 Achiral meteoritic glycine and α-methylalanine, with hydrogen isotope (D/H) chirality, acted as the source of chirality in asymmetric autocatalysis with amplification of ee to afford highly enantioenriched 5-pyrimidyl alkanols.
Achiral meteoritic glycine and α-methylalanine, with hydrogen isotope (D/H) chirality, acted as the source of chirality in asymmetric autocatalysis with amplification of ee to afford highly enantioenriched 5-pyrimidyl alkanols.
Abstract
Achiral meteoritic amino acids, glycine and α-methylalanine, with hydrogen isotope (D/H) chirality, acted as the source of chirality in asymmetric autocatalysis with amplification of ee to afford highly enantioenriched 5-pyrimidyl alkanols.
One of the most fascinating subjects about the prebiotic world is the origin of homochirality, as in l-amino acids and d-sugars.1 According to the theory of the extraterrestrial origin of biological homochirality, the amino acids in meteorites have been considered to play an important role in molecular evolution2 because a variety of prebiotic organic molecules, especially l-enriched amino acids with only slight enantiomeric excess (ee), have been identified.3 Their stable-isotope enrichment (2H, 13C and 15N) supports an extraterrestrial origin for these chiral amino acids.4
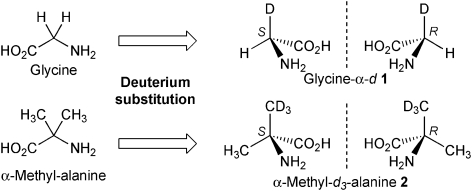
On the other hand, meteorites also include achiral amino acids such as glycine and α-methylalanine that have also been identified as isotopically enriched relative to terrestrial compounds.4c The δ D value of Vienna standard mean ocean water of meteoritic glycine and α-methylalanine appeared to be 399‰ and 3097‰, in contrast to the terrestrial biogenic compounds, which range from 350‰ to 50‰. Although glycine and α-methylalanine composed of 1H are achiral, this is not the case of meteoritic compounds because their hydrogen isotope ratio is high and should include the isotopically chiral components. Deuteration of the methylene group of glycine and one methyl group of α-methylalanine produces the hydrogen isotopically chiral compounds glycine-α-d 1 and α-methyl-d 3-alanine 2 (Fig. 1).
Fig. 1. Generation of chirality by the deuterium substitution of enantiotopic hydrogen in glycine and α-methylalanine.
To the best of our knowledge, there are no reports on the analysis of isotopic chirality of achiral meteoritic amino acids. Analysis of the isotopic chirality in meteoritic achiral molecules is an important subject to help understand the possible role of these compounds as the origin of chirality. In addition, it may expand research on the pathway by which chirality and isotope enrichment are produced in the meteoritic compounds in the universe.
Although there are a few reports on asymmetric reactions,1g,5 and separation6 induced by hydrogen isotope chirality, unlike usual chiral compounds the recognition of the enantiomers solely as a result of isotope labeling still remains difficult. Thus, the development of a highly sensitive method for the detection of isotopic chirality in meteoritic organic compounds, especially amino acids, which are identified in carbonaceous chondrites4c with an achiral framework and isotopic enrichment, is a challenging subject.
We and others have extensively studied asymmetric autocatalysis with amplification of ee.7–11 We have also reported that the external chiral initiator,12 such as chiral natural amino acids,12e in the reaction of pyrimidine-5-carbaldehyde and diisopropylzinc (i-Pr2Zn) controls the absolute configuration of the produced 5-pyrimidyl alkanols and that the ee of the produced pyrimidyl alkanols is high, in conjunction with asymmetric autocatalysis. Chiral deuterated primary alcohols and carbon isotopically chiral compounds can work as chiral triggers of this autocatalytic amplification of ee.13
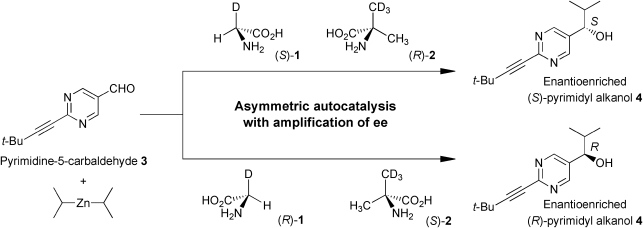
We report here that the isotopically chiral glycine-α-d (1)14 and α-methyl-d 3-alanine (2)15 induce the enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 to afford, in combination with asymmetric autocatalysis, pyrimidyl alkanol 4 with significantly high ee. The absolute configuration of the corresponding alkanol 4 was controlled efficiently by the chirality resulting from hydrogen isotope substitution of 1 and 2 (Scheme 1). Hydrogen isotope enantiomers of compounds 1 and 2 were synthesized by a previously reported method14,15 and the ee was determined from the 1H NMR spectrum of the camphanyl amide of 1 and the α-methoxy-α-(trifluoromethyl)phenylacetamide (MTPA amide) of 2 by the diastereomer method.
