Abstract
Prostaglandin E2 (PGE2) is a bioactive lipid that can elicit a wide range of biological effects associated with inflammation and cancer. The physiological roles of PGE2 are diverse, mediated in part through activation of key downstream signaling cascades via transmembrane EP receptors located on the cell surface. Elevated levels of COX-2 and concomitant overproduction of PGE2 are often found in human cancers. These observations have led to the use of non-steroidal anti-inflammatory drugs (NSAIDs) as chemopreventive agents, particularly for colorectal cancer (CRC). Their long-term use, however, may be associated with gastrointestinal toxicity and increased risk of adverse cardiovascular events, prompting the development of other enzymatic targets in this pathway. This review will focus on recent efforts to target the terminal synthase, mPGES-1, for cancer chemoprevention. The role of mPGES-1 in the pathogenesis of various cancers is discussed. In addition, an overview of recent efforts to develop small molecule inhibitors that target the protein with high selectivity is also be reviewed.
Keywords: mPGES-1, inflammation, PGE2, cancer, mPGES-1 inhibitor
I. Introduction
Prostaglandin E2 (PGE2) is a bioactive lipid that can elicit a wide range of biological effects associated with inflammation and cancer. PGE2 and other prostaglandins (PGs) are derived from the enzymatic release of arachidonic acid (AA), which is rapidly metabolized by cyclo-oxygenase (COX) enzymes and subsequently converted into a panel of PGs by specific terminal PG synthases [1–3]. The physiological roles of PGE2 are diverse, mediated in part through activation of key downstream signaling cascades via transmembrane EP receptors located on the cell surface [4]. Receptor-specific binding can activate diverse pathways that regulate cell proliferation, apoptosis, angiogenesis, inflammation and immune surveillance [3, 5, 6]. Elevated levels of COX-2 and concomitant overproduction of PGE2 are often found in human colon adenomas and in adenocarcinomas [7]. These and other observations have led to the use of non-steroidal anti-inflammatory drugs (NSAIDs) as chemopreventive agents for treatment of cancers, including most recently the selective COX-2 inhibitors (e.g. celecoxib). For example, the regular use of NSAIDs has been shown in clinical trials to markedly reduce the relative risk of developing CRC by up to 40–50% [8–11]. However, long-term clinical use of these agents is not without risk, as they have been associated with gastrointestinal toxicity and an increased risk of adverse cardiovascular events [12–14].
II. The prostaglandin E2 synthase (PTGES) family
In recent years, a significant effort has thus been directed towards the development of other enzymatic targets within the arachidonic acid pathway, including the PGE2 terminal synthases (PGES) [15]. The PGES family of proteins are the terminal synthases for the cloned to production of PGE2, and three different gene products with PGES activity have been date, namely PTGES1, PTGES2 and PTGES3, encoding microsomal PGES-1 (mPGES-1), mPGES-2 and cytosolic PGES (cPGES), respectively [6]. mPGES-1 is a member of the MAPEG (membrane-associated proteins involved in eicosanoid and glutathione metabolism) superfamily [15], showing significant homology with other MAPEG superfamily proteins, including microsomal glutathione-S-transferase (GST)-1-like 1 (MGST-1), 5-lipoxygenase (LOX)-activating protein (FLAP) and leukotriene C4 synthase (LTC4). mPGES-1 requires glutathione (GSH) as an essential cofactor for its enzymatic activity [15]. mPGES-1 expression is typically maintained at minimal levels in most normal tissues, although abundant and constitutive expression is detected in a limited number of organs, such as the lung, kidney, and reproductive organs. The closely related mPGES-2 has broader substrate specificity and exhibits some similarity in function to glutaredoxin and thioredoxin [6]. mPGES-2 is expressed constitutively in a variety of human tissues, and unlike mPGES-1, it is not induced by pro-inflammatory signals. cPGES is also expressed in a ubiquitous manner, and is thought to mediate constitutive PGE2 biosynthesis based on its preferential coupling with COX-1 [16].
While several comprehensive reviews have recently been published describing the enzymatic properties of mPGES-1 and its potential involvement with a range of pathological conditions [6, 17], this review will focus on recent reports that implicate mPGES-1 activity in the pathogenesis of cancer. In addition, we will provide an overview of recent efforts to develop small molecule inhibitors that target the protein with high selectivity.
