial oxygenation was more marked in patients with septic shock.
Keywords: acute respiratory distress syndrome, inhaled nitric oxide, mechanical ventilation, pulmonary hypertension
Introduction
In patients with ARDS and acute pulmonary hypertension, inhaled NO has been shown to selectively dilate pulmonary vessels perfusing ventilated lung areas, and to improve arterial oxygenation [1,2,3,4,5,6,7,8,9]. The `plateau' effect of NO on pulmonary vascular resistance and gas exchange is obtained at various concentrations ranging from 1-40 ppm [2,4,6,7,9,10,11]. In the majority of patients, a major improvement in arterial oxygenation can be obtained with NO concentrations < 5 ppm [4,9,10,11]. In addition, the degree of response as well as the optimal NO dose varies both between individuals and from day to day [11]. In sheep with experimental acute lung injury receiving inhaled NO, a dose-dependent increase in arterial oxygenations is found, with a plateau effect at NO concentrations of 30-60 ppm [12,13]. Nitric oxide concentrations > 30 ppm may result in elevated concentrations of nitrogen dioxide (NO2) and methemoglobin particularly when 100% oxygen is administered together with NO [9]. Because of the potential lung toxicity of NO2, knowledge of the factors influencing the optimal dose of inhaled NO in humans is of critical importance for intensivists. Recently, it has been suggested that the presence of septic shock may decrease responsiveness to inhaled NO [14]: among 25 patients with ARDS and septic shock, only 40% responded to inhaled NO with an improvement in PaO2/FiO2≥ 20%. This proportion was estimated as `abnormally low', although there are no published data reporting the proportion of non-septic patients with ARDS responding to inhaled NO by an increase in PaO2/FiO2> 20%. In the present study, we hypothesized that the presence of septic shock and the administration of vasoconstrictors to patients with ARDS could modify the dose-response to inhaled NO. We wanted to assess whether in NO-responding patients with septic shock, higher NO concentrations were required to obtain a pulmonary effect similar to the one obtained in non-septic patients. In addition, the effect of intravenous norepinephrine on an NO-induced decrease in pulmonary artery pressure and increase in arterial oxygenation was investigated. Therefore, dose–response studies were performed on two groups of critically ill patients with and without septic shock whose lungs were mechanically ventilated for ARDS. All patients enrolled were NO responders and patients with septic shock were exclusively receiving intravenous norepinephrine for hemodynamic support.
Methods
Patients
During an 8 month period, 29 consecutive hypoxemic patients with ARDS, diagnosed on or after admission to the Surgical Intensive Care Unit (SICU) of La Pitié Hospital in Paris (Department of Anesthesiology), were prospectively screened at an early stage of their respiratory disease. Written informed consent was obtained from the patient's next of kin. The study was approved by the Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale of La Pitié-Salpétrière Hospital.
Inclusion criteria were:
1. bilateral infiltrates on a bedside chest radiograph;
2. PaO2≤ 200 mmHg using an FiO2 of 1.0 and zero end-expiratory pressure (ZEEP);
3. bilateral and extensive hyperdensities on a high resolution spiral thoracic CT scan;
4. positive response to inhaled NO, defined as a decrease in MPAP of at least 2 mmHg and an increase in PaO2 (FiO2 1.0, PEEP 10 cmH2O) of at least 40 mmHg after NO inhalation at an inspiratory concentration of 15 ppm.
These response criteria were fixed in order to select patients responding to NO by a decrease in MPAP and an increase in PaO2 of sufficient magnitude to allow the determination of dose-response curves. It was considered that when the variation of the parameter studied (either PaO2 or pulmonary artery pressure) was close or inferior to the precision of measurement, it was not possible to accurately assess the dose-response.
Exclusion criteria were:
1. left ventricular failure, defined as a cardiac index ≤ 21/min/m2 associated with a pulmonary capillary wedge pressure > 18 mmHg and/or a left ventricular ejection fraction < 50% as estimated by bedside transesophageal echocardiography;
2. circulatory shock requiring an exogenous catecholamine other than norepinephrine, or characterized by spontaneous fluctuations of blood pressure despite a constant infusion of norepinephrine;
3. cardiac dysrhythmias;
4. presence of a patent foramen ovale with a right-to-left atrial shunt as assessed by pulsed-wave Doppler transesophageal echocardiography.
These exclusion criteria were intended to eliminate patients with cardiac failure, intracardiac shunt or cardiovascular instability, in whom an accurate evaluation of dose-response to inhaled NO would have been either difficult or heavily biased [15]. Among the 29 patients initially screened for inclusion, 13 had to be excluded (no response to NO, n = 6; left ventricular failure, n = 4; circulatory shock with an unstable arterial pressure, n = 2; atrial fibrillation, n = 1). Finally, 16 patients fulfilling inclusion and exclusion criteria were included. Eight patients were in septic shock and eight patients had no septic shock. Diagnosis of septic shock was made according to the criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference [16], requiring: (1) a systemic response to infection and (2) a systolic blood pressure < 90 mmHg despite adequate fluid resuscitation requiring vasopressor agents. Adult respiratory distress syndrome was diagnosed according to the recent American-European Consensus Conference [17] and its severity was graded according to Murray et al [18].
In each patient the trachea was orally intubated with a HiLo JetTM no 8 Mallinckrodt tube (Inc, Argyle, NY) which incorporates two side ports, one ending at the distal tip of the endotracheal tube and a more proximal port ending 6 cm from the tip. These additional channels were used for continuous monitoring of tracheal pressure and tracheal concentrations of inhaled NO. After inclusion in the study, all patients were sedated and paralysed with a continuous intravenous infusion of fentanyl 250 μg/h, flunitrazepam 1 mg/h and vecuronium 4 mg/h, and their lungs were ventilated using conventional mechanical ventilation (César Ventilator, Taema, France). For each patient, tidal volume and respiratory rate were adjusted to maintain constant minute ventilation throughout the study. An inspiratory time of 30%, a PEEP of 10 cmH2O and an FiO2 of 0.85 were maintained throughout the study period. FiO2 was continuously monitored, using an O2 analyser (Sérès 4000 Aix-en-Provence, France), in order to detect changes resulting from the admixture of inspired gases with NO. All patients were monitored using a fiberoptic thermodilution pulmonary artery catheter (Oximetrix Opticath Catheter, Abbot Critical Care System) and a radial or femoral arterial catheter.
In order to accurately assess the extension of pulmonar hyperdensities, and thereby the severity of ARDS patients were transported to the Department of Radiology (Thoracic Division) for a lung scan. The scan was performed from the apex to the diaphragm using a Tomoscan SR 7000 (Philips, Eindhoven) and a semi-quantitative assessment of parenchymal consolidation in ZEEP was performed according to a technique previously described [4,5,8,9]. CT scans were obtained in all patients except patient 8 who could not be transported to the Department of Radiology because of an unstable pelvic fracture.
Measurements
Systolic and diastolic arterial pressures (SAP and DAP), and systolic and diastolic pulmonary arterial pressures (SPAP and DPAP) were simultaneously measured using the arterial cannula and the fiberoptic pulmonary artery catheter connected to two calibrated pressure transducers (91 DPT-308 Mallinckrodt) positioned at the midaxillary line. Systemic and pulmonary arterial pressures, electrocardiogram (EKG), tracheal pressure (Paw) measured through the distal port of the endotracheal tube, and gas flow and tidal volume (VT) measured using a heated and calibrated Hans Rudolph pneumotachograph, were simultaneously and continuously recorded on a Gould ES 1000 recorder (Gould Instruments, Cleveland, OH) throughout the entire study period, at a paper speed of 1 mm/s.
In all patients, expired CO2 was measured using a nonaspirative calibrated 47210 A infrared capnometer (Hewlett Packard) positioned between the proximal end of the endotracheal tube and the Y piece of the ventilator. Expired CO2 curves were continuously recorded on the Gould ES 1000 recorder at a paper speed of 1 mm/s. After withdrawing an arterial blood sample, the ratio of alveolar dead space (VDA) to VT was calculated as:
VDA/VT = 1 – (PETCO2/PaCO2)
where PETCO2 is end-tidal CO2 measured at the plateau of the expired CO2 curve. Expired CO2 curves were then recorded at a paper speed of 50 mm/s, and only tracings demonstrating a clear end-expiratory plateau, defined as a constant CO2 value for more than 0.5 s at end-expiration, were used to determine PETCO2. In patient 11, VDA/VT was not calculated because no plateau could be identified on the expired CO2 curve. Because ARDS is associated with abnormalities of the pulmonary vasculature (local thrombi and pulmonary vasoconstriction at the early stage and vascular remodeling at the late stage), VDA/VT can be considered as a better index of these vascular lesions than physiologic dead space calculated by the Bohr equation which takes into account the anatomic dead space [19].
