Abstract
A spectrophotometric hemoglobin assay is widely used to estimate the extent of brain hemorrhage by measuring the amount of hemoglobin in the brain. However, this method requires using the entire brain sample, leaving none for histology or other assays. Other widely used measures of gross brain hemorrhage are generally semi-quantitative and can miss subtle differences. Semi-quantitative brain hemorrhage scales may also be subject to bias. Here, we present a method to digitally quantify brain hemorrhage using Photoshop and Image J, and compared this method to the spectrophotometric hemoglobin assay. Male Sprague-Dawley rats received varying amounts of autologous blood injected into the cerebral hemispheres in order to generate different sized hematomas. 24 hours later, the brains were harvested, sectioned, photographed then prepared for the hemoglobin assay. From the brain section photographs, pixels containing hemorrhage were identified by Photoshop® and the optical intensity was measured by Image J. Identification of hemorrhage size using optical intensities strongly correlated to the hemoglobin assay (R=0.94). We conclude that our method can accurately quantify the extent of hemorrhage. An advantage of this technique is that brain tissue can be used for additional studies.
Keywords: cerebral hemorrhage, digital quantification, Photoshop®, Image J, spectrometry, hemoglobin assay
Introduction
Current ways to estimate the amount cerebral hemorrhage include assigning semiquantitative scores (Yenari et al., 1997b; Yenari et al., 1998), counting numbers of brain slice faces containing hemorrhage (Lyden et al., 1990; Thomas et al., 1993), hemoglobin assay (Choudhri et al., 1997), computed tomography (Bardera et al., 2009; Liu et al., 2008), and magnetic resonance imaging (Neumann-Haefelin et al., 2001; Yenari et al., 1997a). All of these methods have disadvantages. For example, semiquantitative scores and brain slice counting are relatively crude measures, and may not accurately estimate subtle differences. CT and MRI scans require specialized equipment, and their accuracy compared to standard histology may vary depending on the sequence used or timing (Neumann-Haefelin et al., 2001). The hemoglobin assay is the most accurate way to quantify the hemorrhage, but this method requires the homogenization of brain tissue, making it unavailable for other purposes. This can be a disadvantage especially when there is a need to study the relationship between hemorrhage and parallel mechanisms. In order to circumvent this problem, we developed a method of digitally measuring the hemorrhage from fresh brain sections that comparably estimates the extent of hemorrhage as the spectrophotometric hemoglobin assay.
1. Materials and Methods
1.1. Animal model and tissue preparation
Male Sprague-Dawley rats (Simonson, Gilroy, CA, USA), 300–500 grams, were housed and treated in a humane manner in strict accordance with institutional guidelines and approval. The cerebral hemorrhage model was generated as described elsewhere with modifications (Hua et al., 2002; Okauchi et al., 2009). In brief, rats were anesthetized with 5% isofluorane delivered into an anesthesia induction chamber with oxygen and air supplied at a ratio of 0.2 L/min oxygen: 0.8 L/min air. Once surgical planes of anesthesia were attained (assessed by absence of hind leg withdrawal to pinch), isoflurane was decreased to 2.5% throughout the remainder of the surgery through Stereotaxic Anesthesia Mask (cat #: 921434, VetEquip, Inc. Pleasanton, CA, USA) mounted on the stereotactic frame (Stoelting, Wood Dale, IL, USA). The left femoral artery was catheterized to obtain autologous blood. Rats were placed in a stereotactic frame and one or two cranial burr holes (1 mm) were drilled by a dental drill (#18000-17, Fine Science Tools Inc. Foster City, CA, USA) on the right coronal suture 3.5 mm lateral to the midline, 1 mm anterior or/and 1 mm posterior to midline. The burr holes were filled with bone wax, and a 25.5 gauge needle was inserted stereotactically into the right hemisphere. Autologous whole blood in volumes of 50 μl (n=8), 100 μl (n=7), 150 μl (n=5), 200 μl (n=6) or 250 μl (n=3) were injected at a rate of 2.5 μl /min using a Harvard 11 Plus Syringe Pumps (Harvard Apparatus Inc, Massachusetts, United States). For animals receiving 50 μl, coordinates were: 2 mm anterior & 3.5 mm lateral to bregma, depth 5.5 mm. For the other groups, two sites were injected with coordinates at 1) 2 mm anterior & 3.5 mm lateral to bregma, depth 5.5 mm; 2) 2 mm posterior & 3.5 mm lateral to bregma, depth 5.5 mm. 20 minutes post injection, the needle was removed. 100 μl of 0.5% bupivocaine was injected at the surgical wound site, and the wound site was then closed. The animals were allowed access to food and water ad libitum. 24 hours after surgery they were euthanized with an overdose of isofluorane, decapitated, and the brains were rapidly removed and sectioned coronally at 2-mm intervals. The sections were transferred to a petri dish, and images of the gross sections were immediately collected by scanning on a digital scanner (HP scanjet 4370, Product Number: L1970A, Hewlett-Packard Company, Palo Alto, CA, USA) including a ruler in the scanning field as a reference. The resolution of each image was 600 dpi and the scanned images were saved as TIF files for the next processing step.
