Abstract
Purpose of review
β-cell death is an important pathogenic component of both type 1 and type 2 diabetes. However, the specific molecular pathways and interactions involved in this process are not understood. Increasing evidence indicates that a type of cell stress called endoplasmic reticulum stress (ER stress) plays an important role in β-cell death. Here we discuss a potential paradigm of ER stress-mediated β-cell death.
Recent findings
Upon ER stress conditions, a signaling network termed the unfolded protein response (UPR) is activated. The UPR regulates adaptive effectors to attenuate ER Stress and restore ER homeostasis promoting cell survival. Paradoxically the UPR also regulates apoptotic effectors. When adaptive effectors fail to attenuate ER stress, these apoptotic effectors take into effect leading to cell death. The nature of this switch between life and death is currently under study.
Summary
Depending on the nature of the stress condition, the UPR either protects β cells or promotes their death. The mechanisms of this switch is not well understood but involves the balance between adaptive and apoptotic factors regulated by the UPR. Herein, we review examples of this UPR balancing act between life and death and the potential mechanisms involved.
Keywords: Endoplasmic Reticulum Stress (ER stress), Unfolded Protein Response (UPR), β-cell death, diabetes
Introduction
Diabetes is a group of disorders defined by hyperglycemia caused by an absolute deficiency (type 1 diabetes) or a relative deficiency of insulin (type 2 diabetes). Insulin, a hormone secreted from pancreatic β cells, functions in lowering blood glucose. Increasing evidence suggests that cellular stress caused by the accumulation of unfolded and misfolded proteins in the endoplasmic reticulum (ER), termed ER stress, is directly related to β-cell dysfunction and death during the progression of type 1 and type 2 diabetes, and Wolfram syndrome, a genetic form of diabetes and neurodegeneration [1–3]. To counteract ER stress, β cells activate cellular signaling pathways termed the unfolded protein response (UPR). Depending on the nature of the stress condition, the UPR either protects β cells or promotes their death. The mechanisms of this switch is not well understood but involves the balance between adaptive and apoptotic factors regulated by the UPR. Herein, we review examples of this UPR balancing act between life and death and the potential mechanisms involved.
Protein folding in the Endoplasmic Reticulum (ER) and ER stress
The ER is an organelle responsible for several important cellular functions including protein and lipid biosynthesis, Ca2+ storage, and cell signaling. Proteins destined for secretion, intracellular organelles, or the plasma membrane, fold into their proper three-dimensional structures in the ER. Protein folding and processing enzymes, as well as the oxidized environment within the ER are required for proteins to properly fold into their functional conformation.
Proinsulin folding in the ER
β cells are specialized for the production and regulated secretion of insulin to control blood glucose levels. In the presence of hyperglycemia, β cells secrete insulin from a readily available pool. At the same time, an increase in insulin release activates proinsulin biosynthesis in the ER of β cells [4]. Therefore, β cells have developed a highly specialized ER to handle this protein load. Human preproinsulin, a precursor for insulin, is synthesized in the cytoplasm containing a signal peptide sequence at its N-terminal, and then is cotranslationally translocated into the lumen of the ER. The signal peptide of preproinsulin is cleaved in the ER to produce proinsulin. In the lumen of the ER, proinsulin undergoes protein folding and three disulfide bonds are formed. Properly folded proinsulin is then delivered to the Golgi apparatus and packaged into secretory granules. The conversion of proinsulin to insulin takes place in the secretory granules and mature insulin is released by exocytosis [5]. The frequent fluctuation of blood glucose levels in humans requires that β cells control proinsulin folding in the ER with exquisite sensitivity.
What is ER stress?
In order for secretory proteins to fold properly, ER homeostasis must be maintained. ER homeostasis is defined by the dynamic balance between the ER protein load and the ER capacity to process this load. ER homeostasis can be perturbed by pathological processes such as viral infections, environmental toxins, inflammatory cytokines, and mutant protein expression, as well as by physiological processes such as aging and postprandial production of proinsulin. Disruption of ER homeostasis causes accumulation of unfolded and misfolded proteins in the ER. This condition is referred as ER stress [6, 7] (Figure 1).
Figure 1. ER stress.
