Abstract
N-Glycolylneuraminic acid (Neu5Gc) is a non-human sialic acid, which may play a significant role in human pathologies, such as cancer, and vascular disease. Further studies into the role of Neu5Gc in human disease is hindered by limited sources of this carbohydrate. Using a chemoenzymatic approach, Neu5Gc was accessed in 6 steps from glucose. The synthesis allows access to gram scale quantities quickly and economically, and produces Neu5Gc in superior quality to commercial sources. Finally, we demonstrate that the synthesized Neu5Gc can be incorporated into the cell glycocalyx of human cells, which do not naturally synthesize this sugar. The synthesis produces Neu5Gc suitable for in vitro or in vivo use.
Keywords: Sialic acid, N-glycolylneuraminic acid, Antigen
Sialic acids (sias) are a family of acidic 9-carbon chain carbohydrates typically found in terminal positions on the cell glycocalyx (the vast coating of glycans that covers all cell surfaces).1 Their biological functions include regulation of processes such as innate immunity, inflammation,2 cell-cell interactions, and neural plasticity. They are also involved in tumor metastasis and pathogen binding. In recent years the significance of this family of glycans to human health and evolution is becoming ever clearer.3 However, advancements have been hindered by a relative lack of available sias, and derivatives thereof, with which to conduct these biological studies. The sia N-glycolylneuraminic acid (Neu5Gc) is a non-human sialic acid, which is biosynthesized from N-acetylneuraminic acid (Neu5Ac) via the enzyme CMP-Neu5Ac hydroxylase (CMAH) encoded by the gene CMAH. Approximately 2-3 million years ago the human CMAH gene was mutated and its product was no longer able to hydroxylate CMP-Neu5Ac to CMP-Neu5Gc.4
Although humans are no longer able to make Neu5Gc, dietary Neu5Gc can still be metabolically incorporated and displayed on the glycocalyx of the human epithelia and associated carcinomas.5 In principle, Neu5Gc could be an important human specific “xeno-autoantigen”. Potential roles in tumorigenesis,6 and vascular pathologies,7 have recently been identified.
To further investigate how dietary Neu5Gc is involved in human-specific disease requires synthetic access to Neu5Gc in large quantities and high purity. Current commercial sources are limited and expensive, contain 1-3% Neu5Ac which can interfere with some biological assays, and their continued support and supply has an uncertain future.8 We have developed a chemo-enzymatic strategy to access Neu5Gc that is high yielding, suitable for use in cell experiments requiring sterile conditions, and allows quick access to gram scale quantities.
Synthetic methods to access the sias have been reported since the 1980's.9 Because of the stereochemical considerations, most methodologies take advantage of monosaccharide starting materials. Auge et al,9a synthesized Neu5Gc in milligram scale starting from mannosamine hydrochloride, introducing the glycolyl moiety using a benzyloxyacetic acid derivative, followed by hydrogenation to yield 2-deoxy-2-[(hydroxyacetyl)amino]-D-Mannopyranose (ManNGc), which was enzymatically converted to Neu5Gc. This is an attractive route for small-scale synthesis, however the 2-deoxy-2-amino-mannose, although commercially available, is expensive and therefore impractical for use on large scale. Some synthetic methods start from little or no chirality within the starting materials.9b-d Kang et al demonstrated a highly diastereoselective synthesis of Neu5Ac by stereoselective functionalization of olefin starting materials.9b Some purely enzymatic synthetic routes have also been described.10 These use enzymatic conversion of the manno derivative, or the gluco derivative via a single or multiple enzyme procedure. Wang et al has recently demonstrated the synthesis of Neu5Ac starting from N-acetyl-D-glucosamine, via two immobilized enzymes, N-acetyl-D-glucosamine 2-epimerase, and N-acetyl-D-neuraminic acid aldolase.10a In addition, bioreactors may also prove an important method to access neuraminic acids. Feirfort et al genetically engineered E.Coli to make excessive quatities of sialylated glycans.11 Although further development would be required here to isolate the pure sialic acid monomer. Although a large body of work has already been undertaken in the synthetic access of the neuraminic acids (examples are summarized in refs 9-11), these studies have not been primarily concerned with a quick, large scale, high purity synthesis of the Neu5Gc analogue.