Scheme 1. Isotopically chiral amino acid induced asymmetric autocatalysis of 5-pyrimidyl alkanol 4 in the addition of i-Pr2Zn to aldehyde 3.
The results of asymmetric autocatalysis triggered by isotopically chiral amino acids are summarized in Table 1. When the i-Pr2Zn addition was performed in the presence of the (S)-glycine-α-d (1) with an ee of 93%, the (S)-5-pyrimidyl alkanol 4 was obtained in a 94% yield with an ee of 96% (Table 1, series I, entry 1). The results were reproducible. Thus, (S)-4 with an ee of 95% was obtained in the presence of (S)-1 (entry 2). The asymmetric autocatalysis using (S)-1 with 94% ee prepared in a different batch supported the reproducibility; that is, (S)-1 induces the formation of (S)-4 with high ee (entries 3, 4). On the other hand, in the presence of the (R)-1 instead of the (S)-1 enantiomer, the reaction between aldehyde 3 and i-Pr2Zn always gave (R)-4 with an ee of 91–93% in a yield of 90–94% (entries 5–8). These results clearly exhibit the correlation between the chirality of the glycine-α-d (1) and the absolute configuration of the resulting alkanol 4.
Table 1. The enantioselectivity and stereochemical correlation between the hydrogen isotope chirality of achiral amino acids, i.e., glycine 1 and α-methylalanine 2, and absolute configuration of the subsequent pyrimidyl alkanol 4 .
| Pyrimidyl alkanol 4 |
||||
| Entry | Amino acida (% ee) | Yieldb (%) | eec (%) | Config. |
| Series I (glycine-α-d1)d | ||||
| 1 | (S)-1 (93) | 94 | 96 | S |
| 2 | (S)-1 (93) | 98 | 95 | S |
| 3 | (S)-1 (94) | 94 | 94 | S |
| 4 | (S)-1 (94) | 95 | 93 | S |
| 5 | (R)-1 (96) | 94 | 93 | R |
| 6 | (R)-1 (96) | 94 | 91 | R |
| 7 | (R)-1 (92) | 90 | 91 | R |
| 8 | (R)-1 (92) | 93 | 92 | R |
| Series II (α-methyl-d3-alanine 2)e | ||||
| 9f | (R)-2 (81) | 95 | 99 | S |
| 10f | (R)-2 (81) | 97 | 98 | S |
| 11 | (R)-2 (81) | 94 | 96 | S |
| 12 | (R)-2 (81) | 92 | 97 | S |
| 13f | (S)-2 (69) | 99 | 98 | R |
| 14f | (S)-2 (69) | 93 | 99 | R |
| 15 | (S)-2 (69) | 84 | 97 | R |
| 16 | (S)-2 (69) | 93 | 96 | R |
aThe ee of 1 and 2 were determined from the 1H NMR spectrum of the camphanyl amide of 1 and the MTPA amide of 2.
bIsolated yield.
cThe ee was determined using HPLC employing a chiral stationary phase.