II. mPGES-1 plays a critical role in cancer cell growth
PGE2 is widely recognized as a bioactive lipid metabolite with potent tumor promotion properties. PGE2 activates a wide range of proliferative signals via its binding to a family of EP receptors [5, 18, 19]. Directly associated with increased PGE2 production, clinical studies have shown increased levels of mPGES-1 present within a number of human cancers, including colon [20, 21], lung [22], stomach [23], pancreas [24], cervix [25], prostate [26], papillary thyroid carcinoma [27], head and neck squamaous carcinoma [28] and brain tumors [29, 30]. These studies are summarized in Table 1. Recently, Seo et al. [31] have reported that elevated levels of mPGES-1 and mPGES-2 are significantly correlated with a worse prognosis in late stages of colorectal cancer, suggesting that the PGE2 synthases may play a key role during cancer progression.
Table 1.
Increased levels of mPGES-1 are common in many human cancers.
| Cancer type | Detection method | Reference |
|---|---|---|
| NSCLC | Western blot, IHC | [22] |
| Colorectal adenomas, carcinomas | Western blot, IHC | [21] |
| Colorectal carcinomas | Western blot, qPCR, IHC | [31] |
| Gastric cancer | Western blot, IHC | [23] |
| Pancreatic cancer | q-PCR, Western blot, IHC | [24] |
| Cervical EpM, SIL, SCC | IHC | [25] |
| Prostate cancer | Western blot | [26] |
| Papillary thyroid carcinoma | IHC | [27] |
| HNSCCC | q-PCR | [28] |
| Low- and High-grade Gliomas | IHC | [30] |
EpM, cervical epithelial metaplasia; SIL, squamous intraepithelial lesions; SCC, squamous cell carcinoma
NSCLC, Non-small cell lung cancer
HNSCCC, Head and neck squamous cell carcinoma
The functional role of mPGES-1 has also been studied in cell culture systems. In one such study, co-transfection of COX-2 and mPGES-1 was found to induce a rapid proliferation in HEK-293 cells [16]. Moreover, HEK-293 cells co-transfected with COX-2 and mPGES-1 were found to form large, well-vascularized tumors when injected into the flanks of nude mice [20]. Two very recent papers have now been published examining the roles of mPGES-1 in lung and prostate cancer cell lines. In the study by Hanaka et al. (2009), mPGES-1 was knocked down using shRNA in a prostate cancer cell line, DU145, and also in the non-small cell lung cancer cell line, A549. Following mPGES-1 knockdown, both cell lines showed a decrease in clonogenic capacity and also exhibited slower growth of xenograft tumors in nude mice [26]. Similarly, Kamei et al. [32] using siRNA silencing of mPGES-1 in Lewis lung carcinoma cells, also showed reduced cell proliferation, attenuated Matrigel invasiveness and increased extracellular matrix adhesion [32]. These studies clearly demonstrate the critical role that is played by mPGES-1 in a variety of cancer cells, and also provide the underlying rationale for strategies that have focused on chemopreventive targeting of this enzyme for cancer suppression.
Elevated levels of mPGES-1 are often observed concomitantly with COX-2 over-expression. In fact, in vitro studies have demonstrated that mPGES-1 is localized at the perinuclear membrane and endoplasmic reticulum and is in general functionally coupled with COX-2 [16, 33, 34], thereby enabling efficient generation of PGE2 during inflammation [16, 35]. Moreover, recent studies have shown that mPGES-1 expression can be specifically induced by lipopolysaccharide (LPS) in rat peritoneal macrophages [36], interleukin-1β (IL-1β) and tumor necrosis factor (TNF)-α in a human lung carcinoma cell line, A549 with or without induction of COX-2 [15, 37]. However, studies with these diverse stimuli have clearly shown that mPGES-1 can also be functionally activated in the absence of induced COX-2 levels [37–39], providing evidence that these two enzymes can be independently regulated. This latter observation is important from the standpoint of drug targeting. It suggests the possibility that the enzymatic activity of mPGES-1 can be pharmacologically targeted with resultant suppression of PGE2 production by mechanisms that circumvent the toxicity associated with inhibition of COX-2 activity.
