Abstract
Background:
The aim of the study was to assess the particle size stability of six parenteral nutrition regimens, fitted to various pathologies, and used by the University Hospital of Limoges. The mixtures contained glucose (30 or 50%), amino acids (Hyperamine®25), and either long-chain triglycerides (20% Intralipide®) or a combination of medium and long-chain triglycerides (20% Médialipide®). The regimens were not supplemented.
Results:
The visual examinations, particle size analysis and physico-chemical tests, carried out during a long storage period, did not reveal any significant evolution of the lipid emulsions. All the tested formulae were stable for 28 days at 4°C plus 24 h at room temperature.
Conclusions:
It was concluded that the choice of lipid emulsions depends, for these formulae, on the metabolic and clinical needs of the treated patients.
Keywords: medium chain triglycerides, parenteral nutrition, stability
Introduction
Parenteral nutrition is often peri-operative. It can be performed with packages containing different nutrients. Knowledge of the stability of these mixtures is necessary, because the infusion of unstable compounds is potentially dangerous [1]. Since Fujita's animal studies in 1971, it has been accepted that there is a relationship between toxicity and particle size [2]. The predominant factors affecting emulsion particle size seem to be pH, electrolyte concentration, and the amino acid and lipid composition of the mixtures [3,4]. A reduced stability is indicated by a low zeta potential, which is a standard parameter used to measure the electrostatic repulsion which prevents aggregation and coalescence of lipid droplets [4,5]. The aim of this study was to test the particle size stability of six parenteral nutrition mixtures fitted to different pathologies (Table 1). The mixtures tested were standard packages, with and without medium chain triglycerides (MCT); packages of low volume for renal or cardiac insufficiency, with and without MCT; packages with low lipid, high protein content with MCT, for mechanical ventilation weaning or stress situations; and high calorie, high protein mixtures with MCT, for situations with high requirements. All the tested formulae were stable for 28 days at 4°C plus 24 h at room temperature.
Table 1.
Contents, distinctive composition and suggested indications of the six tested parenteral nutrition mixtures
| Formulae | ||||||
| Composition | 1 | 2 | 3 | 4 | 5 | 6 |
| 50% Glucose (ml) | 500 | 500 | 650 | 600 | ||
| 30% Glucose (ml) | 750 | 750 | ||||
| 20% Intralipide® (ml) | 500 | 500 | ||||
| 20% Médialipide® (ml) | 500 | 500 | 500 | 600 | ||
| Hyperamine® 25 (ml) | 500 | 500 | 500 | 500 | 750 | 750 |
| Total volume (ml) | 1750 | 1750 | 1500 | 1500 | 1900 | 1950 |
| Nitrogen (g) | 12.8 | 12.8 | 12.8 | 12.8 | 19.2 | 19.2 |
| Glucido-lipidic ratio (% : %) | 47 : 53 | 48 : 52 | 50 : 50 | 50 : 50 | 57 : 43 | 50 : 50 |
| kcal/Nitrogen ratio | 148 | 147 | 156 | 155 | 119 | 124 |
| Glucido-lipidic kcal | 1900 | 1884 | 2000 | 1984 | 2284 | 2381 |
| Distinctive composition | Standard | Standard with MCT | Low volume | Low volume with MCT | Low lipid, high protein content, with MCT | High kcal, high protein content with MCT |
| Suggested indications | Standard | Standard | Cardiac or renal insufficiency | Cardiac or renal insufficiency | Ventilation weaning stress | High requirements |
MCT=medium chain triglycerides. Médialipide® is a registered trademark (in Germany, Lipofundin® MCT/LCT; in Denmark, Norway and Sweden, Vasolipid®).
Material and methods
The mixtures were not supplemented by electrolytes, trace elements or vitamins. The packages, made of ethylene vinyl acetate (EVA) (Nutripoches NPP235: Pharmacia SA, France), contained 50 or 30% glucose (Aguettant and Cooper Laboratories, St Quentin, France), amino acids 15.28 g/100 ml (Hyperamine 25®, B Braun Médical SA, France), and 20% lipids consisting of either medium and long-chain triglycerides (Médialipide®, B Braun Médical SA, France) or only long-chain triglycerides (Intralipide®, Pharmaica, France) (Table 1). The Central Pharmacy of the hospital filled the packages under nitrogen pressure in a clean room inside a laminary horizontal flux hood. Glucose was introduced first into each mixture bag, followed by the amino acids and finally the lipids. Two packages (one analytical and one observation bag for each regimen) were forwarded by air freight in a cold box to the stability control laboratory (B Braun Médical SA, Boulogne, France) within 24 h of production. They were stored in a refrigerator at 4 ± 1° C (circulating air) for 28 days, then for 24 h at room temperature. At the end of this period, each mixture was compared with the corresponding mother emulsion, which contained only lipids from the same batch. The stability tests were performed according to the following methods.