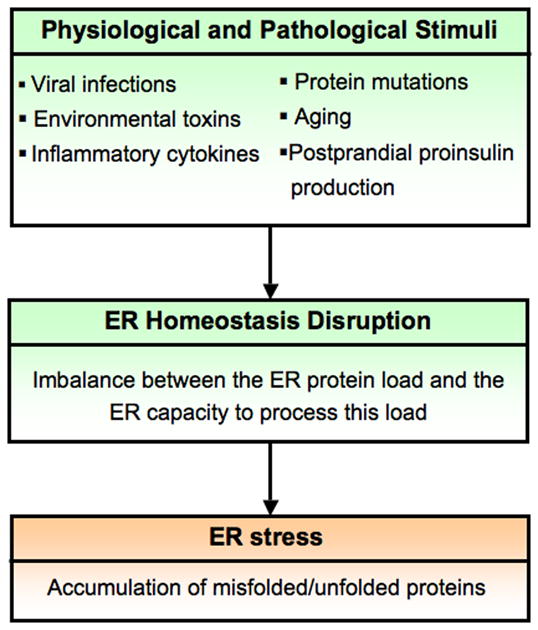
In order for proteins to fold properly within the ER, ER homeostasis must be maintained. ER homeostasis is defined by the dynamic balance between the ER protein load and the ER capacity to process this load. ER homeostasis can be perturbed by physiological and pathological stimuli. Disruption of ER homeostasis causes accumulation of unfolded and misfolded proteins in the ER. This condition is referred as ER stress.
The Unfolded Protein Response (UPR)
Under ER stress conditions, β cells activate a signaling network termed the unfolded protein response (UPR). The UPR is initiated by three ER transmembrane proteins: Inositol Requiring 1 (IRE1), PKR-like ER kinase (PERK), and Activating Transcription Factor 6 (ATF6). These three master regulators sense and interpret protein folding conditions in the ER and translate this information across the ER membrane to regulate downstream effectors [6, 7](Figure 2). These effectors have the following three distinct functions:
Figure 2. The Unfolded Protein Response.
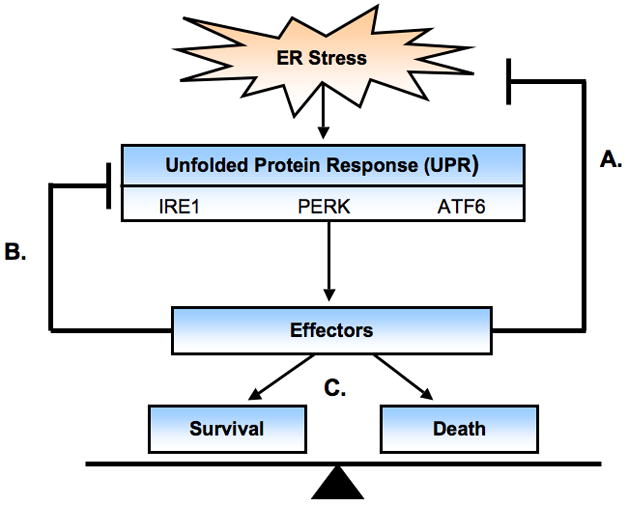
Upon ER stress, a signaling network termed the unfolded protein response (UPR) is activated. The UPR is initiated by three master regulators: IRE1, PERK, and ATF6. Together these transducers regulate three types of effectors with the following functions: homeostatic regulation to attenuate ER stress(a), feedback regulation to turn off the UPR when ER homeostasis is restored (b), and apoptotic regulation balancing both survival and death effectors (c).
-
Homeostatic Regulation
One set of effectors regulated by the UPR elicits three adaptive responses that function to attenuate ER stress and restore ER homeostasis. These responses include the attenuation of protein translation to reduce ER workload and prevent further accumulation of unfolded proteins, upregulation of molecular chaperones and protein processing enzymes to enhance the ER folding activity, and the increase in ER-associated degradation (ERAD) components to promote clearance of unfolded proteins. The effectors involved in these functions include eIF2α for translational attenuation, BiP (GRP78), GRP 94 and PDI for protein folding, and Derlin-1 and HRD1 for protein degradation [8–15].