III. The role of mPGES-1 in gastrointestinal carcinogenesis
Experimental observations developed from cell culture studies, together with the well-recognized role of PGE2 during tumor promotion, have provided the rationale for several recent in vivo studies focused on the impact of mPGES-1 on tumorigenesis. In a recent study from our laboratory, mPGES-1 deficient mice were found to exhibit a significant reduction in the number and size of intestinal tumors generated on an Apc mutant background [40]. Introduction of the Ptges gene deletion onto ApcΔ14/+ mice reduced the number and size of intestinal tumors by up to 75% compared to mice with the wild-type gene [40]. A notable reduction (~50%) in the number of colon tumors was also observed. Interestingly, mPGES-1 deficiency was associated with a disorganized vascular pattern within primary adenomas, confirming a key role for PGE2 in tumor angiogenesis [40]. Consistent with these in vivo observations, recent findings by Kamei et al. [32] showed decreased growth of the Lewis lung carcinoma cell xenograft with concomitant decreases in the density of microvascular networks, the expression of pro-angiogenic vascular endothelial growth factor, and the activity of matrix metalloproteinase-2. However, the mechanism that underlies this defect in neovessel growth has not yet been clarified. In the mPGES-1 knockout study, mPGES-1 deletion resulted in both reduced size and numbers of pre-neoplastic aberrant crypt foci (ACF) following treatment with the colon carcinogen, azoxymethane (AOM) [40]. Importantly, protection of the colonic mucosa was associated with a marked suppression of nuclear β-catenin translocation, a finding that confirms an earlier study from the Gutkind laboratory in which PGE2 was shown to stimulate colon cancer cell growth through the EP2-Akt-GSK3β axis, leading to β-catenin nuclear translocation and accumulation [41]. In direct contrast to these findings, however, Elander et al. [42] reported that genetic deletion of mPGES-1 resulted in accelerated intestinal tumorigenesis in ApcMin/+ mice. Tumor multiplicity in the intestine was increased by approximately 50% (80 vs. 38 tumors/intestine), while tumor burden was also significantly increased (1.64 vs. 1.12 mm) by genetic deletion of Ptges in comparison to the wild-type littermates [42]. Although these findings are inconsistent with the earlier study by Nakanishi et al. [40], it is possible that environmental factors (e.g. bacterial colonization) may play a role in these disparate responses. This possibility is highlighted by the study of Maggio-Price et al. [43] in which SMAD3-deficient mice were sensitized to intestinal tumorigenesis by co-infection with Helicobacter pylori. In fact, Helicoactor pylori infection has been shown to enhance COX-2 and mPGES-1 expression [44]. Moreover, Nardone et al. [45] demonstrated that elevated levels of COX-2 and mPGES-1 were detected with patients with Helicobacter pylori. In any case, clarifying the role of mPGES-1 in intestinal carcinogenesis in pre-clinical studies is a key priority for the further development of small molecule inhibitors as potential chemopreventive agents.
IV. Novel mPGES-1 inhibitors: Drug discovery with mPGES-1 as a molecular target
Despite providing an attractive target for cancer suppression, drug targeting of COX-2 activity has been associated with some clinical concern. For example, the cardiovascular events associated with rofecoxib treatment in the adenoma prevention trial (APPROVe) have been well documented [12, 46]. In patients taking selective COX-2 inhibitors, increased risk of myocardial infarction (MI) and stroke [47, 48], as well as increased mortality after MI [49], may be due to imbalanced production of pro-thrombotic eicosanoids (e.g. increased TxA2) and anti-thrombotic eicosanoids (e.g. decreased PGI2) [50]. However, a detailed analysis of the mPGES-1 knockout mouse model provides new evidence that much of this toxicity associated with COX-2 inhibition can potentially be circumvented. For example, mice deficient in mPGES-1 did not show alterations in the levels of TxA2 PGI2 or in heart tissue after experimentally induced MI [51]. Furthermore, deletion of mPGES-1 did not affect blood pressure when mice were crossed with low-density lipoprotein receptor (LDLR) knockout mice [52]. Moreover, Wu et al. [53] demonstrated absent or reduced levels of myocardial damage after coronary occlusion in mice lacking mPGES-1 compared to mice given the COX-2 inhibitor, celecoxib. Finally, Cheng et al. [54] report that genetic deletion of mPGES-1 in contrast to deletion, disruption, or inhibition of COX-2, does not result in hypertension or a predisposition to thrombosis in normo-lipidemic mice. Taken together, these findings suggest that selective mPGES-1 inhibitors may exhibit only minimal cardio-toxic side effects that are typically associated with COX-2 inhibitors.