Visual observation
The colour and aspect of the mixtures was noted, with each being examined for creaming, oily droplets and the presence or absence of yellow traces. These examinations were performed without shaking the mixtures, and were carried out on the observation bags throughout the entire study. In the analytical bags, they were performed at each stage of analysis before and after the homogenization of the mixtures, which was done by inverting the bags 10 times according to the common conditions of use.
Visual microscopic observations
Visual microscopic observations (Leitz-Diaplan microscope) calculated the number of droplets with diameter > 2.5 μm. The dilution of the emulsions was 1:10 in order to obtain a final lipid concentration of 2 g/100 ml. The observations were made in a cell counting chamber (Thoma's grads) at × 500 magnification. A significant increase in particles with diameter > 5 μm indicated destabilisation and the presence of particles with diameter > 10 μm revealed a breakdown of the emulsion [1]. Polaroid microphotography (× 1000 magnification) confirmed the observations.
Particle size analysis
Particle size analysis was performed with a Coulter Counter TA II, with a 50 μm aperture. The results were expressed in terms of percentage of fat (% of total lipidic fraction) existing as particles in each different diameter class studied. This calculation took into account the number and mean diameter of particles in each class, the fat concentration of the sample, the analytical volume, and the oil density. Two dilutions were studied:
(1) 1:100,000 (final lipid concentration of 2 × 10-4g/100 ml) for particles with diameters between 0.8 and 5.04 μm. This value was then, in reference to internal laboratory values, correlated with the global granulometric state of the emulsions.
(2) 1:1000 (final lipid concentration of 2 × 10-2g/100 ml) for particles with diameter > 1.59μm. The acceptable limit, for this method and this last dilution, was 0.40% of fat in the mixture existing as particles with a diameter > 5 μm [1].
The above tests were performed at D1, D3, D7, D10, D14, D21, D28, D28 + 24 h (D29). pH was tested at every granulometric control stage. Conformity to the formula and osmolality was tested at the beginning and at the end of the study. The chemical analysis performed during the study measured the concentrations of the total nitrogen anhydrous glucose and glycerol, in order to confirm the each mixture conformed to its theoretical formulae (accepted limit ± 10%).
Results and discussion
Addition of ions, pH variation, but also lipid and amino acid composition influence the stability of parenteral nutrition mixtures [3,4,6,7]. The six tested mixtures were not ion-supplemented. The nitrogen content and quality of the triglycerides varied depending on the frequent clinical situation for which the mixture was used. There was no microscopic destabilization from D1 to D29 (no change in the colour or aspect of the mixtures). The mixtures at D29 were microscopically identical to the D1 mother emulsions. Analysis of particle size distribution revealed a difference between Intralipide® and Médialipide® in particles with diameter < 5 μm without by shift in the distribution curves (Figs 1 and 2).
Figure 1.
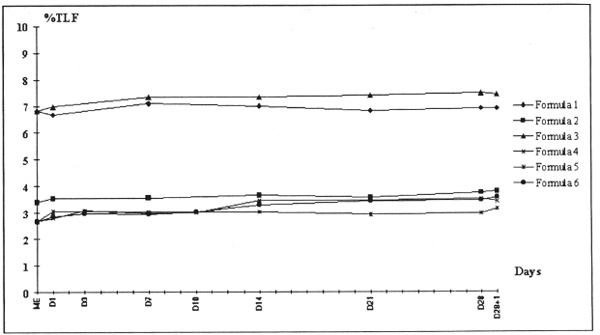
Particle size analysis with Coulter Counter TA II, 50 μ m aperture. The results are expressed as a percentage of the fat (% of total lipidic fraction = TLF). Dilution 1:100,000, particle diameters 0.79 to 5.04 μ m. Mother emulsions (ME) containing Intralipide® 20% are at 6.83% TLF; those containing Medialipide® 20% are at 3.39 and 2.66% TLF.
Figure 2.
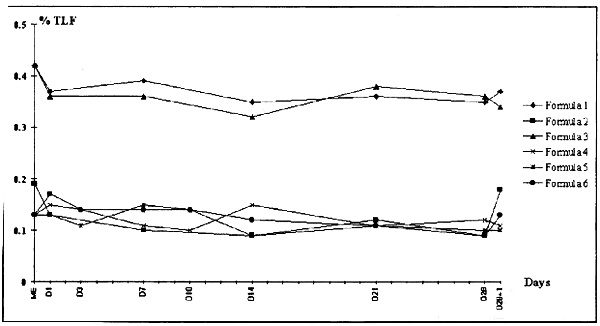
Particle size analysis with Coulter Counter TA II, 50 mm aperture. The results are expressed as a percentage of the fat (% of TLF). Dilution 1:1000, particle diameters 1.59 to 5.04 mm. Intralipide® 20%, ME = 0.42% TLF; Medialipide® 20%, ME = 0.19 and 0.13% TLF.