-
Feedback Regulation
The UPR also regulates a set of effectors that function in negative feedback loops to provide tight control of the UPR and therefore preventing harmful hyperactivation. One example involves an abundant ER chaperone, BiP. BiP binds to the ER luminal domains of the UPR transducers preventing their activation. Upon ER stress, BiP is released and the UPR transducers are activated. In turn, the UPR induces BiP expression to aid in protein folding and also to negatively regulate the UPR master regulators [16–18]. Another example involves PERK signaling regulation by GADD34. PERK plays a role in the attenuation of protein translation through eIF2α phosphorylation to reduce ER protein load. As ER homeostasis is being restored, GADD34 expression is induced by PERK and suppresses PERK signaling through the dephosphorylation of eIF2α restoring protein translation [19].
-
Apoptosis Regulation
Increasing evidence indicates that the UPR regulates both apoptotic and survival effectors. These effectors include CHOP and AATF [20, 21]. CHOP plays an important role in ER stress-mediated β-cell death [22, 23]. It has been shown that CHOP overexpression decreases expression levels of Bcl-2 [24]. Bcl-2 has been shown to inhibits Bax translocation from cytosol to mitochondria [25]. Because Bax is involved in ER stress-mediated cell death [26, 27], CHOP may execute apoptosis by suppressing anti-apoptotic genes, Bcl-2 and enhancing pro-apoptotic component, Bax. We have recently discovered a novel anti-apoptotic effector of the UPR, apoptosis antagonizing transcription factor (AATF). AATF induction is regulated by the UPR and mediates anti-apoptotic effects through transcriptional regulation of AKT1 (Ishigaki et al, In press).
Tolerable versus Unresolvable ER stress
We propose that there are two types of ER stress conditions: tolerable and unresolvable. Under tolerable ER stress conditions, the UPR promotes β-cell survival. In contrast, under unresolvable ER stress conditions, the UPR induces β-cell death.
Tolerable ER stress
Cells are exposed to physiological conditions that induce tolerable ER stress. Under these ER stress conditions, the UPR can restore ER homeostasis promoting cell survival. For instance, when β cells are exposed to transient high glucose proinsulin mRNA translation is increased by several fold therefore increasing the ER protein work load. β cells utilize the UPR in order to handle this load. Several studies indicate that PERK-eIF2α signaling plays a major role in regulating proinsulin mRNA translation under dynamic glucose conditions. Tight control of eIF2α phosphorylation is critical to ensure proper adaptation to increases in ER protein load and to promote β-cell survival [28–31]. In islets from Perk knockout mice, insulin biosynthesis stimulated by high glucose is markedly enhanced as compared to that in control mice [28]. As a consequence, Perk knockout mice develop diabetes because of ER stress-mediated β-cell death. IRE1 is also activated under transient high glucose conditions. Acute IRE1 activation is required for proinsulin biosynthesis and perhaps enhancing ER proinsulin folding capacity [32]. These observations demonstrate that cells utilize the UPR in order to handle physiological disruptions of ER homeostasis therefore promoting their survival.
Unresolvable ER stress and induction of apoptosis by the UPR
When the UPR fails to restore ER homeostasis and attenuate ER stress, UPR activation induces apoptosis. This unresolvable ER stress can be caused by genetic mutations as well as environmental factors. One example is observed in Wolfram syndrome. Wolfram syndrome is a rare autosomal recessive disorder characterized by childhood onset of diabetes mellitus, followed by optic atrophy, deafness and death from neurodegeneration in the third or fourth decades [33–35]. Postmortem studies reveal a non-autoimmune-linked selective loss of β cells [36]. Mutations in the WFS1 gene are responsible for this syndrome [37–39]. The WFS1 protein is localized to the ER and highly expressed in β cells [40, 41] [42]. WFS1 has been shown to be important for mitigating ER stress, and when suppressed, causes unresolvable ER stress in β cells, leading to β-cell death [42–44].
Unresolvable ER stress caused by genetic mutations is also observed in permanent neonatal diabetes. Neonatal diabetes is a rare disorder defined as insulin-requiring hyperglycemia within the first month of life and is typically associated with slowed intrauterine growth. Permanent neonatal diabetes can be caused by several types of mutations. It has recently been shown that mutations in the human insulin gene primarily occurring in critical regions of proinsulin folding can cause this disorder [45]. These mutations presumably lead to improper folding of proinsulin, causing unresolvable ER stress and ultimately leads to β-cell apoptosis. A mouse model of this disease, the Akita mouse, has a dominant cysteine96-to-tyrosine missense mutation in the Ins2 gene [46, 47]. This mutation leads to disruption of disulfide bond formation between the A and B chain of proinsulin, causing insulin to misfold and accumulate in the ER of the β cell [46]. This accumulation of misfolded insulin leads to unresolvable ER stress, β-cell apoptosis, and consequently diabetes [22].