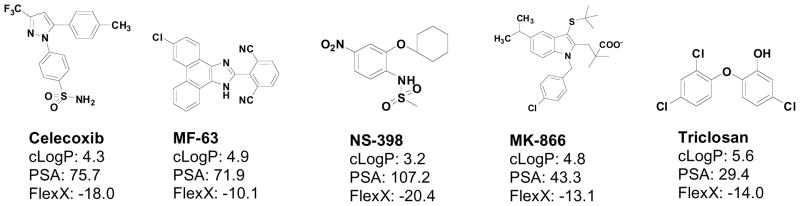
To date, a limited number of compounds have been described that inhibit mPGES-1 activity in vitro. None as yet have been developed as anti-cancer agents. There are several examples of compounds that were developed to target COX-2 but also found to inhibit mPGES-1 activity as well. For example, NS-398 [2-cyclohexyloxy-4-nitrophenyl)-methanesulfonamide], is a COX-2 inhibitor that also inhibits mPGES-1 with an IC50 of ~20mM in vitro [55]. NS-398 was developed in Japan as an aryl-sulfonamide derivative of the anti-inflammatory agent, nimesulide [56]. NS-398 demonstrates approximately 2,000-fold selectivity for COX-2 over COX-1; this is approximately 2-fold higher COX-2 selectivity than that demonstrated for Rofecoxib, and approximately 5-fold higher than that of Celecoxib [57–59]. In animal models, NS-398 was a potent anti-inflammatory agent [60]; however, it has poor bioavailability and has been found to generate hepatotoxic metabolites. Thus, NS-398 has not been further developed as a therapeutic agent. Similarly, 15-deoxy-D12,14-prostaglandin J2 inhibits mPGES-1 with an IC50 of 0.3 mM [61]. Recently, a series of molecules based on the indole FLAP inhibitor, MK-866, have been developed. Several of these compounds show mPGES-1 selectivity compared to their ability to inhibit mPGES-2 or thomboxane synthase, with the lowest IC50 value found being ~3 nM [57]. A group from Germany recently published a short report on pirinixic acid derivatives as novel dual inhibitors of mPGES-1 and 5-LOX [58]. Although exhibiting low micromolar activity in a cell-free assay, these compounds showed only weak inhibition of PGE2 formation (63% at 33mM). Another group of compounds developed by Merck was recently reported to relieve pain in pre-clinical models of inflammation [59]. MF-63 potently inhibited the human mPGES-1 enzyme (IC50 of 1.3 nM), with a high degree (> 1,000-fold) of selectivity over other prostanoid synthases [59]. However, this compound has not yet been tested in animal cancer models. Finally, a well-known compound found in many oral preparations used for its anti-inflammatory effect on the oral mucosa, Triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol), has been reported to inhibit mPGES-1. Triclosan (Irgasan®) is widely used in soaps, deodorants, toothpastes, mouthwashes, and cleaning supplies and its anti-inflammatory effects have been attributed, at least in part, to its inhibition of PGE2 biosynthesis. Thus considering the wide range of molecular structures that are available (Figure 1), there is the strong likelihood that novel structural analogs can ultimately be developed that may show high selectivity against mPGES-1 and effective suppression of PGE2 generation as described under Figure 2.
Figure 1. Structures of known compounds that have mPGES-1 inhibitory action.
Several compounds have been examined for their inhibitory effects against mPGES-1 enzymatic activity in vitro. Solubility parameters (cLogP, Polarity Surface Area: PSA, FlexX) were determined by Sybyl 8.0/FlexX modeling program.
Figure 2. Docking models of inhibitors within the mPGES-1 active site.