In each phase (see experimental protocol), when a steady state was obtained — defined as a leveling of the pulmonary arterial pressure — SAP, DAP, SPAP, DPAP, pulmonary capillary wedge pressure (PWP), right atrial pressure (RAP), VT, Paw and gas flow were recorded at a paper speed of 50 mm/s. Mean arterial pressure (MAP) was calculated as 1/3 SAP + 2/3 DAP. Mean pulmonary artery pressure was measured by planimetry as the mean of four measurements performed at end-expiration. Systolic arterial pressure, DAP, SPAP, DPAP, PWP and RAP were also measured at end-expiration. Cardiac output was measured using the thermodilution technique and a bedside computer allowing the recording of each thermodilution curve (Oximetrix 3 SO2/CO Computer). Four serial 10 ml injections of 5% dextrose solution at room temperature were performed at random during the respiratory cycle [20]. Systemic and pulmonary arterial blood samples were simultaneously withdrawn within 1 min following cardiac output measurements (after discarding an initial 10 ml heparin contaminated aliquot). Arterial pH, PaO2, mixed venous partial pressure of oxygen (PvO2) and PaCO2 were measured using an IL BGETM blood gas analyser. Hemoglobin concentration, methemoglobin concentration, and arterial and mixed venous oxygen saturations (SaO2 and SvO2) were measured using a calibrated OSM3 hemoximeter. Arterial and mixed venous blood samples that showed hemoglobin concentrations differing by more than 0.1 g/100 ml were considered diluted, and the highest hemoglobin concentration was used to calculate oxygen content. Standard formulae were used to calculate cardiac index (CI), PVRI, systemic vascular resistance index (SVRI), right ventricular stroke work index (RVSWI), venous admixture (QVA/QT), arteriovenous oxygen difference [C(av)O2], oxygen delivery (DO2), oxygen extraction ratio (EaO2) and oxygen consumption (VO2).
In all patients, respiratory pressure-volume (P–V) curves were measured using a 1 l syringe (Model Series 5540, Hans Rudolph Inc, Kansas City, MO) according to a previously described technique [8]. Construction of inspiratory and expiratory P–V curves allowed: determination of opening pressure (Pop), static respiratory compliance (Crs) calculated as the slope of the curve between 500-1000 ml, and quasi-static respiratory compliance (Cqs), obtained by dividing the VT by the corresponding airway pressure. Opening pressure could be clearly identified in nine patients and was always ≤ 10 cmH2O. A PEEP of 10 cmH2O was systematically applied to all patients.
Nitric oxide administration
Nitric oxide was released from three different tanks of nitrogen that had NO concentrations of 25, 900 and 2000 ppm, measured using chemiluminescence (Air Liquide, France). Nitric oxide was delivered into the inspiratory limb of the ventilator just after the Fisher-Paykel humidifier, according to a previously described technique [9]. With the aid of a calibrated and heated pneumotachograph (Model Series 3500B, Hans Rudolph Inc, Kansas City, MO) attached to the proximal end of the endotracheal tube, VT was reduced to exactly compensate for the added volume of nitrogen and NO coming from the tank. Thus, VT and minute ventilation delivered to the patients were kept constant for all concentrations of inhaled NO.
Inspiratory, expiratory and mean concentrations of NO and NO2 were continuously measured using a fast response time chemiluminescence apparatus (NOX 4000 Sérès, Aix-en-Provence, France). Intratracheal gas was sampled by continuous aspiration through the proximal side port of the Mallinckrodt endotracheal tube, ie 162 cm from the site of NO administration. The NOX 4000 is a chemiluminescence apparatus specifically designed for medical use. When using an aspiration flow rate of 150 ml/min, the response time - defined as the time necessary to reach 95% of a reference NO concentration - is around 30 s and only mean concentrations of NO can be accurately measured. When an aspiration flow rate of 1000 ml/min is selected, the response time is 0.765 ms and inspiratory and expiratory NO concentrations can be accurately measured. In a previous study, we demonstrated that inspiratory and expiratory concentrations of NO were adequately measured by the NOX 4000 with a precision of 5% [9].
During the study, inspiratory and expiratory NO concentrations were continuously measured and recorded after setting the aspiration flow rate of the NOX 4000 at 1000 ml/min. In addition, in steady state conditions, mean intratracheal NO concentrations were measured by setting the aspiration flow rate of the NOX 4000 at 150 ml/min. When the aspiration flow rate was changed, the tidal volume setting of the ventilator was modified accordingly in order to achieve a constant minute ventilation and stable NO concentration. In order to increase precision, two different operating ranges of measurement were used, depending on the concentrations of NO administered to the patient: an operating range of 0–5 ppm was selected for inspiratory tracheal concentrations of 0.15, 0.45, 1.5 and 4.5 ppm, and an operating range of 0–200 ppm for inspiratory tracheal concentrations of 15, 45 and 150 ppm. When 0–5 ppm was selected, calibration was performed using a tank of NO with a reference concentration of 0.945 ppm (CFPO, Air Liquide, France); when 0–200 ppm was selected, calibration was performed using a tank of NO with a reference concentration of 22.8 ppm (CFPO, Air Liquide, France). Nitrogen oxides (NOX) were calibrated using the same reference tanks according to the manufacturer's instructions. The oxygen analyser of the NOX 4000 was used for continuous monitoring of oxygen concentration in order to ensure that a constant FiO2 was maintained during NO inhalation, whatever the concentration administered.
Protocol
In each patient, the protocol consisted of three consecutive phases. At each phase hemodynamic and respiratory parameters were measured.
Phase 1: PEEP without NO (control 1)
Baseline measurements were made following a 1 h steady state of conventional mechanical ventilation using the following ventilatory settings: FiO2 0.85, PEEP 10 cmH2O, inspiratory time 30%, respiratory frequency 16 ± 2 bpm, VT 728 ± 32 ml.
Phase 2: PEEP 10 cm H2O with NO at increasing inspiratory concentrations (dose–response curve)
Using the same ventilatory settings as in phase 1, seven inspiratory tracheal concentrations of NO, chosen according to a logarithmic scale, were randomly administered: 0.15, 0.45, 1.5, 4.5, 15, 45 and 150 ppm. Because concentrations of 45 and 150 ppm were associated with a longlasting increase in blood methemoglobin concentration, which interfered with the calculation of venous and arterial O2 content and pulmonary shunt, they were not included in the randomization, but were always administered as the last concentrations. For each inspiratory tracheal concentration of NO, expiratory and mean intratracheal concentrations of NO were measured and recorded. In addition, VT and FiO2 were adjusted at the ventilator level in order to maintain a constant minute ventilation and an FiO2 of 0.85 as assessed by the pneumotachograph and the oxygen analyser. For each inspiratory NO concentration, hemodynamic and respiratory measurements were recorded after a 15 min steady state.
Phase 3: PEEP 10 cm H2O without NO (control 2)
At the end of a 1 h steady state following the discontinuation of NO 150 ppm, hemodynamic and respiratory paramaters were measured at the same ventilator settings as in phase 1.
Statistical analysis
Cardiorespiratory parameters at control were compared between groups using a Student's t-test for unpaired data. The cardiorespiratory effects of NO were analysed in each group using contrast analysis (control values were compared with values obtained using graded concentrations of NO). In both groups of patients, the existence of a dose-related effect was investigated using a one-way analysis of variance for repeated measures including only the different concentrations of NO. Dose–response curves of NO on hemodynamic and respiratory parameters in the presence or absence of septic shock were analysed using a two-way analysis of variance for one within and one grouping factor, ie factor `group (absence or presence of septic shock)' and factor `dose of NO'. Interaction between these two factors allowed us to test the hypothesis that the effect of NO differed depending on the presence or absence of septic shock. The significance level was fixed at 5%, but due to the nature of the analysis of variance, we used the criterion of Huynh and Feld rather than the classical F value [21]. Calculations were made using Super ANOVA statistical software (Abanus Concepts, Inc). All values are expressed as mean ± SEM.
Results
Patients
Among the 16 men enrolled in the study, eight were admitted to the SICU following multiple trauma and eight following postoperative complications after major surgical procedures (vascular surgery, n = 1; cardiac surgery, n = 3; orthopedic surgery, n = 1; digestive surgery, n = 2; neurosurgery, n = 1). Eight patients were in septic shock, defined as the presence of an identified infectious foci associated with arterial hypotension requiring the continuous intravenous administration of norepinephrine [16]. Norepinephrine was administered in doses ranging between 1 and 5 mg/h. All patients were studied at the early phase of ARDS (first 5 days). As shown in Tables 1 and 2, all patients had ARDS characterized by arterial hypoxemia, increased QVA/QT, pulmonary artery hypertension, reduced respiratory compliance, and consolidation of lung parenchyma involving at least 45% of total lung volume. Initial clinical hemodynamic and respiratory parameters were not statistically different between patients with and without septic shock.