Unresolvable ER stress can be also caused by environmental factors. Several studies demonstrate that chronic exposure to long chain free fatty acids (FFAs) or cytokines induce β-cell apoptosis [48–52]. Treatment of β cell lines with the free fatty acid palmitate or the cytokines interleukin-1β and interferon-γ induce unresolvable ER stress and UPR activation contributing to β-cell death. The underlying mechanisms are currently under study.
Binary Switch that regulates life and death
The UPR regulates both adaptive and apoptotic effectors. The balance between these effectors depends on the nature of the ER stress whether it is tolerable or unresolvable as discussed above. Thus, the UPR acts as a binary switch between life and death (Figure 3). What are the mechanisms of this switch? Although our understanding of this switch is far from complete, there are several important clues to elucidate these mechanisms.
Figure 3. Binary switch between life and death.
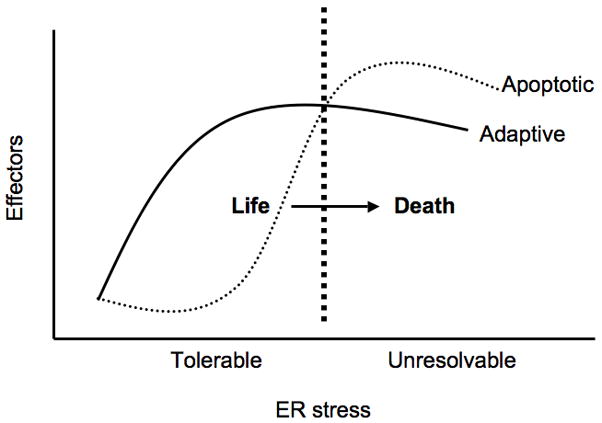
The UPR regulates both adaptive/survival and apoptotic effectors. Under tolerable ER stress conditions, the expression and activation of adaptive effectors outweigh the induction of apoptotic effectors therefore promoting cell survival. However under unresolvable ER stress conditions in which ER homeostasis cannot be restored, expression and activation of apoptotic effectors outweigh adaptive effectors leading to cell death. Thus the UPR acts as a binary switch between life and death. The underlying mechanisms of this switch are not well understood but may involve hyperactivation of the UPR sensors, dysregulation of UPR negative feedback loops, and differential regulation of adaptive/survival and apoptotic effectors at both transcriptional and post-transcriptional levels.
Regulation of Survival and Death Factors by IRE1
Upon sensing ER stress, IRE1 undergoes oligomerization and trans-autophosphorylation activating its endoribonuclease domain. Activated IRE1 cleaves an intron from the mRNA encoding X-box binding protein 1 (XBP1) [53–55]. The spliced variant of XBP1 mRNA encodes a transcriptional activator for several UPR genes including chaperones, protein folding catalysts, and ERAD components [56, 57]. Apart from homeostatic functions, IRE1 also regulates apoptotic effectors. In the presence of unresolvable ER stress, IRE1 activates JNK through ASK1 and elicits apoptosis [58, 59]. This pathway has been shown to block the function of the anti-apoptotic Bcl-2 by phosphorylating it, thus causing apoptosis in β cells [60, 61]. IRE1 is also involved in the decay of mRNAs encoding ER homeostatic proteins, including PDI, and BiP [62–65]. Thus, IRE1 could be one of the major determinants of the switch between life and death.
Regulation of Survival and Death Factors by PERK
PERK has been shown to protect β cells from ER stress-mediated cell death through the attenuation of protein translation [28, 66]. In addition, we have recently found that PERK upregulates a novel anti-apoptotic effector, apoptosis antagonizing transcription factor (AATF) and mediates survival in part through the transcriptional regulation of AKT1 (Ishigaki, S., et al, In press). In contrast, PERK also regulates expression of CHOP which is an important effector of ER stress-mediated β-cell death [20–23, 67]. Thus, PERK could be another important determinant of the switch between life and death.
Potential mechanisms of the UPR binary switch between life and death
Perhaps under tolerable ER stress conditions, feedback regulators such as BiP and Gadd34 turn off UPR pathways to tip the balance in favor of survival effectors. However under unresolvable ER stress conditions, the UPR master regulators are hyperactivated bypassing negative regulation therefore tipping the balance towards apoptosis effectors.