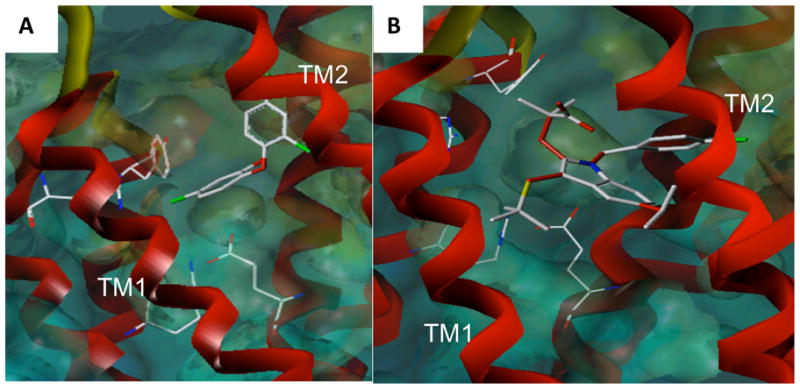
Docking studies were performed using Sybyl 8.0/FlexX modeling program for triclosan (A) and MK-886 (B). mPGES-1 crystal structure coordinates (3dww) were used. The active site residues interacting with both inhibitors include: Arg110, Arg126, Glu77 and Tyr117.
V. Summary and conclusions
As illustrated throughout this review, PGE2 is an important bioactive lipid that plays a fundamental role in a variety of signaling pathways. Acting primarily through its interactions with the EP receptor family, PGE2 elicits control over a range of cellular responses, ranging from angiogenesis to migration and proliferation [6]. While PGE2 is also important in maintaining GI barrier function and integrity, its elevated production within the GI mucosa likely contributes to cancer pathogenesis [62]. Abundant data that has been generated both in cell culture systems as well as in pre-clinical mouse tumor models have provided considerable impetus for the continued development and refinement of mPGES-1 inhibitors. The strongest case for targeting mPGES-1 is based on promising chemoprevention trials that have targeted COX-2 for the suppression of colon adenomas. A major limitation of these trials, however, has been the unfortunate adverse events associated with long-term suppression of COX-2 activity, due in part to the reduced levels of other key prostanoids. Thus, the ability to specifically target mPGES-1, the terminal synthase in the production of PGE2, without affecting the tissue levels of other important prostanoid molecules, offers the potential for therapeutic benefit without the potential toxicity associated with COX-2 inhibition.
Acknowledgments
Financial support: This work was supported by NIH grant 5R01CA114635 (DWR) the SPORE pilot project (to EJM) and the ACS-IRG (to EJM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–1829. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Narumiya S. Prostanoids in health and disease; lessons from receptor-knockout mice. Adv Exp Med Biol. 2002;507:593–597. doi: 10.1007/978-1-4615-0193-0_90. [DOI] [PubMed] [Google Scholar]
- 5.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 7.Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 9.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 10.Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- 11.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 12.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 13.Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Song WL, Cheng Y, Fitzgerald GA. Microsomal prostaglandin E synthase-1 inhibition in cardiovascular inflammatory disease. J Intern Med. 2008;263:500–505. doi: 10.1111/j.1365-2796.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Narumiya S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat. 2002;68–69:557–573. doi: 10.1016/s0090-6980(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 19.Ushikubi F, Sugimoto Y, Ichikawa A, Narumiya S. Roles of prostanoids revealed from studies using mice lacking specific prostanoid receptors. Jpn J Pharmacol. 2000;83:279–285. doi: 10.1254/jjp.83.279. [DOI] [PubMed] [Google Scholar]
- 20.Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J Biol Chem. 2003;278:19396–19405. doi: 10.1074/jbc.M213290200. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimatsu K, Golijanin D, Paty PB, et al. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001;7:3971–3976. [PubMed] [Google Scholar]
- 22.Yoshimatsu K, Altorki NK, Golijanin D, et al. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001;7:2669–2674. [PubMed] [Google Scholar]
- 23.Gudis K, Tatsuguchi A, Wada K, et al. Clinical significance of prostaglandin E synthase expression in gastric cancer tissue. Hum Pathol. 2007;38:1826–1835. doi: 10.1016/j.humpath.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Hasan S, Satake M, Dawson DW, et al. Expression analysis of the prostaglandin E2 production pathway in human pancreatic cancers. Pancreas. 2008;37:121–127. doi: 10.1097/MPA.0b013e31816618ba. [DOI] [PubMed] [Google Scholar]