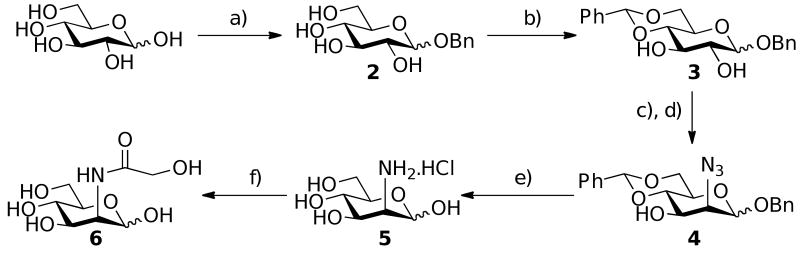
Retrosynthetic analysis of Neu5Gc (Scheme 1) presents a synthetic pathway from the readily available and cheap monosaccharide D-glucose. Orthogonal protection of the pyranose ring followed by triflation prepares the molecule for selective stereochemical inversion to the manno stereochemistry. Global deprotection and appending of the glycolyl moiety prepares the molecule for final enzymatic conversion to the product Neu5Gc 1.
Scheme 1.
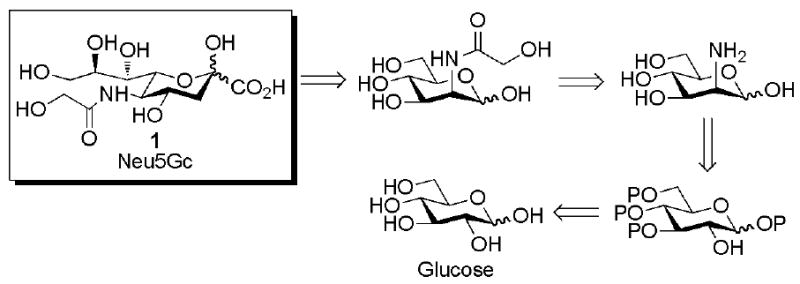
Retrosynthetic analysis of Neu5Gc synthesis reveals glucose as a suitable starting material. P = protecting group.
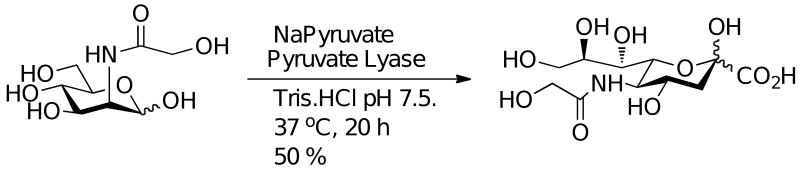
Scheme 2 outlines the synthetic route taken. Reaction of D-glucose with benzyl alcohol in the presence of acetyl chloride produced benzyl D-glucopyranoside 2 in 86 % yield, which was further modified to produce the 4,6 benzylidiene derivative 3 in 84 % yield, by reaction with benzaldehyde dimethylacetyl and a catalytic amount of (+)-camphor-10-sulfonic acid. Triflation of the 2-OH group of 3 using triflic anhydride at -30 °C12 followed by displacement with sodium azide selectively inverted the stereochemistry to afford the 2-azido-2-deoxy-mannopyranoside, 4 in 72 % yield. A global deprotection strategy was envisaged using Pd/C and H2(g), however, initial experiments showed only reduction of the azide moiety to form the amine product (table 1, entry 1). No reduction of benzyl moieties occurred, thought to be due to inhibition of the catalyst by the amine product (compare table 1, entry 1 and 2), an observation that has been made previously.13 It has been demonstrated that this problem can be overcome using large amounts of Pd/C,14 however, this is only practical for small scale synthesis (5-10 mg). We initially changed catalyst to the more rigorous pearlmans catalyst, Pd(OH)2/C, which has been successfully used to reduce more difficult O-benzyl moieties. However, this was unsuccessful (table 1, entry 3). Access to 5 was successfully achieved by addition of 1 M hydrochloric acid to the reaction mixture (table 1, entry 4), which afforded the product after filtration through celite in 89 % yield. One previous example of this has been demonstrated.15 Preparation of ManNGc 6 was achieved in 94 % yield by reaction with acetoxyacetyl chloride under mild basic conditions and reduced temperature. Final conversion of 6 to the target 1, was achieved using an aldolase isolated from Pasteurella multocida, using a previously described method.16 Conversion of ManNGc to Neu5Gc using this methodology has previously been reported on a milligram scale.9a,16 Reaction of 6 with the aldolase in the presence of an excess of pyruvate, afforded Neu5Gc 1 in 50 % yield (scheme 3). The starting material 6, could be recovered during the purification step (see SI) and used again as a substrate for the aldolase.
Scheme 2.