Table 1.
Initial clinical characteristics of the 16 patients
| Patients without septic shock | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Age | 26 | 35 | 67 | 69 | 35 | 25 | 55 | 48 |
| SAPS | 17 | 9 | 17 | 13 | 10 | 10 | 12 | 12 |
| LISS | 2.3 | 3 | 3 | 3 | 2.3 | 2.8 | 2.5 | 3 |
| Outcome | S | S | D | D | S | S | D | S |
| Cause of ARDS | BPN | BPN | BPN | BPN | Pulmonary contusion | BPN | Mesenteric infarction | BPN |
| COPD | No | No | Yes | No | No | No | Yes | No |
| % of lung consolidation | 63 | 51 | 72 | 43 | 55 | 64 | 89 | nd |
| CT scan abnormalities | BCLL | BCLL | BCLL | BCLL | BCLL + DPH | BCLL + DPH | DPH | nd |
| Patients with septic shock | ||||||||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Age | 17 | 59 | 61 | 42 | 63 | 47 | 67 | 67 |
| SAPS | 6 | 8 | 10 | 16 | 5 | 7 | 10 | 14 |
| LISS | 2 | 2.8 | 3.5 | 3 | 1.8 | 2 | 2.8 | 2.5 |
| Outcome | S | S | D | S | S | S | S | D |
| Cause of ARDS | BPN | BPN | BPN | Peritonitis | Post CPB | BPN | BPN | Septic shock |
| COPD | No | Yes | No | No | No | No | No | Yes |
| % of lung consolidation | 49 | 72 | 70 | 50 | 57 | 58 | 49 | 48 |
| CT scan abnormalities | BCLL | BCLL + DPH | BCLL | BCLL | BCLL | BCLL + DPH | BCLL + DPH | BCLL |
S = survived; D = deceased; BPN = bronchopneumonia; LISS = lung injury severity score; SAPS = simplified acute physiologic score; ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; nd = not determined (unstable spine fractures); BCLL = bilateral consolidation of lower lobes; DPH = disseminated `patchy' hyperdensities; CPB = cardiopulmonary bypass.
Table 2.
Initial hemodynamic and respiratory characteristics of the 16 patients: intermittent positive pressure ventilation, ZEEP and FiO2= 1.0
| Patients without septic shock | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean ± SEM | |
| PaCO2 (mmHg) | 66 | 45 | 41 | 41 | 46 | 49 | 58 | 56 | 50 ± 3 |
| VDA/VT (%) | 39 | 26 | 26 | 35 | 18 | 45 | 46 | 33 | 34 ± 4 |
| PaO2 (mmHg) | 58 | 107 | 111 | 104 | 81 | 49 | 188 | 64 | 95 ± 16 |
| QVA/QT (%) | 53 | 43 | 34 | 29 | 46 | 71 | 36 | 53 | 46 ± 5 |
| Cqs (ml/cmH2O) | 44 | 57 | 52 | 50 | 36 | 25 | 57 | - | 46 ± 4 |
| Crs (ml/cmH2O) | 50 | 56 | 55 | 82 | 29 | 19 | 84 | 58 | 54 ± 8 |
| MPAP (mmHg) | 21 | 31 | 20 | 43 | 27 | 28 | 19 | 36 | 28 ± 3 |
| PVRI (dyn s/cm5m2) | 168 | 265 | 443 | 1329 | 298 | 215 | 246 | 286 | 406 ± 135 |
| PCWP (mmHg) | 4 | 11 | 6 | 3 | 7 | 2 | 7 | 10 | 6 ± 1 |
| CI (l/min/m2) | 8.3 | 6.1 | 2.6 | 2.4 | 5.3 | 9.7 | 3.9 | 7.2 | 5.7 ± 1 |
| Patients with septic shock | |||||||||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Mean ± SEM | |
| PaCO2 (mmHg) | 55 | 56 | 56 | 57 | 39 | 44 | 33 | 50 | 48 ± 3 |
| VDA/VT (%) | 48 | 38 | 33 | 42 | 33 | 23 | 25 | 39 | 35 ± 3 |
| PaO2 (mmHg) | 130 | 68 | 59 | 57 | 145 | 106 | 88 | 77 | 88 ± 11 |
| QVA/QT (%) | 50 | 53 | 50 | 51 | 36 | 43 | 41 | 40 | 47 ± 2 |
| Cqs (ml/cmH2O) | 43 | 58 | 30 | 26 | 83 | 52 | 39 | 59 | 49 ± 6 |
| Crs (ml/cmH2O) | 50 | 56 | 48 | 39 | 77 | 57 | 57 | 59 | 56 ± 4 |
| MPAP (mmHg) | 24 | 37 | 45 | 31 | 21 | 39 | 27 | 27 | 32 ± 3 |
| PVRI (dyn s/cm5m2) | 399 | 590 | 489 | 377 | 360 | 471 | 321 | 652 | 442 ± 40 |
| PWP (mmHg) | 4 | 14 | 13 | 5 | 10 | 9 | 14 | 4 | 9 ± 1 |
| CI (l/min/m2) | 3.9 | 3.1 | 5.3 | 5.4 | 2.4 | 5.1 | 3.3 | 2.9 | 4.3 ± 1 |
VDA/VT = alveolar dead space; QVA/QT = venous admixture; Cqs = quasi-static respiratory compliance; Crs = respiratory compliance (slope of the P-V curve above the lower inflection point); MPAP = mean pulmonary arterial pressure; PVRI = pulmonary vascular resistance index; PCWP = pulmonary capillary wedge pressure; CI = cardiac index.
NO concentrations
Table 3 shows that inspiratory intratracheal NO concentrations were 1.5–2 times greater than mean intratracheal NO concentrations. Expiratory concentrations of NO progressively increased with mean NO concentrations. For an inspiratory NO concentration of 0.15 ppm, expired NO was not detectable. For an inspiratory NO concentration of 0.45 ppm, expired NO could be measured in 15 patients. From inspiratory NO concentrations of 1.5 ppm, expired NO could be measured in all patients.
Table 3.
Mean (FNO), inspiratory (FINO) and expiratory (FENO) intratracheal NO concentrations, mean NO2 intratracheal concentrations and methemoglobin (MetHb) blood levels measured in 16 patients with ARDS receiving increasing concentrations of inhaled NO at FiO2 0.85
| NO (ppm) | |||||||
| 0.15 | 0.45 | 1.5 | 4.5 | 15 | 45 | 150 | |
| FNO (ppm) | 0.102 ± 0.004 | 0.32 ± 0.011 | 1.05 ± 0.02 | 2.98 ± 0.06 | 10.4 ± 0.2 | 26 ± 0.8 | 100 ± 4 |
| FINO (ppm) | 0.15 ± 0.006 | 0.45 ± 0.073 | 1.5 ± 0.2 | 4.5 ± 0.3 | 15.3 ± 1.2 | 45.2 ± 0.9 | nd |
| FENO (ppm) | 0.004 ± 0.0005 | 0.1 ± 0.02 | 0.6 ± 0.05 | 1.95 ± 0.1 | 6 ± 0.2 | 17 ± 0.9 | nd |
| NO2 (ppm) | 0.02 ± 0.004 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.02 | 0.3 ± 0.1 | 0.8 ± 0.3 | 4 ± 0.9 |
| MetHb (%) | 0.9 ± 0.1 | 1 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1 ± 0.1 | 1.4 ± 0.2 | 3.8 ± 0.5 |
Values are given as mean ± SEM. nd = not determined.
Hemodynamic and respiratory effects of NO in patients without septic shock
As shown in Tables 4 and 5, NO induced a significant dose-dependent decrease in MPAP, SPAP, DPAP, PVRI,RVSWI and QVA/QT with a significant and dose-dependent increase in PaO2/FiO2. As shown in Figs 1,2,3, a plateau effect was observed at inspiratory NO concentrations of 4.5 ppm for MPAP, PVRI, QVA/QT and PaO2/FiO2. All other hemodynamic and respiratory parameters did not vary significantly. Hemodynamic and respiratory parameters returned to control values after the cessation of inhaled NO.
Table 4.