- 25.Herfs M, Herman L, Hubert P, et al. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol Immunother. 2009;58:603–614. doi: 10.1007/s00262-008-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanaka H, Pawelzik SC, Johnsen JI, et al. Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proc Natl Acad Sci U S A. 2009;106:18757–18762. doi: 10.1073/pnas.0910218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omi Y, Shibata N, Okamoto T, Obara T, Kobayashi M. Immunohistochemical Demonstration of Membrane-bound Prostaglandin E(2) Synthase-1 in Papillary Thyroid Carcinoma. Acta Histochem Cytochem. 2009;42:105–109. doi: 10.1267/ahc.09014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho M, Leon X, Fernandez-Figueras MT, Quer M, Vila L. Prostaglandin E(2) pathway in head and neck squamous cell carcinoma. Head Neck. 2008;30:1175–1181. doi: 10.1002/hed.20850. [DOI] [PubMed] [Google Scholar]
- 29.Baryawno N, Sveinbjornsson B, Eksborg S, et al. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro Oncol. 2008;10:661–674. doi: 10.1215/15228517-2008-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattila S, Tuominen H, Koivukangas J, Stenback F. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology. 2009;29:156–165. doi: 10.1111/j.1440-1789.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 31.Seo T, Tatsuguchi A, Shinji S, et al. Microsomal prostaglandin E synthase protein levels correlate with prognosis in colorectal cancer patients. Virchows Arch. 2009;454:667–676. doi: 10.1007/s00428-009-0777-z. [DOI] [PubMed] [Google Scholar]
- 32.Kamei D, Murakami M, Sasaki Y, et al. Microsomal prostaglandin E synthase-1 in both cancer cells and hosts contributes to tumour growth, invasion and metastasis. Biochem J. 2009 doi: 10.1042/BJ20090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarus M, Kubata BK, Eguchi N, Fujitani Y, Urade Y, Hayaishi O. Biochemical characterization of mouse microsomal prostaglandin E synthase-1 and its colocalization with cyclooxygenase-2 in peritoneal macrophages. Arch Biochem Biophys. 2002;397:336–341. doi: 10.1006/abbi.2001.2614. [DOI] [PubMed] [Google Scholar]
- 34.Stark K, Bylund J, Torma H, Sahlen G, Oliw EH. On the mechanism of biosynthesis of 19-hydroxyprostaglandins of human seminal fluid and expression of cyclooxygenase-2, PGH 19-hydroxylase (CYP4F8) and microsomal PGE synthase-1 in seminal vesicles and vas deferens. Prostaglandins Other Lipid Mediat. 2005;75:47–64. doi: 10.1016/j.prostaglandins.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and -2. J Biol Chem. 1995;270:24019–24023. doi: 10.1074/jbc.270.41.24019. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto H, Naraba H, Murakami M, et al. Concordant induction of prostaglandin E2 synthase with cyclooxygenase-2 leads to preferred production of prostaglandin E2 over thromboxane and prostaglandin D2 in lipopolysaccharide-stimulated rat peritoneal macrophages. Biochem Biophys Res Commun. 1997;230:110–114. doi: 10.1006/bbrc.1996.5894. [DOI] [PubMed] [Google Scholar]
- 37.Thoren S, Jakobsson PJ. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Inhibition by NS-398 and leukotriene C4. Eur J Biochem. 2000;267:6428–6434. doi: 10.1046/j.1432-1327.2000.01735.x. [DOI] [PubMed] [Google Scholar]
- 38.Stichtenoth DO, Thoren S, Bian H, Peters-Golden M, Jakobsson PJ, Crofford LJ. Microsomal prostaglandin E synthase is regulated by proinflammatory cytokines and glucocorticoids in primary rheumatoid synovial cells. J Immunol. 2001;167:469–474. doi: 10.4049/jimmunol.167.1.469. [DOI] [PubMed] [Google Scholar]
- 39.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi M, Montrose DC, Clark P, et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 2008;68:3251–3259. doi: 10.1158/0008-5472.CAN-07-6100. [DOI] [PubMed] [Google Scholar]