Synthesis of ManNGc. a) benzyl alcohol, acetyl chloride, 86 %, b) benzyl-dimethylacetyl, (+)-camphor-10-sulfonic acid, acetonitrile, 84 %, c) pyridine, triflic anhydride, dichloromethane, d) sodium azide, dimethylformamide, 72 % (over two steps), e) Pd(OH)2/C(cat), H2(g), HCl(aq), methanol, 89 %, f) acetoxyacetyl chloride, sodium bicarbonate, water, 94 %.
Table 1.
O-linked Hydrogenation in the presence of an amine, achieved using HCl(aq) within the reaction mixture.
| Entry | Starting material | Catalyst | Solvent | Yield (%)[a] |
|---|---|---|---|---|
| 1 | 4 | Pd/C | MeOH | ND |
| 2 | 3 | Pd(OH)2/C | MeOH | 100 |
| 3 | 4 | Pd(OH)2/C | MeOH | ND |
| 4 | 4 | Pd(OH)2/C | MeOH/HCl(aq) | 100 |
The yield of the fully deprotected product (determined by t.l.c). ND = not detected.
Scheme 3.
Enzymatic synthesis of Neu5Gc from ManNGc.
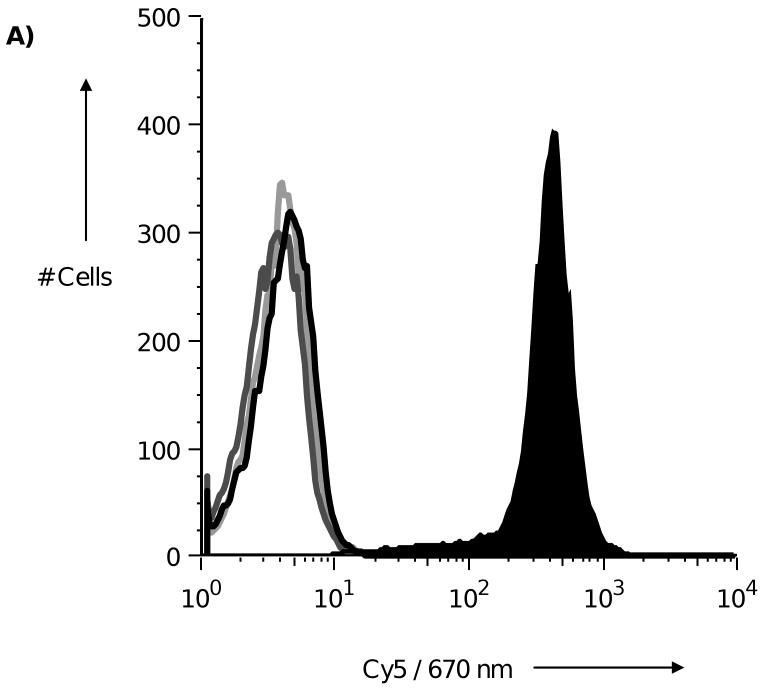
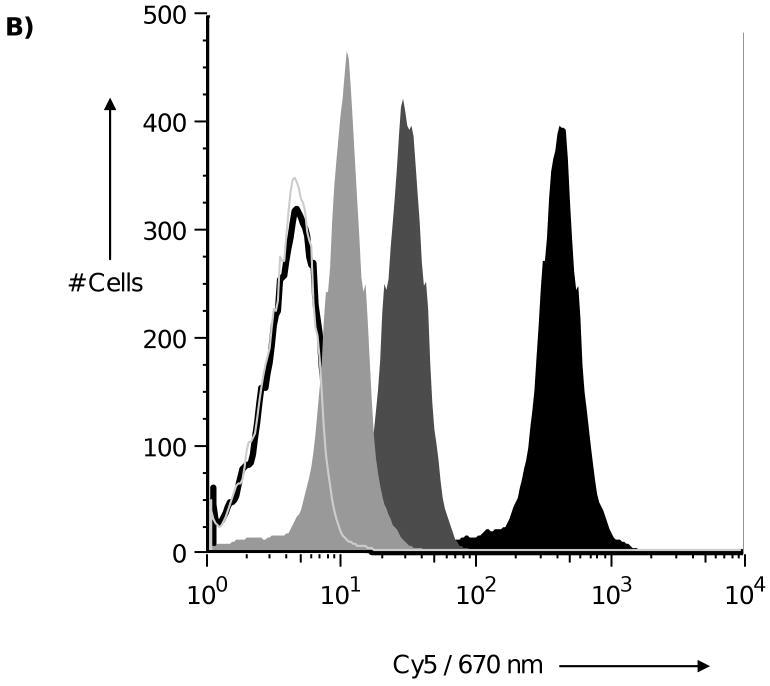
Comparison of the commercial Neu5Gc and synthesized versions by amperometric analysis (see SI figure S1-3) revealed the methodology produced Neu5Gc which had no trace of Neu5Ac, compared to approximately 3 % seen in commercial sources. Although humans can no longer make Neu5Gc, human tissue samples, including carcinomas, have been shown to have significant levels of Neu5Gc on their surface.17 A likely explanation for this is incorporation from dietary sources such as red meat products. To test whether Neu5Gc could be incorporated into the cell glycocalyx of human cells, human monocytic cell line THP-1 was grown in media containing either Neu5Ac, synthesized Neu5Gc, or neither. After three days the cells were stained with a recently described anti-Neu5Gc IgY,18 and analysed by flow cytometry (Figure 1). Neu5Gc fed cells showed incorporation of Neu5Gc into the cell surface (1A). Moreover, the anti-Neu5Gc IgY staining seen in Neu5Gc fed cells could be inhibited by addition of free Neu5Gc. 20 mM free Neu5Gc showed complete inhibition (Figure 1B). In addition, cells fed the synthesized Neu5Gc showed comparable cell proliferation to unfed cells (cell proliferation was measured using a cytometer. Cells fed synthesized Neu5Gc, or commercial Neu5Ac proliferated in a comparable manner to unfed cells), indicating that the synthesized material contained no trace amounts of cytotoxic materials.
Figure 1.