Hemodynamic effects of increasing inspiratory concentrations of inhaled NO in eight patients with ARDS and without septic shock
| NO (ppm) | ||||||||||
| Control 1 | 0.15 | 0.45 | 1.5 | 4.5 | 15 | 45 | 150 | Control 2 | P value* | |
| SPAP (mmHg) | 45 ± 5 | 38 ± 5 | 37 ± 5 | 35 ± 4 | 36 ± 5 | 34 ± 5 | 33 ± 4 | 33 ± 4 | 43 ± 5 | 0.0001 |
| DPAP (mmHg) | 19 ± 2 | 17 ± 3 | 16 ± 2 | 15 ± 2 | 16 ± 2 | 15 ± 2 | 16 ± 2 | 15 ± 2 | 19 ± 2 | 0.0001 |
| MPAP (mmHg) | 29 ± 3 | 25 ± 3 | 24 ± 3 | 24 ± 3 | 24 ± 3 | 23 ± 3 | 23 ± 3 | 23 ± 3 | 28 ± 4 | 0.0001 |
| PVRI (dyn s/cm5m2) | 431 ± 105 | 383 ± 94 | 345 ± 90 | 340 ± 82 | 338 ± 85 | 321 ± 74 | 311 ± 83 | 305 ± 77 | 438 ± 122 | 0.0001 |
| HR (beats/min) | 94 ± 6 | 89 ± 6 | 88 ± 6 | 88 ± 7 | 90 ± 6 | 91 ± 6 | 88 ± 6 | 90 ± 6 | 90 ± 7 | 0.3188 |
| CI (l/min/m2) | 4.3 ± 0.5 | 4 ± 0.4 | 4.2 ± 0.5 | 4.1 ± 0.5 | 4.1 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.5 | 0.8806 |
| RVSWI (g/m2) | 13 ± 1 | 11 ± 1 | 11 ± 1 | 11 ± 1 | 10 ± 1 | 10 ± 1 | 10 ± 1 | 10 ± 1 | 13 ± 1 | 0.0001 |
| RAP (mmHg) | 7 ± 2 | 7 ± 1 | 8 ± 2 | 7 ± 1 | 7 ± 2 | 7 ± 1 | 7 ± 2 | 7 ± 2 | 7 ± 2 | 0.8382 |
| PCWP (mmHg) | 9 ± 2 | 8 ± 1 | 8 ± 2 | 8 ± 1 | 9 ± 1 | 8 ± 1 | 9 ± 1 | 9 ± 2 | 9 ± 1 | 0.1125 |
| MAP (mmHg) | 84 ± 4 | 76 ± 6 | 79 ± 3 | 81 ± 4 | 86 ± 3 | 82 ± 3 | 83 ± 4 | 83 ± 5 | 81 ± 5 | 0.1603 |
| SVRI (dyn s/cm5m2) | 1589 ± 215 | 1432 ± 198 | 1499 ± 201 | 1601 ± 224 | 1720 ± 229 | 1563 ± 195 | 1653 ± 247 | 1651 ± 240 | 1631 ± 239 | 0.1339 |
NO = nitric oxide; SPAP = systolic pulmonary arterial pressure; DPAP = diastolic pulmonary arterial pressure; MPAP = mean pulmonary arterial pressure; PVRI = pulmonary vascular resistance index; HR = heart rate; CI = cardiac index; RVSWI = right ventricular stroke work index; RAP = right atrial pressure; PCWP = pulmonary capillary wedge pressure; MAP = mean arterial pressure; SVRI = systemic vascular resistance index. Values are given as mean ± SEM.*P value for the one-way analysis of variance (dose–response curve).
Table 5.
Respiratory effects of increasing inspiratory concentrations of inhaled NO in eight patients with ARDS and without septic shock
| NO (ppm) | ||||||||||
| Control 1 | 0.15 | 0.45 | 1.5 | 4.5 | 15 | 45 | 150 | Control 2 | P value* | |
| PaO2/FiO2 (mmHg) | 162 ± 23 | 221 ± 27 | 220 ± 26 | 245 ± 27 | 261 ± 31 | 275 ± 28 | 278 ± 30 | 290 ± 48 | 177 ± 28 | 0.0001 |
| QVA/QT (%) | 33 ± 3 | 29 ± 1 | 30 ± 2 | 27 ± 2 | 27 ± 2 | 27 ± 2 | 27 ± 2 | 28 ± 4 | 33 ± 3 | 0.0122 |
| SvO2 (%) | 65 ± 3 | 67 ± 3 | 68 ± 4 | 66 ± 3 | 68 ± 3 | 70 ± 3 | 70 ± 3 | 67 ± 3 | 65 ± 4 | 0.1753 |
| DO2 (ml/min/m2) | 440 ± 45 | 427 ± 38 | 446 ± 43 | 441 ± 48 | 435 ± 44 | 452 ± 43 | 441 ± 44 | 433 ± 47 | 422 ± 49 | 0.9511 |
| VO2 (ml/min/m2) | 146 ± 9 | 141 ± 11 | 142 ± 10 | 147 ± 11 | 138 ± 10 | 137 ± 6 | 134 ± 10 | 144 ± 12 | 140 ± 12 | 0.4556 |
| PaCO2 (mmHg) | 43 ± 2 | 41 ± 2 | 41 ± 2 | 41 ± 2 | 41 ± 2 | 42 ± 2 | 42 ± 2 | 43 ± 2 | 43 ± 2 | 0.1204 |
| PETCO2 (mmHg) | 30 ± 1 | 29 ± 2 | 29 ± 2 | 30 ± 2 | 30 ± 2 | 29 ± 2 | 30 ± 2 | 30 ± 2 | 29 ± 2 | 0.6522 |
| VDA/VT (%) | 31 ± 3 | 29 ± 4 | 30 ± 4 | 27 ± 3 | 27 ± 4 | 30 ± 3 | 30 ± 4 | 29 ± 4 | 33 ± 3 | 0.2898 |
QVA/QT = venous admixture; SvO2 = mixed venous oxygen saturation; VO2 = oxygen consumption; DO2 = oxygen delivery; PETCO2 = end tidal CO2; VDA/VT = alveolar dead space. Values are given as mean ± SEM. *P value for the one-way analysis of variance (dose-response curve).
Figure 1.
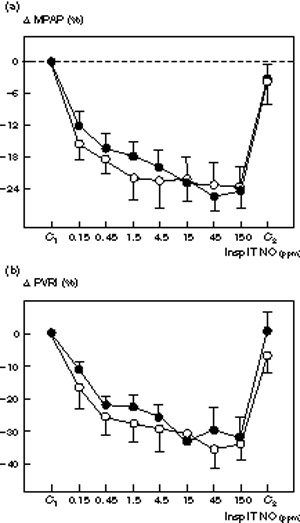
Comparative changes in (a) mean pulmonary artery pressure (ΔMPAP) and (b) pulmonary vascular resistance index (ΔPVRI) induced by increasing inspiratory intratracheal concentrations of inhaled NO (Insp IT NO) in the presence (n = 8, ●) or absence (n = 8, ○) of septic shock in 16 patients with ARDS. Mean pulmonary artery pressure and PVRI were measured: (1) before NO administration (C1); (2) following seven randomized concentrations of NO between 0.15 and 150 ppm, and (3) after the cessation of NO (C2). In both groups, NO induced a significant and dose-dependent decrease in MPAP and PVRI (P< 0.01). Change in MPAP and Δ PVRI are expressed as percentage variation from the control value. In both groups, a plateau effect was observed for MPAP and PVRI from NO concentrations of 4.5 ppm. No interaction between the factors `group' and `does of NO' was found using the two-way analysis of variance, suggesting that the NO dose-response was not affected by the presence of septic shock.
Figure 2.
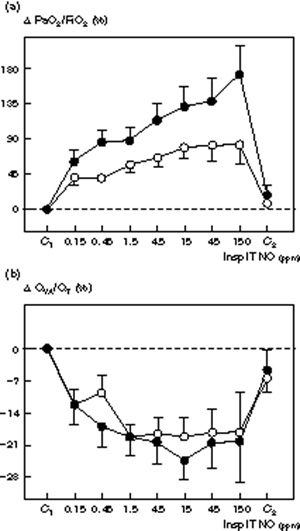
Changes in (a) PaO2/FiO2 (Δ PaO2/FiO2 and (b) venous admixture (QVA/QT) induced by increasing inspiratory intratracheal concentrations of inhaled NO (Insp IT NO) in the presence (n = 8, ●) or absence (n = 8, ○) of septic shock in 16 patients with ARDS. PaO2/FiO2 and QVA/QT were measured: (1) before NO administration (C1); (2) following seven randomized concentrations of NO between 0.15 and 150 ppm, and (3) after cessation of NO (C2). Δ PaO2/FiO2 and QVA/QT are expressed as percentage variation from the control value. In both groups, NO induced a significant and dose–dependent increase in PaO2/FiO2 and a decrease in QVA/QT (P< 0.01). In both groups, a plateau effect was observed for the NO-induced decrease in QVA/QT from NO concentrations of 1.5 ppm. In patients with septic shock, NO-induced increases in PaO2 did not show any plateau whereas in patients without septic shock a plateau effect was observed from NO concentrations of 4.5 ppm. An interaction between the factors 'group' and 'dose of NO' was found using the two-way analysis of variance (P = 0.035) suggesting that the profile of the NO dose–response curve was affected by the presence of septic shock.