dThe molar ratio was 1/3/i-Pr2Zn = 0.05/1.05/2.2. The aldehyde 3 and i-Pr2Zn were added in three separate portions. Experimental details are as follows (entry 1): i-Pr2Zn (0.15 mL of 1 M methylcyclohexane (MCH) solution, 0.15 mmol) was added to (S)-1 (3.8 mg, 0.05 mmol) and the mixture was stirred for 12 h at room temperature. After the addition of i-Pr2Zn (0.5 mL of 1 M MCH solution, 0.5 mmol,) at 0 °C, a MCH (2.0 mL) solution of 3 (9.4 mg, 0.05 mmol) was added over a period of 1 h and the mixture was stirred for 6 h at 0 °C. After toluene (3.2 mL) and i-Pr2Zn (0.4 mL of 1 M toluene solution, 0.4 mmol) were added, the reaction mixture was stirred for 10 min. Then, a toluene (1.5 mL) solution of 3 (37.6 mg, 0.2 mmol) was added over a period of 1 h at 0 °C, and the mixture was stirred for 2 h. Moreover, after toluene (14.4 mL) and i-Pr2Zn (1.6 mL of 1 M toluene solution) were added and the mixture was stirred for 10 min, a toluene (4.0 mL) solution of 3 (150.6 mg, 0.8 mmol) was added over a period of 1 h. After stirring for 1 h, the reaction was quenched with 1 M aqueous hydrochloric acid (5 mL) at 0 °C. After neutralization with saturated aqueous sodium hydrogen carbonate (15 mL), the mixture was filtered through Celite, and the filtrate was extracted with ethyl acetate. The combined organic fractions were dried over anhydrous sodium sulfate and evaporated in vacuo. Purification of the residue using silica gel thin layer chromatography (hexane–ethyl acetate = 2/1, v/v) gave (S)-4 (229 mg, 0.9849 mmol, 96% ee) in 94% yield.
eThe molar ratio was 2/3/i-Pr2Zn = 0.075/0.625/1.35. The aldehyde 3 and i-Pr2Zn were added in four separate portions.
fEach pair of reactions (9/13 and 10/14) was performed using the same apparatus to exclude any effect other than that of isotopically chiral amino acid 2.
Next, we examined the highly enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 using the (R)- and (S)-α-methyl-d 3-alanine (2). In the presence of (R)-α-methyl-d 3-alanine (2), (S)-5-pyrimidyl alkanol 4 was induced, and was obtained with an ee of 96–99% (series II, entries 9–12). On the other hand, in the presence of (S)-2, the reaction between aldehyde 3 and i-Pr2Zn always gave (R)-alkanol 4 with an ee of 96–99% in a yield of 84–99% (entries 13–16). To exclude any effect other than that of the α-methyl-d 3-alanine (2), both reactions (entries 9, 13 and 10, 14) induced by (R)- and (S)-2 were performed using the same apparatus to give the (S)- and (R)-alkanols 4, respectively.
The chirality of these enantiomers is mainly due to the very small difference between the lengths of the C–D and C–H bonds.16 The high ee observed in the above asymmetric reactions may be explained as follows. In the initial step of the reaction, the isotopically chiral amino acid (or its zinc salt) induces a slight enantiomeric imbalance in the addition of i-Pr2Zn to 3. The resulting isopropylzinc alkoxide of 4 has a small ee, which possesses the absolute configuration corresponding to that of the isotopically chiral amino acid. Then, the ee is enhanced during the subsequent asymmetric autocatalysis followed by hydrolysis, which affords alkanol 4 with high ee.13
In conclusion, the enantioselective addition of i-Pr2Zn to pyrimidine-5-carbaldehyde 3 was achieved by utilizing the hydrogen isotopic chirality of achiral amino acids, that is, glycine-α-d (1) and α-methyl-d 3-alanine (2). The observed ee of the produced pyrimidyl alkanol 4 was amplified up to 99% ee in conjunction with asymmetric autocatalysis. This is the first example of a highly enantioselective reaction induced by the chirality resulting from deuterium substitution of amino acids, which are detected in meteorites as achiral isotopically enriched molecules. We believe that the asymmetric autocatalysis significantly increases the value of the implications of isotope substitution in achiral meteoritic amino acids in the study of the origin and prebiotic evolution of biological homochirality.
Footnotes
†This article is part of a ChemComm ‘Catalysis in Organic Synthesis’ web-theme issue showcasing high quality research in organic chemistry. Please see our website (http://www.rsc.org/chemcomm/organicwebtheme2009) to access the other papers in this issue.