Synthetic Neu5Gc is metabolised by human THP-1 cells and displayed in terminal positions on the cell glycocalyx. 1A) Synthesized Neu5Gc can be metabolized and displayed on terminal positions of the cell glycocalyx of human cells. THP-1 cells, a human monocytic cell line that cannot synthesize Neu5Gc, was fed with synthesized Neu5Gc, Neu5Ac, or no additional glycan. After three days the cells were fixed and stained for Neu5Gc using a primary IgY. Open black: IgY isotype control, closed black: Neu5Gc fed, open dark grey: Neu5Ac fed, open light grey: not fed. 1B) The binding of anti-Neu5Gc IgY was specific for Neu5Gc. Binding of the IgY to cells fed with Neu5Gc could be blocked with ‘free’ Neu5Gc. 20 mM ‘free’ Neu5Gc was sufficent to completely block IgY binding. Solid black: no ‘free’ Neu5Gc, solid dark grey: 0.2 mM ‘free’ Neu5Gc, solid light Grey: 2 mM free Neu5Gc, open light grey: 20 mM free Neu5Gc, open black: IgY isotype control.
In summary, we have produced a chemoenzymatic synthesis of the human carbohydrate antigen Neu5Gc. The synthetic route is quick and allows access to gram scale quantities in high yield and purity. Given the uncertain future of commercial sources and the known Neu5Ac contamination issue, this methodology provides an alternative means to access this important sugar. We have also demonstrated that the synthesized Neu5Gc is appropriate for use in sterile in vitro conditions and can be loaded into the glycocalyx of human cells in vitro. We have also used it within in vivo models with no reported atypical effects (unpublished data). We envisage the synthesis is flexible enough to allow a wide range of ManNGc or Neu5Gc derivatives to be made which may prove useful to other areas of glycobiology research.
1. Experimental
1.1. General Methods
‘Petrol’ refers to the fraction of petroleum ether in the boiling range of 35-60 °C. ‘Brine’ refers to a saturated aqueous solution of sodium chloride. Proton nuclear magnetic resonance (δH) were recorded on a Jeol ECA 500 (500 MHz). 500 MHz spectra were assigned using COSY. All chemical shifts are quoted on the δ-scale in ppm, using residual solvent as the internal standard. Low resolution mass spectra were recorded on a Micromass Platform 1 spectrometer using electron spray (ES) ionisation with methanol as carrier solvent. Flash column chromatography was performed using sorbsil C60 40/60 silica gel. Thin layer chromatography (t.l.c) was performed using Merck Kieselgel 60F254 pre-coated aluminium backed plates. Plates were visualised using UV Lamp (λmax = 354 or 365 nm), or 5 % sulphuric acid in methanol. DCM was dried over molecular beads (8-12 mesh). Other anhydrous solvents were purchased from Fluka and Sigma.