Figure 3.
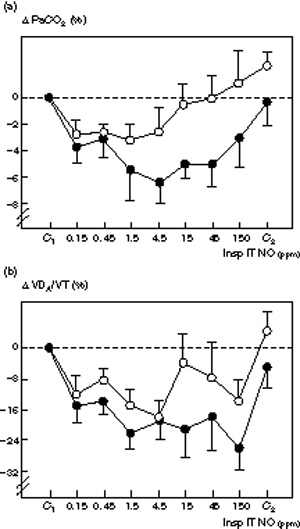
Comparative changes in (a) PaCO2 (Δ PaCO2) and (b) alveolar dead space (ΔVDA/VT) induced by increasing inspiratory intratracheal concentrations of inhaled NO (Insp IT NO) in the presence (n = 7, filled circle) or absence (n = 8, ○) of septic shock in 15 patients with ARDS. PaCO2 and VDA/VT were measured: (1) before NO administration (C1); (2) following seven randomized concentrations of NO between 0.15 and 150 ppm, and (3) after the cessation of NO (C2). Δ PaCO2 and Δ VDA/VT are expressed as percentage variation from the control value. In each condition, minute ventilation was kept constant by adjusting the tidal volume. In both groups, NO induced a decrease in PaCO2 and VD A/VT which was statistically significant but dose-dependent in patients who only had septic shock (P < 0.02).
Hemodynamic and respiratory effects of NO in patients with septic shock
Hemodynamic and respiratory effects of increasing inspiratory concentrations of NO in patients with septic shock are summarized in Tables 6 and 7. A significant dose-dependent decrease in SPAP, DPAP, MPAP, PVRI, RVSWI, PaCO2, VDA/VT and QVA/QT and a significant dose-dependent increase in PaO2/FiO2 were observed. The maximum decrease in mean PVRI, PaCO2 and VDA/VT was obtained for an inspiratory NO concentration of 4.5 ppm (Fig 3). The maximum increase in PaO2/FiO2 was obtained for an inspiratory NO concentration of 150 ppm (Figs 1 and 2). All other hemodynamic and respiratory parameters did not vary significantly. Hemodynamic and respiratory parameters returned to control values after the cessation of NO inhalation.
Table 6.
Hemodynamic effects of increasing inspiratory concentrations of inhaled NO in eight patients with ARDS and with septic shock
| NO (ppm) | ||||||||||
| Control 1 | 0.15 | 0.45 | 1.5 | 4.5 | 15 | 45 | 150 | Control 2 | P value* | |
| SPAP (mmHg) | 48± 5 | 43± 5 | 42± 4 | 40± 4 | 39± 3 | 38± 3 | 36± 3 | 36± 3 | 48± 4 | 0.0001 |
| DPAP (mmHg) | 23± 2 | 19± 2 | 20± 2 | 19± 2 | 18± 2 | 18± 2 | 17± 2 | 17± 2 | 22± 2 | 0.0001 |
| MPAP (mmHg) | 32± 3 | 28± 3 | 28± 3 | 27± 3 | 26± 3 | 26± 2 | 24± 2 | 24± 2 | 31± 3 | 0.0001 |
| PVRI (dyn s/cm5m2) | 513± 60 | 395± 39 | 399± 37 | 383± 45 | 352± 35 | 355± 27 | 351± 25 | 362± 33 | 484± 50 | 0.0001 |
| HR (/min) | 91± 8 | 89± 9 | 91± 8 | 95± 8 | 90± 9 | 89± 9 | 89± 7 | 89± 8 | 93± 7 | 0.5331 |
| CI (I/min/m2) | 3.3± 0.3 | 3.3± 0.3 | 3.2± 0.3 | 3.5± 0.3 | 3.3± 0.3 | 3.2± 0.3 | 3.2± 0.3 | 3.1± 0.3 | 3.2± 0.3 | 0.2356 |
| RVSWI (g/m2 | 11± 2 | 10± 2 | 10± 2 | 9± 2 | 9± 2 | 8± 1 | 8± 1 | 7± 1 | 10± 2 | 0.0003 |
| RAP (mmHg) | 10± 2 | 10± 2 | 10± 2 | 9± 2 | 10± 2 | 10± 2 | 9± 2 | 9± 1 | 10± 2 | 0.2142 |
| PCWP (mmHg) | 11± 2 | 11± 1 | 12± 2 | 11± 2 | 11± 2 | 12± 2 | 11± 2 | 10± 2 | 11± 2 | 0.4322 |
| MAP (mmHg) | 74± 5 | 75± 3 | 78± 5 | 77± 3 | 79± 4 | 74± 3 | 73± 4 | 73± 4 | 75± 4 | 0.3197 |
| SVRI (dyn s/cm5m2) | 1709± 236 | 1685± 187 | 1767± 177 | 1695± 184 | 1827± 258 | 1705± 170 | 1731± 219 | 1788± 279 | 1698± 163 | 0.8766 |
NO = nitric oxide; SPAP = systolic pulmonary arterial pressure; DPAP = diastolic pulmonary arterial pressure; MPAP = mean pulmonary arterial pressure; PVRI = pulmonary vascular resistance index; HR = heart rate; CI = cardiac index; RVSWI = right ventricular stroke work index; RAP = right aterial pressure; PCWP = pulmonary capillary wedge pressure; MAP = mean arterial pressure; SVRI = systemic vascular resistance index. Values are given as mean ± SEM. *P value for the one-way analysis of variance (dose-response curve).
Table 7.
Respiratory effects of increasing inspiratory concentrations of inhaled NO in eight patients with ARDS and with septic shock
| NO (ppm) | ||||||||||
| Control 1 | 0.15 | 0.45 | 1.5 | 4.5 | 15 | 45 | 150 | Control 2 | P value* | |
| PaO2/FiO2 (mmHg) | 128± 18 | 199± 20 | 229± 25 | 232± 28 | 255± 23 | 270± 18 | 283± 24 | 313± 23 | 148± 21 | 0.0001 |
| QVA/QT (%) | 37± 2 | 32± 2 | 30± 1 | 29± 1 | 29± 1 | 27± 1 | 29± 1 | 29± 3 | 35± 1 | 0.0001 |
| SVO2 (%) | 67± 4 | 72± 2 | 73± 2 | 73± 3 | 74± 3 | 74± 3 | 74± 2 | 73± 3 | 69± 3 | 0.0012 |
| DO2 (ml/min/m2) | 416± 38 | 425± 31 | 437± 40 | 455± 41 | 430± 25 | 420± 29 | 417± 35 | 407± 35 | 413± 31 | 0.315 |
| VO2 (ml/min/m2) | 126± 13 | 113± 10 | 114± 9 | 124± 16 | 110± 11 | 114± 12 | 109± 9 | 118± 18 | 121± 11 | 0.2372 |
| PaCO2 (mmHg) | 44± 3 | 43± 3 | 42± 2 | 41± 3 | 41± 2 | 41± 3 | 41± 2 | 42± 3 | 43± 2 | 0.0114 |
| PETCO2 (mmHg) | 30± 2 | 31± 2 | 31± 2 | 30± 2 | 30± 2 | 31± 2 | 30± 2 | 32± 3 | 30± 2 | 0.0829 |
| VDA/VT (%) | 30± 4 | 25± 3 | 25± 3 | 24± 4 | 24± 4 | 24± 4 | 25± 4 | 23± 5 | 28± 4 | 0.0008 |
QVA/QT = venous admixture; SVO2 = mixed venous oxygen saturation; VO2 = oxygen consumption; DO2 = oxygen delivery; PETCO2 = end tidal CO2; VDA/VT = alveolar dead space Values are given as mean ± SEM. *P value for the one-way analysis of variance (dose-response curve).