For particles with diameter > 5 μm, the values were the same for Intralipide® and Médialipide® (Fig 3). In all mixtures, no evolution (granulometric destablization or breakdown of the emulsion) was observed over the 29 days, regardless of the formula tested (Figs 1,2,3). All the mixtures conformed to the theoretical compositions, and there was not evolution during storage (Table 2). Osmolality and pH were not significantly different from D1 to D29 (Table 3).
Figure 3.
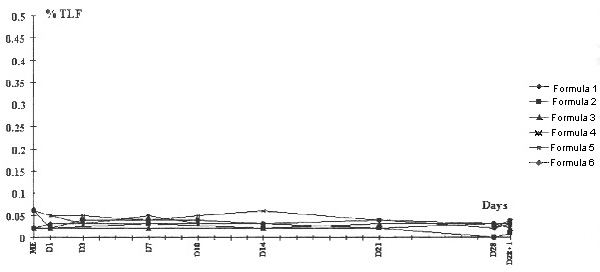
Particle size analysis with Coulter Counter TA II, 50 μ m aperture. The results are expressed as a percentage of the fat (% of TLF). Dilution 1:1000, particle diameters > 5.04 μ m. Intralipide® 20%, ME = 0.02% TLF; Medialipide® 20%, ME = 0.02 and 0.06% TLF.
Table 2.
Theoretical and D28 + 1 values of total nitrogen, anhydrous glucose and glycerol. None of the formulae at D28 + 1 exceed theoretical values ± 10%
| Total nitrogen (g/l) | Anhydrous glucose (g/l) | Glycerol (g/l) | ||||
| Formulae | Theoretical | D28 + 1 | Theoretical | D28 + 1 | Theoretical | D28 + 1 |
| 1 | 7.31 | 7.49 | 128.6 | 127.5 | 6.43 | 6.65 |
| 2 | 7.31 | 7.21 | 128.6 | 127.3 | 7.14 | 7.48 |
| 3 | 8.53 | 8.58 | 166.7 | 178.6 | 7.50 | 7.28 |
| 4 | 8.53 | 8.49 | 166.7 | 155.5 | 8.33 | 8.53 |
| 5 | 10.11 | 10.17 | 171.05 | 164.8 | 6.58 | 6.60 |
| 6 | 9.85 | 9.75 | 153.85 | 148.8 | 7.69 | 8.15 |
Table 3.
pH at D1 and D28 +1 for the six mixtures tested
| Formulae | ||||||
| Day | 1 | 2 | 3 | 4 | 5 | 6 |
| D1 | 6.56 | 6.55 | 6.62 | 6.48 | 6.42 | 6.47 |
| D28 + 1 | 6.59 | 6.56 | 6.59 | 6.57 | 6.51 | 6.58 |
Conclusion
All the stability tests complied — the six ternary unsupplemented controlled mixtures were stable and acceptable for normal therapeutic use after a long storage period, ie 28 days at 4°C, plus 24 h at room temperature. Therefore the choice of triglyceride mixture used in defined solely by the clinical and metabolic requirements of each regimen.
References
- Driscoll DF, Bhargava HN, Li L, Zaim RH, Babayan VK, Bistrian BR. Physicochemical stability of total nutrient admixtures. Am J Health-Syst Pharm. 1995;52:623–634. doi: 10.1093/ajhp/52.6.623. [DOI] [PubMed] [Google Scholar]
- Fujita T, Sumaya T, Yokoyama K. Fluorocarbon emulsion as a candidate for artificial blood. Eur Surg Res. 1971;3:436–453. doi: 10.1159/000127590. [DOI] [PubMed] [Google Scholar]
- Bullock L, Fitzgerald JF, Walter WV. Emulsion stability in total nutrient admixtures containing a pediatric amino acid formulation. J Parenter Enteral Nutr. 1992;16:64–68. doi: 10.1177/014860719201600164. [DOI] [PubMed] [Google Scholar]
- Müller RH, Heinemann S. Fat emulsions for parenteral nutrition. III: Lipofundin MCT/LCT regimens for total parenteral nutrition (TPN) with low electrolyte load. Int J Pharm. 1994;101:175–189. [Google Scholar]
- Barnett MI, Cosslett AG, Duffield JR, Evans DA, Hall SB, Williams DR. Parenteral nutrition. Pharmaceutical problems of compatibility and stability. Drug Safety. 1990;5 (suppl 1):101–106. doi: 10.2165/00002018-199000051-00016. [DOI] [PubMed] [Google Scholar]
- Gerbaud D, Lorieul F, Veber C, Bourillet F. Stabilité comparée d´une émulsion TCM/TCL et de deux émulsions TCL dans onze mélanges nutritifs Grenoble, France: Xéme Symposium de la SFNEP. 1990.
- Ricard C, Fortune R, Florent M, Bardet L. Granulométrie moyenne et distribution granulométrique des émulsions lipidiques injectables, paramàtres de stabilité pour la préparation des mélanges destinés à la nutrition parentérale totale. J Pharm Clin. 1996;15:72–80. [Google Scholar]