1.2. Benzyl D-glucopyranoside (2)19
Glucose (68 g, 0.38 mmol) was slurried in benzyl alcohol (400 mL, 3.7 mol). Acetyl chloride (35 mL, 0.45 mmol) was added slowly over 10 min. Reaction mixture was heated to 50 °C and stirred for 48 h, after which t.l.c (EtOAc:MeOH 9:1) showed significant consumption of starting material (Rf 0.0) and formation of the product (Rf 0.4). The reaction mixture was dried by vacuum distillation (110 °C). The resulting crude product 2 was recrystallised from acetonitrile to yield a mixture of anomers as a white amorphous material (88 g, 86 %).
1H NMR (CD3OD, 500 MHz) (assigned for the major anomer; alpha) δ = 3.23-3.36 (m, 2H, H-6, H-6′), 3.39 (dd, 1H, J1,2 3.7 Hz, J2,3 9.7 Hz, H-2), 3.64-3.69 (m, 2H, H-3, H-5), 3.76 (dd, J 2.3 Hz, J 11.8 Hz, 1H, H-4), 4.52 (d, 1H, Ja,b 11.7 Hz, PhHaHbO), 4.74 (d, 1H, Ja,b 11.7 Hz, PhHaHbO), 4.86 (d, 1H, J1,2 3.7 Hz, H-1), 7.23-7.41 (m, 5H, Ph). m/z (ESI+) 288 (M+NH4+, 100 %), 293 (M+Na+, 35 %). HRMS m/z (ES-) Calcd. For C13H18O6 (M-H) 269.1020. Found 269.1019.
1.3. Benzyl 4-6-O-(phenylmethylene)-D-glucopyranoside (3)20
1-Benzyl-D-glucopyranoside (17g, 63 mmol) was slurried in acetonitrile (150 mL) and benzyl-dimethylacetal (40.5 g, 0.26 mol). (+)-Camphor-10-sulfonic acid (154 mg, 0.67 mmol) was added and the reaction mixture stirred at room temperature for three hours after which t.l.c. (100 % EtOAc) showed complete consumption of the starting material (Rf 0.3) and the formation of the product (Rf 0.8). The reaction mixture was quenched with triethylamine (1.8 mL, 17.8 mmol) and dried under vacuum. The crude product was purified by flash silica chromatography (Petrol:EtOAc 1:1) to yield the product 3 as a white gum (18.9 g, 84 %).
1H NMR (CDCl3, 500 MHz) (assigned for the major anomer; alpha) δ = 3.49 (at, 1H, J 9.5 Hz, H-4), 3.63 (dd, 1H, J1,2 4.0 Hz, J2,3 9.2 Hz, H-2), 3.71 (at, 1H, J 10.4 Hz, H-6′), 3.83 (atd, 1H, J 5.2 Hz, 10.0 Hz, H-5), 3.95 (at, 1H, J 9.2, H-3), 4.21 (dd, 1H, J5,6′ 4.3 Hz, J6,6′ 10.3 Hz, H-6′), 4.56 (d, 1H, Ja,b 11.8 Hz, PhHaHbO), 4.67 (d, 1H, Ja,b 11.7 Hz, PhHaHbO), 5.01 (d, 1H, J1,2 4.0 Hz, H-1), 5.51 (s, 1H, PhCH), 7.30-7.40 (m, 10H, 2 Ph). m/z (ESI+) 359 (M+H+, 70 %), 376 (M+NH4+, 70 %). HRMS m/z (ES+) Calcd. For C20H22O6 (M+H+) 359.1411. Found 359.1413.
1.4. Benzyl 2-azido-2-deoxy-4,6-O-(phenylmethylene)-D-mannopyranoside (4)21
Pyridine (8.8 g, 111.6 mmol), was dissolved in dry DCM (300 mL), followed by addition of trifluoromethanesulfonic anhydride (8.6 g, 30.7 mmol) and cooled to -30 °C. 1-Benzyl, 4-6-O-(phenylmethylene)-D-glucopyranoside (10 g, 27.9 mmol) was dissolved in DCM (250 mL) and added dropwise over 30 min under anhydrous conditions to the cooled reaction mixture. After 2h t.l.c. (Petrol:EtOAc 4:1 showed almost complete consumption of the starting material (Rf 0.0), and the formation of the unisolated intermediate (Rf 0.7). The reaction mixture was quenched with brine and the organic layer dried with sodium sulfate, filtered, and concentrated under vacuum (< 30°C). The crude intermediate (oil) was dissolved in dry dimethylformamide (500 mL) and sodium azide (5.4 g, 83.7 mmol) added to the solution. The reaction mixture was heated to 50 °C and stirred under argon for 16 h, after which time t.l.c. (Petrol: EtOAc 10 %) showed the formation of the product (Rf 0.4). The reaction mixture was quenched with excess water, and the product extracted with diethyl ether, dried over magnesium sulfate, filtered and concentrated under vacuum. The crude product was purified by flash silica column chromatography (Petrol:EtOAc 9:1) to yield the product 4 as a clear/colourless oil (7.7 g, 72 %).