Effects of septic shock on dose–response curves
At control, hemodynamic and respiratory parameters were the same for both groups. Dose-response curves of inhaled NO for MPAP, PVRI, RVSWI, PaCO2 and QVA/QT were not significantly different between patients with and without septic shock (Figs 1,2,3). As shown in Fig 2, the effect of inhaled NO on PaO2/FiO2 was significantly increased by the presence of septic shock. In patients with septic shock, inhaled NO increased PaO2/FiO2 by 190%, the maximum effect being obtained at an inspiratory NO concentration of 150 ppm. In patients without septic shock, inhaled NO increased PaO2/FiO2 by 81%, the maximum effect being obtained at an inspiratory NO concentration of 4.5 ppm. Using a two-way analysis of variance, a significant interaction was found for the factor group (P = 0.047).
Individual variability of dose–response curves
As shown in Figs 4 and 5, dose-response curves demonstrated marked variability between individuals. In patients without septic shock, the decrease in MPAP varied from 11 to 45% whereas the increase in PaO2/FiO2 varied from 30 to 220% (Fig 4). In five patients a clear plateau could be identified for the decrease in MPAP (Fig 4c), whereas MPAP continued to decrease with higher NO concentrations in three (Fig 4a). Different patterns were observed for PaO2/FiO2: in four patients the PaO2/FiO2 ratio deteriorated at the highest inspiratory NO concentrations (Fig 4d), whereas in the other four PaO2/FiO2 continued to increase (Fig 4b). The patients whose PaO2/FiO2 ratio continued to increase with the highest NO concentrations demonstrated a clear plateau effect in MPAP at NO concentrations of 4.5 ppm suggesting that the effects of NO on gas exchange and pulmonary circulation can be dissociated. In patients with septic shock (Fig 5), the decrease in MPAP varied from 8 to 32% whereas the increase in PaO2 varied from 60 to 380%. In five patients, a clear plateau could be identified on the dose-response curve of MPAP (Fig cc) whereas it continued to decrease with higher NO concentrations in three (Fig 4a). In two patients, PaO2/FiO2 deteriorated at the highest inspiratory NO concentrations (Fig 5d) whereas in the other six, PaO2/FiO2 continued to increase (Fig 5b). As observed in patients without septic shock, the effects of NO on arterial oxygenation and pulmonary artery pressure were dissociated. In two patients only (patients 10 and 11), dose-response curves were characterized by a concurrent dose-dependent decrease in MPAP and an increase in PaO2/FiO2 in the range of 0.15 to 150 ppm inhaled NO.
Figure 4.
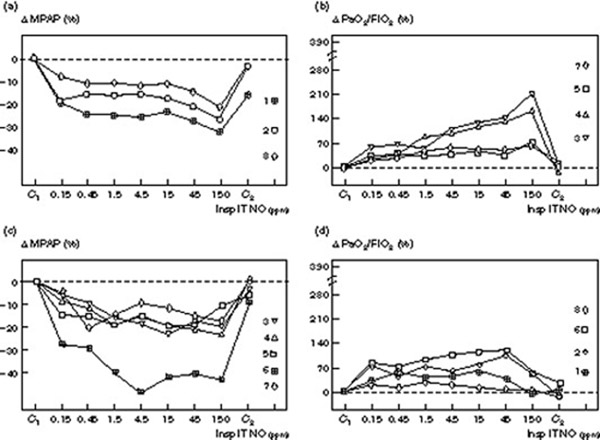
Individual changes in MPAP and PaO2/FiO2 induced by increasing inspiratory intratracheal concentrations of inhaled NO (Insp IT NO) in eight patients with ARDS and without septic shock. Mean pulmonary artery pressure was measured: (1) before NO administration (C1); (2) following seven randomized concentrations of NO between 0.15 and 150 ppm, and (3) after the cessation of NO (C2). Changes are expressed as percentage variation from C1 (Δ MPAP and Δ PaO2/FiO2) and each patient is represented by a different symbol with a number corresponding to the numbers shown in Tables 1 and 2. In (a) and (b) patients without plateau effect on the dose–response curve are represented. In (c) and (d) patients with a plateau effect on the MPAP dose–response curve and showing a deterioration of their PaO2/FiO2 at the highest NO concentrations are represented.
Figure 5.
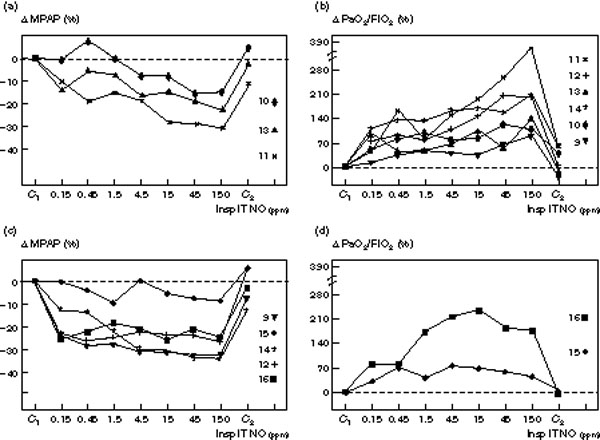
Individual changes in mean pulmonary artery pressure (MPAP) and PaO2/FiO2 induced by increasing inspiratory intratracheal concentrations of inhaled NO (Insp IT NO) in eight patients with ARDS and septic shock. MPAP was measured: (1) before NO administration (C1); (2) following seven randomized concentrations of NO between 0.15 and 150 ppm, and (3) after the cessation of NO (C2). Changes are expressed as a percentage variation from C1 (Δ MPAP and Δ PaO2/FiO2 and each patient is represented by a different symbol with a number corresponding to the numbers shown in Tables 1 and 2. In (a) and (b) patients without plateau effect on the dose-response curve are represented. In (c) and (d) patients with a plateau effect on the MPAP dose-response curve and showing a deterioration of their PaO2/FiO2 at the highest NO concentrations are represented.
Toxic effects of increasing concentrations of inhaled NO
As shown in Table 3, methemoglobin and NO2 significantly increased at inspiratory NO concentrations of 15 ppm. A mean intratracheal NO2 concentration of 4 ± 0.9 ppm and a mean methemoglobin concentration of 3.8 ± 0.5% were observed at an inspiratory NO concentration of 150 ppm.
Discussion
The main results of this study can be summarised as follows:
1. the dose-response relationship between inhaled NO and pulmonary vascular effects is not influenced by the presence of septic shock in patients with ARDS;
2. pulmonary vascular and gas exchange effects are frequently dissociated;
3. for the same pulmonary vascular effect, inhaled NO-induced improvement in arterial oxygenation is of greater magnitude in patients with ARDS and septic shock receiving norepinephrine;
4. dose–response curves are characterized by a wide variability between patients, although for most, 90% of the maximum effect is obtained with NO concentrations ≤ 4.5 ppm. This latter result is in accordance with five recent studies demonstrating a plateau effect at inspiratory NO concentrations < 10 ppm [4,9,10,11,22].
Factors influencing individual dose–response curves
During mechanical ventilation, intratracheal NO concentrations fluctuate according to the phase of respiration [9], the inspiratory concentration being greater than the expiratory concentration because NO is absorbed at the alveolar level. In the present study, NO concentrations delivered to the patient were determined by sampling the endotracheal gas using a fast response chemiluminescence apparatus in order to accurately measure inspiratory NO concentration [9]. If used, slow response chemiluminescence would have underestimated the true inspiratory NO concentration by averaging it together with the expiratory level, as probably occurred in two of our previous studies [4,23]. Another reason for determining the inspiratory NO concentration in this way was the method of NO administration used. Continuous administration of NO through the initial part of the inspiratory limb during volume controlled ventilation invariably results in fluctuation of the NO concentration within the inspiratory limb due to a 'bolus' effect [24,25]. Although mixing of NO increases with distance from the site of administration [24], a fast response analyser is required to accurately measure the peak NO concentration during the inspiratory phase. We previously demonstrated in an in vitro experiment, that the NOX 4000 was able to measure rapid fluctuations of NO concentrations with a precision ≥ 95% [9].
In the present study, two different patterns of dose-response curves were observed. In 10 patients (five in each group) a plateau effect for MPAP could be identified at NO concentrations ranging between 0.45 and 4.5 ppm. In six patients (three in each group) MPAP continued to decrease with the highest NO concentrations (Figs 4 and 5). These different variation profiles did not appear to be related to the presence of septic shock.