Switching may also involve regulation of the effectors themselves at the transcriptional and post-transcriptional levels. It has been shown that survival is favored during mild and tolerable ER stress as a consequence of the intrinsic instabilities of mRNAs and proteins that promote apoptosis compared to those that facilitate protein folding and adaptation. As a consequence, the expression of apoptotic proteins is short-lived as cells adapt to stress [68]. This observation indicates that post-transcriptional mechanisms regulating the ratio between survival and apoptotic effectors are important for controlling life and death of β cells.
Conclusion
Analysis of ER stress-mediated β-cell death as the balancing act between survival and apoptotic effectors of the UPR allows new insight into the mechanisms of β-cell death during the progression of diabetes. The complete understanding of the switching process between life and death will enable us to predict when apoptotic effectors outweigh survival effectors and prevent β cell death. Systems approaches using genomics including SNP analysis, transcriptomics, and proteomics are necessary to the complete understanding. To verify our concept and develop a novel therapeutic modality, we also need to incorporate experimental and clinical data into our binary switch model. Furthermore, studying this switch between life and death will have a direct impact on future therapies for diabetes.
Acknowledgments
This work was supported in part by grants from NIH-NIDDK (R01DK067493), the Diabetes and Endocrinology Research Center at the University of Massachusetts Medical School (5 P30 DK32520), the Juvenile Diabetes Research Foundation International and the Worcester Foundation for Biomedical Research to F. Urano.
References
* of special interest
** of outstanding interest
- 1.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 2.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–33. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.07.003. This is a review on the role of ER stress in β-cell dysfunction and death during the progression of diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes CJ. Processing of the insulin molecule. In: LeRoith D, Taylor SI, Olefsky JM, editors. Diabetes Mellitus. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 27–50. [Google Scholar]
- 5.Rhodes CJ, Shoelson S, Halban PA. Insulin Biosynthesis, Processing, and Chemistry. In: Kahn CR, Weir GC, King GL, et al., editors. Joslin’s Diabetes Mellitus. Boston: Joslin Diabetes Center; 2005. pp. 65–82. [Google Scholar]
- 6.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 7.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–76. doi: 10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Ernst V, Levin DH, London IM. Inhibition of protein synthesis initiation by oxidized glutathione: activation of a protein kinase that phosphorylates the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978;75:4110–4. doi: 10.1073/pnas.75.9.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 10.Sorger PK, Pelham HR. The glucose-regulated protein grp94 is related to heat shock protein hsp90. J Mol Biol. 1987;194:341–4. doi: 10.1016/0022-2836(87)90380-9. [DOI] [PubMed] [Google Scholar]
- 11.Kozutsumi Y, Segal M, Normington K, et al. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–4. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 12.Bulleid NJ, Freedman RB. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988;335:649–51. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- 13.Gardner RG, Swarbrick GM, Bays NW, et al. Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol. 2000;151:69–82. doi: 10.1083/jcb.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander R, Jarosch E, Urban J, et al. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2:379–84. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- 15.Schuberth C, Buchberger A. Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat Cell Biol. 2005;7:999–1006. doi: 10.1038/ncb1299. [DOI] [PubMed] [Google Scholar]
- 16.Okamura K, Kimata Y, Higashio H, et al. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem Biophys Res Commun. 2000;279:445–50. doi: 10.1006/bbrc.2000.3987. [DOI] [PubMed] [Google Scholar]
- 17.Bertolotti A, Zhang Y, Hendershot LM, et al. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biology. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 19.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–22. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–53. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 21.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. Journal of Clinical Investigation. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10845–50. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy KM, Ranganathan V, Farnsworth ML, et al. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7:102–11. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 26.Zong WX, Li C, Hatzivassiliou G, et al. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong WX, Lindsten T, Ross AJ, et al. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–6. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Molecular Cell. 2001;7:1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Feng D, Li Y, et al. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–7. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Scheuner D, Mierde DV, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–64. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 31.Scheuner D, Song B, McEwen E, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Molecular Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 32.Lipson KL, Fonseca SG, Ishigaki S, et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–54. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Wolfram DJ, Wagener HP. Diabetes mellitus and simple optic atrophy among siblings: report of four cases. May Clin Proc. 1938;1:715–718. [Google Scholar]