1H NMR (assigned for the major anomer, alpha) (CDCl3, 500 MHz) δ = 3.81 (m, 2H, H-5, H-6′), 3.91 (at, 1H, J 9.5 Hz, H-4), 4.02 (dd, 1H, J1,2 1.4 Hz, J2,3 4.0 Hz, H-2), 4.22 (m, 1H, H-6), 4.32 (dd, 1H, J2,3 3.9 Hz, J3,4 9.6 Hz, H-3), 4.50 (d, 1H, Ja,b 11.7 Hz, PhCHaHbO), 4.70 (d, 1H, Ja,b 11.7 Hz, PhCHaHbO), 4.88 (d, 1H, J1,2 1.3 Hz, H-1), 5.58 (s, 1H, PhCH), 7.30-7.40 (m, 10H, 2 Ph). m/z (ESI+) 384 (M+H+, 100 %), 401 (M+NH4+, 65 %), 406 (M+Na+, 35 %).
1.5. 2-Amino-2-deoxy-D-mannose hydrochloride (5)
1-Benzyl-2-azido-2-deoxy-4,6-O-(phenylmethylene)-D-mannopyranoside (3.3 g, 8.6 mmol), was dissolved in methanol (100 mL) and 2 M HCl (aq) (8.6 mL, 17.2 mmol of HCl) was added. Pd(OH)2/C (825 mg) was added to the reaction mixture. The reaction mixture was saturated with H2 (g) and stirred for 48 h under H2 (g) after which time t.l.c. (EtOAc:MeOH 7:3) showed complete consumption of the starting material (Rf 1.0), and the formation of the product (Rf 0.3). The reaction mixture was filtered through celite, and washed with methanol. The clear/colourless solution was concentrated under vacuum. The product was dissolved in water (50 mL) and washed with Et2O (3 × 50 mL). Thr aqueous layer was dried under vacuum to yield the product 5 as a clear/colourless gum (1.6 g, 89 %).
1H NMR (D2O, 500 MHz) (assigned for the major anomer) δ = 3.31 (ddd, 1H, J5,6′ 2.3 Hz, J5,6 5.7 Hz, J4,5 10.0 Hz, H-5), 3.38 (at, 1H, J 9.5 Hz, H-4), 3.56 (dd, 1H, J1,2 1.4 Hz, J2,3 4.6 Hz, H-2), 3.60 (dd, 1H, J5,6 5.3 Hz, J6,6′ 12.3 Hz, H-6), 3.75 (dd, 1H, J5,6′ 2.3 Hz, J6,6′ 12.3 Hz, H-6′), 3.83 (dd, 1H, J2,3 4.9, J3,4 9.4, H-3), 5.01 (d, 1H, J1,2 1.8 Hz, H-1). m/z (ESI+) 180 (M+H+, 90 %). HRMS m/z (ES+) Calcd. For C6H14NO5 (M+H+) 180.0866. Found 180.0865. Spectral data was in agreement with a commercial standard (mannosamine standard purchased from Sigma).
1.6. 2-Deoxy-2-[(hydroxyacetyl)amino]-D-Mannose (6)9j
2-Amino-2-deoxy-D-mannopyranoside (1.4 g, 6.5 mmol) was dissolved in a water (60mL) with sodium bicarbonate (10.8 g, 130 mmol) and cooled in an ice bath. Acetoxyacetyl chloride (4.2 mL, 39 mmol) was added slowly to the reaction mixture. After 30 min t.l.c (EtOAc: MeOH 7:3) showed complete consumption of the starting material (Rf 0.0) and the formation of the product (Rf 0.4). The reaction mixture was neutralized with mixed bed resin and concentrated under vacuum to yield 6 as a white gum (1.0 g, 94 %).
1H NMR (D2O, 500 MHz) (assigned for the major anomer) δ = 3.30 (m, 1H, H-5), 3.45 (at, 1H, J 10.0 Hz, H-4), 3.58 (dd, 1H, J5,6 5.2 Hz, J6,6′ 12.0 Hz, H-6), 3.69-3.73 (m, 2H, H-2, H-6′), 3.78 (m, 1H, H-3), 4.02 (s, 2H, COCH2OH), 4.91 (d, 1H, J1,2 1.4 Hz, H-1). m/z (ESI+) 260 (M+Na+, 100 %). HRMS m/z (ES+) Calcd. For C8H15NO7Na (M+Na+) 260.0741. Found 260.0742.