- 41.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 42.Elander N, Ungerback J, Olsson H, Uematsu S, Akira S, Soderkvist P. Genetic deletion of mPGES-1 accelerates intestinal tumorigenesis in APC(Min/+) mice. Biochem Biophys Res Commun. 2008;372:249–253. doi: 10.1016/j.bbrc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. Embo J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nardone G, Rocco A, Vaira D, et al. Expression of COX-2, mPGE-synthase1, MDR-1 (P-gp), and Bcl-xL: a molecular pathway of H pylori-related gastric carcinogenesis. J Pathol. 2004;202:305–312. doi: 10.1002/path.1512. [DOI] [PubMed] [Google Scholar]
- 46.Baron JA, Sandler RS, Bresalier RS, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372:1756–1764. doi: 10.1016/S0140-6736(08)61490-7. [DOI] [PubMed] [Google Scholar]
- 47.Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs. 2000;59:957–980. doi: 10.2165/00003495-200059040-00017. [DOI] [PubMed] [Google Scholar]
- 48.Matheson AJ, Figgitt DP. Rofecoxib: a review of its use in the management of osteoarthritis, acute pain and rheumatoid arthritis. Drugs. 2001;61:833–865. doi: 10.2165/00003495-200161060-00019. [DOI] [PubMed] [Google Scholar]
- 49.Fosslien E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Clin Lab Sci. 2000;37:431–502. doi: 10.1080/10408360091174286. [DOI] [PubMed] [Google Scholar]
- 50.Futaki N, Yoshikawa K, Hamasaka Y, et al. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen Pharmacol. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- 51.Degousee N, Fazel S, Angoulvant D, et al. Microsomal prostaglandin E2 synthase-1 deletion leads to adverse left ventricular remodeling after myocardial infarction. Circulation. 2008;117:1701–1710. doi: 10.1161/CIRCULATIONAHA.107.749739. [DOI] [PubMed] [Google Scholar]
- 52.Wang M, Zukas AM, Hui Y, Ricciotti E, Pure E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc Natl Acad Sci U S A. 2006;103:14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D, Mennerich D, Arndt K, et al. Comparison of microsomal prostaglandin E synthase-1 deletion and COX-2 inhibition in acute cardiac ischemia in mice. Prostaglandins Other Lipid Mediat. 2009;90:21–25. doi: 10.1016/j.prostaglandins.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–1399. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arai I, Hamasaka Y, Futaki N, et al. Effect of NS-398, a new nonsteroidal anti-inflammatory agent, on gastric ulceration and acid secretion in rats. Res Commun Chem Pathol Pharmacol. 1993;81:259–270. [PubMed] [Google Scholar]
- 56.Quraishi O, Mancini JA, Riendeau D. Inhibition of inducible prostaglandin E(2) synthase by 15-deoxy-Delta(12,14)-prostaglandin J(2) and polyunsaturated fatty acids. Biochem Pharmacol. 2002;63:1183–1189. doi: 10.1016/s0006-2952(02)00844-4. [DOI] [PubMed] [Google Scholar]
- 57.Riendeau D, Aspiotis R, Ethier D, et al. Inhibitors of the inducible microsomal prostaglandin E2 synthase (mPGES-1) derived from MK-886. Bioorg Med Chem Lett. 2005;15:3352–3355. doi: 10.1016/j.bmcl.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 58.Koeberle A, Zettl H, Greiner C, Wurglics M, Schubert-Zsilavecz M, Werz O. Pirinixic acid derivatives as novel dual inhibitors of microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. J Med Chem. 2008;51:8068–8076. doi: 10.1021/jm801085s. [DOI] [PubMed] [Google Scholar]
- 59.Xu D, Rowland SE, Clark P, et al. MF63 [2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)-isophthalonitrile], a selective microsomal prostaglandin E synthase-1 inhibitor, relieves pyresis and pain in preclinical models of inflammation. J Pharmacol Exp Ther. 2008;326:754–763. doi: 10.1124/jpet.108.138776. [DOI] [PubMed] [Google Scholar]
- 60.Wallace JL. Selective COX-2 inhibitors: is the water becoming muddy? Trends Pharmacol Sci. 1999;20:4–6. doi: 10.1016/s0165-6147(98)01283-8. [DOI] [PubMed] [Google Scholar]
- 61.San Juan AA, Cho SJ. 3D-QSAR study of microsomal prostaglandin E2 synthase (mPGES-1) inhibitors. J Mol Model. 2007;13:601–610. doi: 10.1007/s00894-007-0172-0. [DOI] [PubMed] [Google Scholar]
- 62.DuBois RN, Giardiello FM, Smalley WE. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol Clin North Am. 1996;25:773–791. doi: 10.1016/s0889-8553(05)70274-0. [DOI] [PubMed] [Google Scholar]