References
- (a) Mislow K. Collect. Czech. Chem. Commun. 2003;68:849–864. [Google Scholar]; (b) Feringa B. L., van Delden R. A. Angew. Chem., Int. Ed. 1999;38:3418–3438. doi: 10.1002/(sici)1521-3773(19991203)38:23<3418::aid-anie3418>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]; (c) Bolli M., Micula R., Eschenmoser A. Chem. Biol. 1997;4:309–320. doi: 10.1016/s1074-5521(97)90074-0. [DOI] [PubMed] [Google Scholar]; (d) Bonner W. A. Origins Life Evol. Biosphere. 1995;25:175–190. doi: 10.1007/BF01581581. [DOI] [PubMed] [Google Scholar]; (e) Girard C., Kagan H. B. Angew. Chem., Int. Ed. 1998;37:2923–2959. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2922::AID-ANIE2922>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]; (f) Zepik H., Shavit E., Tang M., Jensen T. R., Kjaer K., Bolbach G., Leiserowitz L., Weissbuch I., Lahav M. Science. 2002;295:1266–1269. doi: 10.1126/science.1065625. [DOI] [PubMed] [Google Scholar]; (g) Green M. M., Park J.-W., Sato T., Teramoto A., Lifson S., Selinger R. L. B., Selinger J. V. Angew. Chem., Int. Ed. 1999;38:3139–3154. doi: 10.1002/(sici)1521-3773(19991102)38:21<3138::aid-anie3138>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]; (h) Ribó J. M., Crusats J., Sagues F., Claret J. M., Rubires R. Science. 2001;292:2063–2066. doi: 10.1126/science.1060835. [DOI] [PubMed] [Google Scholar]; (i) Barabás B., Caglioti L., Micskei K., Zucchi C., Pályi G. Origins Life Evol. Biosphere. 2008;38:317–327. doi: 10.1007/s11084-008-9138-1. [DOI] [PubMed] [Google Scholar]; (j) Guijarro A. and Yus M., The Origin of Chirality in the Molecules of Life, The Royal Society of Chemistry, Cambridge, 2009 [Google Scholar]
- (a) Notz W., List B. J. Am. Chem. Soc. 2000;122:7386–7387. [Google Scholar]; (b) Pizarello S., Weber A. L. Science. 2004;303:1151–1151. doi: 10.1126/science.1093057. [DOI] [PubMed] [Google Scholar]; (c) Córdova A., Engqvist M., Ibrahem I., Casas J., Sundén H. Chem. Commun. 2005:2047–2049. doi: 10.1039/b500589b. [DOI] [PubMed] [Google Scholar]
- (a) Kvenvolden K. Nature. 1970;228:923–926. doi: 10.1038/228923a0. [DOI] [PubMed] [Google Scholar]; (b) Cronin J. R., Pizzarello S. Science. 1997;275:951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]; (c) Engel M. H., Nagy B. Nature. 1982;296:837–840. [Google Scholar]; (d) Glavin D. O., Dworkin J. P. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5487–5492. doi: 10.1073/pnas.0811618106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Epstein S., Krishnamurthy R. V., Cronin J. R., Pizzarello S., Yuen G. U. Nature. 1987;326:477–479. doi: 10.1038/326477a0. [DOI] [PubMed] [Google Scholar]; (b) Engel M. H., Macko S. A. Nature. 1997;389:265–268. doi: 10.1038/38460. [DOI] [PubMed] [Google Scholar]; (c) Pizzarello S., Huang Y. Geochim. Cosmochim. Acta. 2005;69:599–605. [Google Scholar]
- (a) Pracejus H. Tetrahedron Lett. 1966;7:3809–3813. [Google Scholar]; (b) Horeau A., Nouaille A., Mislow K. J. Am. Chem. Soc. 1965;87:4957–4958. [Google Scholar]