1.7. N-Glycolylneuraminic acid (1)9a
2-Deoxy-2-[(hydroxyacetyl)amino]-D-mannopyranose (2.4 g, 9.68 mmol), sodium pyruvate (24.8 g, 48.34 mmol), and pyruvate lyase (1553 μl, 33.76 mg/mL, from pasteorella multocida (PmNanA), plasmid gifted from Dr. Xi Chen) were dissolved in TRIS-HCl (524 mL, 100 mM) and pH confirmed to be 7.5. The reaction mixture was incubated at 37 °C with shaking for 20 h. TBA analysis was used to confirm formation of the product, and predicted a 50 % conversion. The reaction solution was passed through a Dowex -50 column (diameter = 2.5 cm, height = 15 cm). The column was washed with water (5 × 75 mL). The combined eluent and washes were passed though an AG 1×8 ion exchange column (diameter = 2.5 cm, height = 100 cm). The column was washed with 10 mM formic acid (7 × 500 mL). The product was eluted from the column with 1 M formic acid (approx 1 L). The clear / colourless eluent was concentrated under vacuum to yield the product as a white gum (1.6 g, 50 %).
1H NMR (D2O, 500 MHz) (assigned for the major anomer) δ = 1.73 (t, 1H, J 11.7 Hz, H-3), 2.17 (dd, 1H, J3′,4 4.9 Hz, J3,3′ 13.2 Hz, H-3′), 3.38 (t, 1H, J 9.2 Hz, H-7), 3.46 (dd, 1H, J8,9 6.3 Hz, J9,9′ 12.0 Hz, H-9), 3.59-3.62 (m, 1H, H-8), 3.68 (dd, 1H, J8,9′ 2.5 Hz, J9,9′ 11.7 Hz, H-9′), 3.85 (t, 1H, J 10.3 Hz, H-5), 3.98-4.07 (m, 2H, H-4, H-6), 4.00 (s, 2H, COCH2OH). m/z (ESI+) 324 (M-H+, 100 %).
1.8. Neu5Gc / Neu5Ac feeding
THP-1 cells were grown in Dulbecco's Modified Eagle Medium with 5 % human serum. Freshly passaged cells were incubated with 1.9 mM Neu5Gc or Neu5Ac, or just media for three days. Cells were lifted from culture flasks using 2 mM ethylenediaminetetraacetic acid (EDTA, 10-15 min, r.t.), and immediately pelleted (centrifudge 1400 rpm) and the supernatent removed. The cell pellet was resuspended in 3 % paraformaldehyde (20 min, r.t.). After fixing, cells were pelleted then resuspended in 1 % fish geletin (sigma) in phosphate buffered saline (PBS) (blocking buffer). Once prepared 400,000 cells were used for each staining reaction, at 4 °C. The cell pellet was resuspended in 100 μL of either affinity purified chicken anti-Neu5Gc antibody,17 or a control chicken IgY (Jackson ImmunoResearch, West Grove, PA) diluted 1:5000 in blocking buffer and incubated for 20 min at 4 °C. Cells were pelleted, then washed with 500 μL of blocking buffer, and pelleted. Cells were resuspended in 100 μL Cy5-conjugated Donkey-anti-chicken IgY (Jackson ImmunoResearch, West Grove, PA), diluted 1:1000 in blocking buffer, incubated for 20 min at 4 °C in the dark, then washed as above. The stained cells were resuspended in 500 μL PBS, the data collected on a FACSCalibur (BD Biosciences Immunocytometry Systems, San Jose, CA) and analysed with Flowjo software (Tree Star, Ashlan, OR). Competition assays were done by addition of ‘free’ Neu5Gc during the primary antibody staining step.
Supplementary Material
Acknowledgments
We thank, Sandra L. Diaz and Christopher J. Gregg for useful discussions and technical help; Prof Xi Chen for the aldolase enzyme plasmid; Biswa Choudhury and Natasha Naidu for pulsed amperometric analysis of compounds; Dr. Katie J. Doores and Dr. Ieuan G. Davies for critical reading of the manuscript; and, the Cancer Research Institute/Samuel and Ruth Engelberg Fellowship for funding (O. M. T. P). This work was supported by NIH grant R01GM32373 to A.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varki A. Nature. 2007;446(7139):1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Lewis AL, Cho JL, Miller M, Varki A. Blood. 2007;109(10):4280–4287. doi: 10.1182/blood-2006-08-039255.. For a review see, Crocker P, Paulson JC, Varki A. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056.