Although the mean pulmonary vascular effect of inhaled NO was not affected by the presence of septic shock, the resulting improvement in arterial oxygenation was of a greater magnitude in patients with septic shock (Fig 2). The reasons for this difference are not clear. It can be hypothesized that the same degree of inhaled NO-induced vasodilation of the pulmonary vessels perfusing ventilated lung areas resulted in a greater redistribution of pulmonary blood flow in patients with septic shock. This implies that for the same extent of lung consolidation, basal pulmonary blood flow perfusing non-ventilated lung areas was greater in patients with septic shock. As a matter of fact, although the percentage of lung consolidation tended to be greater in patients without septic shock (63 vs 57%), their mean PaO2 tended to be higher (95 ± 16 vs 88 ± 11 mmHg), suggesting some degree of hypoxic pulmonary vasoconstriction impairment in the non-ventilated lung areas of patients with septic shock. It is well known that acute lung infection and septic shock may impair hypoxic pulmonary vasoconstriction through the massive release from activated endothelium of vasodilating mediators such as prostaglandins and endogenous NO, and hence result in disproportionately high shunting and hypoxemia [26,27,28,29,30,31,32]. In addition, exogenous catecholamines, used to maintain arterial pressure during septic shock, interfere with hypoxic pulmonary vasoconstriction: vasodilators like isoproterenol or dobutamine tend to inhibit hypoxic pulmonary vasoconstriction whereas vasoconstrictors like dopamine, epinephrine or norepinephrine tend to reinforce hypoxic pulmonary vasoconstriction. In the present study, patients with circulatory shock receiving vasodilating inotrops were excluded in order to eliminate the interferences between these agents, inhaled NO and hypoxic pulmonary vasoconstriction.
Confirming a previous study [11], an important interpatient variability was found in both groups of patients (Figs 4 and 5). Several factors may account for this variability: at the time of investigation, endogenous vasoconstricting mediators involved in pulmonary artery hypertension were probably different between patients. In animal studies, NO dose–response curves depend on the model of acute lung injury and on the pathophysiology of pulmonary artery hypertension [33,34,35]. In patients treated with extracorporeal membrane oxygenation, dose–response curves of inhaled NO on MPAP have been found to be in the range of 1–100 ppm [2]. It has been suggested that pulmonary vasoconstrictors are continuously activated by the extracorporeal circuit and released into the circulation, thus contributing to pulmonary hypertension [36,37,38,39]. Therefore, it is conceivable that higher concentrations of NO are necessary to obtain the maximum effect of NO on pulmonary artery pressure. In the present study, dose-response curves in the range of 0.15 to 150 ppm were observed in three patients without septic shock and in three patients with septic shock. By analogy with the dose-response curves obtained in patients on extracorporeal membrane oxygenation, it can be hypothesized that the presence of large amounts of circulating pulmonary vasoconstrictors in these patients led to the need for greater NO concentrations. The variability of circulating vasoactive mediators from one day to another has been recently advocated to explain the variability of the dose-response to NO on different days in the same patient [11].
There are three factors that could have a potential influence on the responsiveness of patients with ARDS to inhaled NO: (1) the anatomical remodeling of the pulmonary circulation; (2) the reduction of the lung volume accessible to gas, and (3) the presence of septic shock.
External compression of the pulmonary vessels by PEEP, thickening of pulmonary arterial walls observed in the late stage of ARDS, and thrombosis [40] contribute to further increase pulmonary artery pressure which becomes less and less sensitive to inhaled NO. If the alveolar space available for distribution of NO is reduced, only a small number of pulmonary vessels can be reached, thus limiting the efficiency of NO. Because all patients were enrolled in the study during the first 5 days of acute respiratory failure, it is unlikely that wall thickening was an important limiting factor of NO efficiency. However, a major reduction in lung volume was likely to account for the limited effect of NO observed in some patients with lung consolidation > 70% (patients 3, 7 and 10 in Figs 4 and 5). Recently, it has been suggested that the presence of septic shock may impair responsiveness to inhaled NO [14]. However, due to the small number of patients included in this study and the absence of a control group, further studies are required to confirm this interesting hypothesis.
Finally, the maximum pulmonary vascular effect and the dose–response of inhaled NO on pulmonary artery pressure depends on many diverse factors that may be associated in a given patient: type and concentration of circulating pulmonary vasoconstrictors and vasodilators (endogenous and exogenous); relative importance of 'fixed' and 'nonfixed' components of pulmonary artery hypertension; and loss of lung volume. The results of the present study show that during the early stage of ARDS, inspiratory NO concentrations around 5 ppm provide the maximum decrease in pulmonary artery pressure in the majority of patients whereas higher concentrations are necessary in a minority of patients.
Dissociation between pulmonary vascular effects and effects on gas exchange
Quantitatively, the effects of NO on pulmonary artery pressure and arterial oxygenation were well correlated in 75% of patients. In 11 subjects (patients 5 to 8 and 10 to 16) quantitative variations in PaO2 and pulmonary artery pressure were in agreement: a decrease in MPAP > 20% of the control value was associated with an increase in PaO2/FiO2 > 130% of the control value and vice versa. In five subjects (patients 1 to 4 and patient 9) inhaled NO-induced changes in MPAP and PaO2/FiO2 were quantitatively dissociated. Patient 9 illustrates this (Fig 5) — although among patients with septic shock he had the greatest NO-induced decrease in MPAP, his PaO2/FiO2 ratio only increased by 70%. These results clearly suggest that, although linked, NO-induced pulmonary vascular effects and effects on arterial oxygenation can be dissociated in patients with ARDS. In patients with septic shock, pulmonary arterial pressure plateaued at 15 ppm whereas PaO2/FiO2 continued to increase at higher NO concentrations. This is in apparent contrast with two previous dose-response studies showing that the increase in PaO2 in patients with ARDS generally occurs at an inspiratory NO concentration range lower than the one necessary to decrease pulmonary artery pressure [2, 11]. Further, undetectable changes in pulmonary artery pressure may induce pulmonary blood flow redistribution and changes in arterial oxygenation [2,3,11]. Recently, however, Lowson et al [10] found, as did this study, that PaO2 continued to increase whereas pulmonary artery pressure and pulmonary vascular resistance plateaued at NO concentrations > 0.1 ppm. In fact, among six dose-response studies already published [2,4,9,10,11,22] only two [2,11] have suggested that NO concentrations required to improve PaO2 are less than those required to decrease pulmonary artery pressure. At high concentrations, it may be that NO reaches pulmonary vessels perfusing non-ventilated lung areas and worsens arterial oxygenation by inhibiting hypoxic pulmonary vasoconstriction as observed in patients 1, 2, 6, 8 and 15. This 'spillover' of NO into the pulmonary circulation could occur either by diffusion through the lung structures or directly by transportation in the blood stream [41].
In conclusion, in patients with ARDS the presence of septic shock treated by norepinephrine administration does not modify the inhaled NO-induced pulmonary artery vascular effect but amplifies the resulting improvement in arterial oxygenation. Although dose–response curves are characterized by a wide inter-patient variability, 90% of the pulmonary vascular effect is obtained for NO concentrations ≤ 4.5 ppm in patients with or without septic shock. The use of such low concentrations precludes any potential toxicity due to the generation of high concentrations of NO2 and methemoglobin. In many patients, the pulmonary vascular effect and effect on gas exchange, although linked, are dissociated suggesting that redistribution of pulmonary blood flow does not exclusively depend on the intensity of the pulmonary vasodilating effect. In a minority of patients, inspiratory NO concentrations > 5 ppm may be necessary to obtain the maximum improvement in arterial oxygenation.
Acknowledgments
Acknowledgements
The authors thank Dr Liliane Bodin, Dr Pierre Kalfon and Dr Pascale Poète for their contribution to the study; the nurses of the Surgical Intensive Care Unit and the technicians of the Department of Radiology for their active participation; E Vicaut for his statistical advice; and Véronique Connan for her secretarial assistance in preparing the manuscript.
This paper was presented in part at the 36th Congrès National d'Anesthésie et de Réanimation, Paris, France, 30 September–2 October 1994 and at the third congress of the European Society of Anaesthesiologists, Paris, France, 29 April–3 May 1995.