- (a) Kimata K., Kobayashi M., Hosoya K., Araki T., Tanaka N. J. Am. Chem. Soc. 1996;118:759–762. [Google Scholar]; (b) Pirkle W. H., Gan K. Z. Tetrahedron: Asymmetry. 1997;8:811–814. [Google Scholar]; (c) Harris III R. N., Sundararaman P., Djerassi C. J. Am. Chem. Soc. 1983;105:2408–2413. [Google Scholar]
- Soai K., Shibata T., Morioka H., Choji K. Nature. 1995;378:767–768. [Google Scholar]
- (a) Sato I., Urabe H., Ishiguro S., Shibata T., Soai K. Angew. Chem., Int. Ed. 2003;42:315–317. doi: 10.1002/anie.200390105. [DOI] [PubMed] [Google Scholar]; (b) Shibata T., Yonekubo S., Soai K. Angew. Chem., Int. Ed. 1999;38:659–661. doi: 10.1002/(SICI)1521-3773(19990301)38:5<659::AID-ANIE659>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; (c) Shibata T., Morioka H., Hayase T., Choji K., Soai K. J. Am. Chem. Soc. 1996;118:471–472. [Google Scholar]; (d) Lutz F., Kawasaki T., Soai K. Tetrahedron: Asymmetry. 2006;17:486–490. [Google Scholar]
- (a) Podlech J., Gehring T. Angew. Chem., Int. Ed. 2005;44:5776–5777. doi: 10.1002/anie.200501742. [DOI] [PubMed] [Google Scholar]; (b) Bolm C., Bienewald F., Seger A. Angew. Chem., Int. Ed. Engl. 1996;35:1657–1659. [Google Scholar]; (c) Todd M. H. Chem. Soc. Rev. 2002;31:211–222. doi: 10.1039/b104169j. [DOI] [PubMed] [Google Scholar]; (d) Avalos M., Babiano R., Cintas P., Jiménez J. L., Palacios J. C. Chem. Commun. 2000:887–892. [Google Scholar]; (e) Buschmann H., Thede R., Heller D. Angew. Chem., Int. Ed. 2000;39:4033–4036. doi: 10.1002/1521-3773(20001117)39:22<4033::aid-anie4033>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]; (f) Mikami K., Yamanaka M. Chem. Rev. 2003;103:3369–3400. doi: 10.1021/cr000260z. [DOI] [PubMed] [Google Scholar]; (g) Gridnev I. D. Chem. Lett. 2006;35:148–153. [Google Scholar]; (h) Caglioti L., Zucchi C., Pályi G. Chim. Oggi. 2005;23:38–43. [Google Scholar]
- (a) Soai K., Shibata T., Sato I. Bull. Chem. Soc. Jpn. 2004;77:1063–1073. [Google Scholar]; (b) Soai K., Shibata T., Sato I. Acc. Chem. Res. 2000;33:382–390. doi: 10.1021/ar9900820. [DOI] [PubMed] [Google Scholar]; (c) Soai K., Kawasaki T. Chirality. 2006;18:469–478. doi: 10.1002/chir.20289. [DOI] [PubMed] [Google Scholar]; (d) Soai K., Kawasaki T. Top. Curr. Chem. 2008;284:1–33. [Google Scholar]; (e) Soai K. and Kawasaki T., in Organometallic Chirality, ed. G. Palyi, C. Zucchi and L. Caglioti, Mucchi Editore, Modena, 2008, ch. 6, pp. 107–125 [Google Scholar]
- (a) Sato I., Omiya D., Igarashi H., Kato K., Ogi Y., Tsukiyama K., Soai K. Tetrahedron: Asymmetry. 2003;14:975–979. [Google Scholar]; (b) Gridnev I. D., Serafimov J. M., Brown J. M. Angew. Chem., Int. Ed. 2004;43:4884–4887. doi: 10.1002/anie.200353572. [DOI] [PubMed] [Google Scholar]; (c) Blackmond D. G. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5732–5736. doi: 10.1073/pnas.0308363101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Islas J. R., Lavabre D., Grevy J.-M., Lamoneda R. H., Cabrera H. R., Micheau J.-C., Buhse T. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13743–13748. doi: 10.1073/pnas.0503171102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Saito Y., Hyuga H. J. Phys. Soc. Jpn. 2004;73:33–35. [Google Scholar]; (f) Lente G. J. Phys. Chem. A. 2005;109:11058–11063. doi: 10.1021/jp054613f. [DOI] [PubMed] [Google Scholar]; (g) Micskei K., Pota G., Caglioti L., Palyi G. J. Phys. Chem. A. 2006;110:5982–5984. doi: 10.1021/jp0614502. [DOI] [PubMed] [Google Scholar]; (h) Klankermayer J., Gridnev I. D., Brown J. M. Chem. Commun. 2007:3151. doi: 10.1039/b705978g. [DOI] [PubMed] [Google Scholar]; (i) Lutz F., Igarashi T., Kawasaki T., Soai K. J. Am. Chem. Soc. 2005;127:12206–12207. doi: 10.1021/ja054323c. [DOI] [PubMed] [Google Scholar]; (j) Lutz F., Igarashi T., Kinoshita T., Asahina M., Tsukiyama K., Kawasaki T., Soai K. J. Am. Chem. Soc. 2008;130:2956–2958. doi: 10.1021/ja077156k. [DOI] [PubMed] [Google Scholar]; (k) Brown J. M., Gridnev I., Klankermayer J. Top. Curr. Chem. 2008;284:35–65. [Google Scholar]; (l) Lavabre D., Micheau J.-C., Islas J. R., Buhse T. Top. Curr. Chem. 2008;284:67–96. [Google Scholar]; (m) Saito Y., Hyuga H. Top. Curr. Chem. 2008;284:97–118. [Google Scholar]; (n) Schiaffino L., Ercolani G. Angew. Chem., Int. Ed. 2008;47:6832–6835. doi: 10.1002/anie.200802450. [DOI] [PubMed] [Google Scholar]
- (a) Shibata T., Yamamoto J., Matsumoto N., Yonekubo S., Osanai S., Soai K. J. Am. Chem. Soc. 1998;120:12157–12158. [Google Scholar]; (b) Soai K., Osanai S., Kadowaki K., Yonekubo S., Shibata T., Sato I. J. Am. Chem. Soc. 1999;121:11235–11236. [Google Scholar]; (c) Kawasaki T., Sato M., Ishiguro S., Saito T., Morishita Y., Sato I., Nishino H., Inoue Y., Soai K. J. Am. Chem. Soc. 2005;127:3274–3235. doi: 10.1021/ja0422108. [DOI] [PubMed] [Google Scholar]; (d) Kawasaki T., Tanaka H., Tsutsumi T., Kasahara T., Sato I., Soai K. J. Am. Chem. Soc. 2006;128:6032–6033. doi: 10.1021/ja061429e. [DOI] [PubMed] [Google Scholar]; (e) Sato I., Ohgo Y., Igarashi H., Nishiyama D., Kawasaki T., Soai K. J. Organomet. Chem. 2007;692:1783–1787. [Google Scholar]; (f) Kawasaki T., Suzuki K., Hakoda Y., Soai K. Angew. Chem., Int. Ed. 2008;47:496–499. doi: 10.1002/anie.200703634. [DOI] [PubMed] [Google Scholar]; (g) Kawasaki T., Omine T., Suzuki K., Sato H., Yamagishi A., Soai K. Org. Biomol. Chem. 2009;7:1073–1075. doi: 10.1039/b823282b. [DOI] [PubMed] [Google Scholar]
- (a) Sato I., Omiya D., Saito T., Soai K. J. Am. Chem. Soc. 2000;122:11739–11740. [Google Scholar]; (b) Kawasaki T., Matsumura Y., Tsutsumi T., Suzuki K., Ito M., Soai K. Science. 2009;324:492–495. doi: 10.1126/science.1170322. [DOI] [PubMed] [Google Scholar]
- (a) Walker J. R., Curley, Jr. W. Tetrahedron. 2001;57:6695–6701. [Google Scholar]; (b) Williams R. M., Zhai D., Sinclair Peter J. J. Org. Chem. 1986;51:5021–5022. [Google Scholar]
- cf.Seebach D., Fadel A., Helv. Chim. Acta, 1985, 68, 1243–1250 [Google Scholar]
- (a) Bartell L. S., Roth E. A., Hollowell C. D., Kuchitsu K., Young, Jr. J. E. J. Chem. Phys. 1965;42:2683–2686. [Google Scholar]; (b) Bartell L. S., Roskos R. R. J. Chem. Phys. 1966;44:457–463. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.