- 34.Barrett TG, Bundey SE. Wolfram (DIDMOAD) syndrome. J Med Genet. 1997;34:838–41. doi: 10.1136/jmg.34.10.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346:1458–63. doi: 10.1016/s0140-6736(95)92473-6. [DOI] [PubMed] [Google Scholar]
- 36.Karasik A, O’Hara C, Srikanta S, et al. Genetically programmed selective islet beta-cell loss in diabetic subjects with Wolfram’s syndrome. Diabetes Care. 1989;12:135–8. doi: 10.2337/diacare.12.2.135. [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Tanizawa Y, Wasson J, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nature Genetics. 1998;20:143–8. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- 38.Strom TM, Hortnagel K, Hofmann S, et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet. 1998;7:2021–8. doi: 10.1093/hmg/7.13.2021. [DOI] [PubMed] [Google Scholar]
- 39.Khanim F, Kirk J, Latif F, Barrett TG. WFS1/wolframin mutations, Wolfram syndrome, and associated diseases. Hum Mutat. 2001;17:357–67. doi: 10.1002/humu.1110. [DOI] [PubMed] [Google Scholar]
- 40.Takeda K, Inoue H, Tanizawa Y, et al. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet. 2001;10:477–84. doi: 10.1093/hmg/10.5.477. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann S, Philbrook C, Gerbitz KD, Bauer MF. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum Mol Genet. 2003;12:2003–12. doi: 10.1093/hmg/ddg214. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca SG, Fukuma M, Lipson KL, et al. WFS1 Is a Novel Component of the Unfolded Protein Response and Maintains Homeostasis of the Endoplasmic Reticulum in Pancreatic {beta}-Cells. J Biol Chem. 2005;280:39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- 43.Ishihara H, Takeda S, Tamura A, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13:1159–70. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 44.Riggs AC, Bernal-Mizrachi E, Ohsugi M, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–21. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 45.Stoy J, Edghill EL, Flanagan SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–4. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Takeuchi T, Tanaka S, et al. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Journal of Clinical Investigation. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbach N, Rathkolb B, Kemter E, et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes. 2007;56:1268–76. doi: 10.2337/db06-0658. [DOI] [PubMed] [Google Scholar]
- 48.Karaskov E, Scott C, Zhang L, et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 49.Cnop M, Ladriere L, Hekerman P, et al. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem. 2007;282:3989–97. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- 50.Cunha DA, Hekerman P, Ladriere L, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–18. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirot P, Eizirik DL, Cardozo AK. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia. 2006;49:1229–36. doi: 10.1007/s00125-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 52.Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–61. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida H, Matsui T, Yamamoto A, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 54.Shen X, Ellis RE, Lee K, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 55.Calfon M, Zeng H, Urano F, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida H, Matsui T, Hosokawa N, et al. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4:265–71. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 57.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–59. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 59.Nishitoh H, Matsuzawa A, Tobiume K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–55. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maundrell K, Antonsson B, Magnenat E, et al. Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J Biol Chem. 1997;272:25238–42. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- 61.Park J, Kim I, Oh YJ, et al. Activation of c-Jun N-terminal kinase antagonizes an anti-apoptotic action of Bcl-2. J Biol Chem. 1997;272:16725–8. doi: 10.1074/jbc.272.27.16725. [DOI] [PubMed] [Google Scholar]
- 62**.Lipson KL, Ghosh R, Urano F. The Role of IRE1alpha in the Degradation of Insulin mRNA in Pancreatic beta-Cells. PLoS ONE. 2008;3:e1648. doi: 10.1371/journal.pone.0001648. This paper shows that chronic high glucose causes IRE1 α hyperactivation, leading to insulin mRNA degradation. This is the first report integrating ER stress signaling and glucose toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Han D, Lerner AG, Vande Walle L, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–75. doi: 10.1016/j.cell.2009.07.017. This paper shows that IRE1α acts as a binary switch between life and death of ER stressed cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hollien J, Lin JH, Li H, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–7. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 66.Harding HP, Zhang Y, Bertolotti A, et al. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 67*.Song B, Scheuner D, Ron D, et al. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89. doi: 10.1172/JCI34587. This study shows that deletion of an apoptotic component of the UPR promotes β-cell survival in various diabetes mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]