References
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol ZM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Gerlach H, Rossaint R, Pappert D, Falke KJ. Time-course and dose-response of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndrome. Eur J Clin Invest. 1993;23:499–502. doi: 10.1111/j.1365-2362.1993.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Gerlach H, Pappert D, Lewandowski K, Rossaint R, Falke KJ. Long-term inhalation with evaluated low doses of nitric oxide for selective improvement in oxygenation in patients with adult respiratory distress syndrome. Intensive Care Med. 1993;19:443–449. doi: 10.1007/BF01711084. [DOI] [PubMed] [Google Scholar]
- Puybasset L, Rouby JJ, Mourgeon E, et al. Inhaled nitric oxide in acute respiratory failure: dose–response curves. Intensive Care Med. 1994;20:319–327. doi: 10.1007/BF01720903. [DOI] [PubMed] [Google Scholar]
- Puybasset L, Stewart T, Rouby JJ, et al. Inhaled nitric oxide reverses the increase in pulmonary vascular resistance induced by permissive hypercapnia in patients with ARDS. Anesthesiology. 1994;80:1254–1267. doi: 10.1097/00000542-199406000-00013. [DOI] [PubMed] [Google Scholar]
- Bigatello LM, Huford WE, Kacmarek RM, Roberts JD, Zapol ZM. Prolonged inhalation of low concentrations of nitric oxide in patients with adult respiratory distress syndrome. Anesthesiology. 1994;80:761–770. doi: 10.1097/00000542-199404000-00007. [DOI] [PubMed] [Google Scholar]
- Young JD, Brampton WJ, Knighton JD, Finfer SR. Inhaled nitric oxide in acute respiratory failure in adults. Br J Anaesth. 1994;73:499–502. doi: 10.1093/bja/73.4.499. [DOI] [PubMed] [Google Scholar]
- Puybasset L, Rouby JJ, Mourgeon E, et al. Factors influencing cardiopulmonary effects of inhaled nitric oxide in acute respiratory failure. Am J Respir Crit Care Med. 1995;152:318–328. doi: 10.1164/ajrccm.152.1.7599840. [DOI] [PubMed] [Google Scholar]
- Lu Q, Mourgeon E, Law-Koune JD, et al. Dose-response curves of inhaled nitric oxide with and without almitrine in nitric oxide responding patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:929–943. doi: 10.1097/00000542-199511000-00005. [DOI] [PubMed] [Google Scholar]
- Lowson S, Rich MGF, Mc Ardle PA, Jaidev J, Morris GN. The response to varying concentrations of inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesth Analg. 1996;82:574–581. doi: 10.1097/00000539-199603000-00026. [DOI] [PubMed] [Google Scholar]
- Lundin S, Westfelt UN, Stenqvist O, et al. Response to nitric oxide inhalation in early acute lung injury. Intensive Care Med. 1996;22:728–734. doi: 10.1007/BF01709513. [DOI] [PubMed] [Google Scholar]
- Dyar O, Young JD, Xiong L, Howell S, Johns E. Dose–response relationship for inhaled nitric oxide in experimental pulmonary hypertension in sheep. Br J Anaesth. 1993;71:702–708. doi: 10.1093/bja/71.5.702. [DOI] [PubMed] [Google Scholar]
- Rovira I, Chen TY, Winkler M, Kawai N, Bloch ZD, Zapol WM. Effects of inhaled nitric oxide on pulmonary hemodynamics and gas exchange in an ovine model of ARDS. J Appl Physiol. 1994;76:345–355. doi: 10.1152/jappl.1994.76.1.345. [DOI] [PubMed] [Google Scholar]
- Krafft P, Fridrich P, Fitzgerald RD, Koc D, Steltzer H. Effectiveness of nitric oxide inhalation in septic ARDS. Chest. 1996;109:486–493. doi: 10.1378/chest.109.2.486. [DOI] [PubMed] [Google Scholar]
- Fellahi JL, Mourgeon E, Goarin JP. Inhaled nitric oxide-induced closure of a patent foramen ovale in a patient with acute respiratory distress syndrome and life-threatening hypoxemia. Anesthesiology. 1995;83:635–638. doi: 10.1097/00000542-199509000-00028. [DOI] [PubMed] [Google Scholar]
- American COllege of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes and clinical trial coordination. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Murray JF, Matthay MA, Luce JM, Fick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Fletcher R, Jonson B, Cumming G, Brew J. The concept of dead space with special reference to the single breath test for carbon dioxide. Br J Anaesth. 1981;53:77–88. doi: 10.1093/bja/53.1.77. [DOI] [PubMed] [Google Scholar]
- Chioléro R, Mavrocordatos P, Bracco D, Schutz Y, Cayeux C, Revelly JP. O2 consumption by the Fick method. Methodologic factors. Am J Respir Crit Care Med. 1994;149:1118–1122. doi: 10.1164/ajrccm.149.5.8173750. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design, 2nd edn New York: Mc Graw-Hill. 1971.
- Finer NN, Etches PC, Kamstra B, Tierney AJ, Peliowski A, Ryan CA. Inhaled nitric oxide in infants referred for extracorporeal membrane oxygenation: dose–response. J Pediatr. 1994;124:302–308. doi: 10.1016/s0022-3476(94)70324-8. [DOI] [PubMed] [Google Scholar]
- Samama CM, Diaby M, Fellahi JL, et al. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:56–65. doi: 10.1097/00000542-199507000-00007. [DOI] [PubMed] [Google Scholar]
- Mourgeon E, Rouby JJ, et al. Distribution of inhaled nitric oxide during sequential and continuous administration into the inspiratory limb of the ventilator. Intensive Care Med. 1997 doi: 10.1007/s001340050421. [DOI] [PubMed] [Google Scholar]
- Imanaka H, Hess D, Kirmse M, et al. Inaccuracies of nitric oxide delivery systems during adult mechanical ventilation. Anesthesiology. 1997;86:676–688. doi: 10.1097/00000542-199703000-00021. [DOI] [PubMed] [Google Scholar]
- Graham LM, Vasil A, Vasil ML, Voelkel NF, Stenmark KR. Decreased pulmonary vasoreactivity in an animal model of chronic pseudomonas pneumonia. Am Rev Respir Dis. 1990;142:221–229. doi: 10.1164/ajrccm/142.1.221. [DOI] [PubMed] [Google Scholar]
- Spapen H, Vincken W. Pulmonary arterial hypertension in sepsis and the adult respiratory distress syndrome. Acta Clin Belg. 1992;47:30–41. doi: 10.1080/17843286.1992.11718207. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Grover RF. Blockade of acute hypoxic pulmonary hypertension by endotoxin. J Appl Physiol. 1974;36:328–332. doi: 10.1152/jappl.1974.36.3.328. [DOI] [PubMed] [Google Scholar]
- Newman JH, Mc Murtry IF, Reeves JT. Blunted pulmonary pressure response to hypoxia in perfused, ventilated lungs from oxygen toxic rats: possible role of prostaglandins. Prostaglandins. 1980;22:1–20. doi: 10.1016/0090-6980(81)90050-2. [DOI] [PubMed] [Google Scholar]
- Demling RH, Smith M, Gunther R. The effects of prostacyclin infusion on endotoxin induced lung injury. Surgery. 1981;89:257–263. [PubMed] [Google Scholar]
- Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–2047. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Jr, Chen TY, Kawai N, et al. Inhaled nitric oxide reverses pulmonary vasoconstriction in the hypoxic and acidotic newborn lamb. Circ Res. 1993;72:246–254. doi: 10.1161/01.res.72.2.246. [DOI] [PubMed] [Google Scholar]
- Dyar O, Young JD, Xiong L, Howell S, Johns E. Dose-response relationship for inhaled nitric oxide in experimental hypertension in sheep. Br J Anaesth. 1993;71:702–708. doi: 10.1093/bja/71.5.702. [DOI] [PubMed] [Google Scholar]
- Berger JI, Gibson RL, Redding GJ, Standaert TA, Clarke WR, Truog WE. Effect of inhaled nitric oxide during group B streptococcal sepsis in piglets. Am Rev Respir Dis. 1993;147:1080–1086. doi: 10.1164/ajrccm/147.5.1080. [DOI] [PubMed] [Google Scholar]
- Fratacci MD, Frostell CG, Chen TY, Wain JC, Robinson DR, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator of heparin-protamine vasoconstriction in sheep. Anesthesiology. 1991;75:990–999. doi: 10.1097/00000542-199112000-00011. [DOI] [PubMed] [Google Scholar]
- Chenoweth DE, Cooper SW, Hugli TE, et al. Complement activation during cardiopulmonary bypass. N Engl J Med. 1981;304:497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- Ylikorkala O, Saarela E, Viinikka L. Increased prostacyclin and thromboxane production in man during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1981;82:245–247. [PubMed] [Google Scholar]
- Colman RW. Platelet and neutrophil activation in cardiopulmonary bypass. Ann Thorac Surg. 1990;49:32–34. doi: 10.1016/0003-4975(90)90352-7. [DOI] [PubMed] [Google Scholar]
- Jahr J, Grände PO. Pulmonary and hemodynamic effects of extracorporeal circulation in the cat and the beneficial effect of prostacyclin. Intensive Care Med. 1992;18:118–122. doi: 10.1007/BF01705045. [DOI] [PubMed] [Google Scholar]
- Jones R, Langleben D, Reid LM. Patterns and mechanisms of remodeling of the pulmonary circulation in acute and subacute lung injury. In The Pulmonary Circulation and Acute Lung Injury Edited by Said SI Mount Kisko, NY: Futura Publishing Company. 1991. pp. 179–242.
- Jia L, Bonaventura C, Bonaventura J, Stanuler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]