- 3.Varki A. Trends Mol Med. 2008;14(8):351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varki A. Am J Phys Anthropol. 2000:309–309. [Google Scholar]
- 5.Bardor M, Nguyen DH, Diaz SL, Varki A. J Biol Chem. 2005;280(6):4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 6.Hedlund M, Padler-Karavani V, Varki N, Varki A. Proc Natl Acad Sci USA. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, Chen X, Witztum JL, Varki NM, Varki A. Blood. 2009;114(25):5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commercial sources are limited, and may not continue. Source: Inalco pharmaceuticals. Website: www.inalcopharm.com.
- 9.Examples of chemical procedures to produce neuraminic acids and derivatives thereof: Auge C, David S, Gautheron C, Malleron A, Cavaye B. New J Chem. 1988;12:733–744.Kang SH, Choi Hw, Kim JS, Youn JH. Chem Commun. 2000:227–228.Danishefsky SJ, DeNinno MP. J Org Chem. 1986;51:2615–2617.Danishefsky SJ, DeNinno MP, Chen SL. J Am Chem Soc. 1988;110(12):3929–3940.Hartlieb S, Gunzel A, Gerardy-Schahn R, Munster-Kuhnel AK, Kirschning A, Drager G. Carbohyd Res. 2008;343:2075–2082. doi: 10.1016/j.carres.2008.02.003.Takahashi T, Tsukamoto H, Kurosaki M, Yamada H. Synlett. 1997:1065–1066.Li LS, Wu YL, Wu Y. Org Lett. 2000;2(7):891–894. doi: 10.1021/ol0055418.Mack H, Brossmer R. Tetrahedron Lett. 1992;33(14):1867–1870.Hong Z, Liu L, Hsu CC, Wong CH. Angew Chem Int Ed. 2006;45:7417–7421. doi: 10.1002/anie.200601555.Choi SK, Lee S, Whitesides GM. J Org Chem. 1996;61(25):8739–8745. doi: 10.1021/jo9614856.
- 10.Examples of enzymatic methods to make neuraminic acids: Wang TH, Chen YY, Pan HH, Wang FP, Cheng CH, Lee WC. BMC Biotechnol. 2009;9:63. doi: 10.1186/1472-6750-9-63.Kuboki A, Okazaki H, Sugai T, Ohta H. Tetrahedron. 1997;53(7):2387–2400.Humphrey AJ, Fremann C, Critchley P, Malykh Y, Schauer R, Bugg TDH. Bioorg Med Chem. 2002;10:3175–3185. doi: 10.1016/s0968-0896(02)00213-4.Kim MJ, Hennen WJ, Sweers HM, Wong CH. J Am Chem Soc. 1988;110:6481–6486.Huang S, Yu H, Chen X. Angew Chem Int Ed. 2007;46:2249–2253. doi: 10.1002/anie.200604799.
- 11.Fierfort N, Samain E. J Biotechnol. 2008;134:261–265. doi: 10.1016/j.jbiotec.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Chambers DJ, Evans GR, Fairbanks AJ. Tetrahedron Asymmetry. 2003;14:1767–1769. [Google Scholar]
- 13.Czech BP, Bartsch RA. J Org Chem. 1984;49:4076–4078. [Google Scholar]
- 14.(a) Crossman A, Smith TK, Ferguson MAJ, Brimacombe JS. Tetrahedron Lett. 2005;46:7419–7421. [Google Scholar]; (b) Liu X, Kwon YU, Seeberger PH. J Am Chem Soc. 2005;127:5004–5005. doi: 10.1021/ja042374o. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto N, Kanie O, Huang YY, Fujii R, Watanabe H, Shimamura M. Chem Biol. 2005;12:677–683. doi: 10.1016/j.chembiol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B, Chen X. Appl Microbiol Biotechnol. 2008;79:963–970. doi: 10.1007/s00253-008-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Plasmid kindly gifted from Professor Xi Chen.
- 17.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, Brinkman-Van der Linden ECM, Varki A, Varki NM. PLos ONE. 2009;4(1):1–10. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonda Y, Krainz K, Liebner F, Potthast A, Rosenau T, Karakawa M, Nakatsubo F. Eur J Org Chem. 2008:475–484. [Google Scholar]
- 20.Lubineau A, Thieffry A, Veyrieres A. Carbohydr Res. 1976;46:143–148. [Google Scholar]
- 21.Paulsen H, Helpap B, Lorentzen JP. Leibigs Ann Chem. 1987:431–437. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.