1.2. Isolation of the hemorrhagic portion of the images
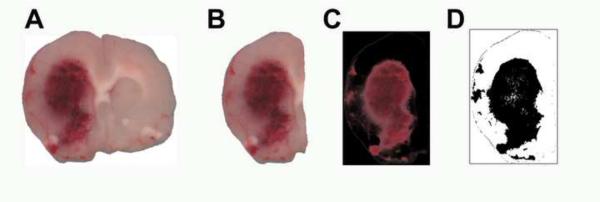
After brain section images were collected, areas of hemorrhage were isolated from the images using Photoshop 6.0 (Adobe, San Jose, CA). Digital images (Fig 1A) taken from scanned brain sections were opened into Photoshop. The image of the ipsilateral brain section (Fig 1B) was selected and copied to the clipboard. A new image was created, and the contents of the clipboard pasted twice into the new image to create two identical layers, layer 1 and layer 2. This was done in order to place the brain image onto a transparent layer in a new image. Next, the background (currently empty) layer was deleted, leaving only the pasted layers of the brain image. Using the Magic Wand tool, the image space surrounding the brain section was selected and deleted in layers 1 and 2, thus isolating only the brain tissue to be analyzed (it is helpful to photograph the brain tissue on a white background to easily isolate the brain from the background in Photoshop). Next, all hemorrhage was converted to black. This was accomplished by selecting layer 2, selecting the menu item Adjustments->Replace color, and using the dropper selector to select a color from the center of the hemorrhage. One sample may not be enough to select the entire hemorrhage if the areas of hemorrhage are not confluent, so it may be necessary to add selections using the dropper with the plus next to it. Once the area(s) of hemorrhage was selected, the lightness is reduced to −100 to blacken the brain area and the fuzziness set to 65 to allow for more gradations in the color values being converted to black. Once the color replacement was applied, the blending options in the layers dialog is changed to “difference”, thus revealing only the portions of the brain section containing blood. In order to make measurement of the hemorrhage easier, a new layer was created, filled with black, and moved to the bottom of the layer stack, making all of the image black, except for the selected hemorrhage area. The resulting image was then saved as a TIFF file with no layers (Fig 1C). (For detailed instructions, see the supplementary materials)
Figure 1. Image Processing by Photoshop and Image J.
A digital image of cerebral hemorrhage scanned on a Hewlett Packard scanner (A). The ipsilateral brain section with cerebral hemorrhage selected for measurement is shown (B). The brain section after processing by Photoshop showing pixels indicate only regions of cerebral hemorrhage (C). The digital image was further processed by Image J with the hemorrhage now appearing white, and is ready for digital quantification (D).
1.3. Measurement of the hemorrhage area
To quantify the hemorrhage area, the processed image was loaded into ImageJ v. 1.31 (available as freeware from http://rsbweb.nih.gov/ij/) and converted into a 16-bit image through the menu option Image->Type->16-bit. Then, the image was thresholded using the menu option Image->Adjust->Threshold. The type was set to Black & White and the top slider moved to a value of 34 (the researcher can set this to a value useful to their dataset, but the same value should be used in all images being analyzed from an experiment). The resulting thresholded image is binary and will only show the hemorrhage region (Fig 1D). To measure the area and number of pixels, the menu option Analyze->Measure was selected, the values recorded and the process repeated on subsequent images (supplementary materials and figures). The total hemorrhage volume was calculated by summing the hemorrhagic area in each section and multiplying by the thickness of each section. The `Set Scale' function in Image J was used to convert pixel numbers to standard units (http://rsbweb.nih.gov/ij/docs/menus/analyze.html#scale). To determine inter rater reliability, two independent observers measured 4 representative brains using the above method.
1.4. Spectrophotometric Hemoglobin Assay
This hemoglobin assay is similar to that previously published by Choudri et al (Choudhri et al., 1997). After brain sections were scanned, the sections from the ipsilateral cerebral hemisphere were collected from each animal. Distilled water (1ml) was added to each hemisphere, and the tissue was homogenized for 30 strokes in a Dounce homogenizer and centrifuged at 13000 rpm for 30 minutes. The hemoglobin containing supernatant was collected, and 80 μl of Drabkin's solution (Sigma) was added to a 20 μL aliquot. After incubating at room temperature for fifteen minutes the optical density of the solution at 550 nm wavelength was measured to assess hemoglobin content. Measurement of each sample was repeated four times. In order to verify that the measured absorbance by this method reflected the amount of hemoglobin, human erythrocyte hemoglobin (Sigma) of known concentrations ranging from 0.156 mg/ml to 20 mg/ml) were analyzed simultaneously along with each brain tissue hemoglobin assay in order to generate a standard curve. Thus, the hemoglobin concentration of each brain was calculated from the standard curve. To convert these values to volume, the hemoglobin concentration was multiplied by the lysate volume (on the average, 1.3 ml) then divided by a previously published hemoglobin concentration in rat blood (12 g/dl (Williamson and Ets, 1926)).
1.5. Pearson Correlation Study and Linear Regression
A Pearson correlation and linear regression analysis was applied to compare results from the two methods studied using SigmaStat v. 3.11 (Systat Software, Inc. Chicago, IL, USA). Student's T-test was applied to compare differences between 2 groups, and sample size calculations were performed based on these data. Kappa statistics to estimate inter rater reliability was computed using MedCalc v. 11.2.1.0 (MedCalc Software, Mariakerke, Belgium). All data are expressed as Mean±SE.
3. Results and Discussion
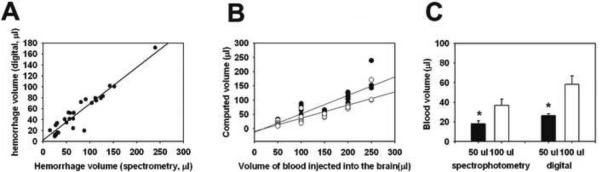
Increasing volumes of injected autologous blood not surprisingly led to larger hemotoma sizes. A strong proportional relationship was observed between brain hemorrhage volume determined from brain hemoglobin concentration measured using the spectrophotometric hemoglobin assay and the digitally calculated hemorrhage volume (Fig. 2A). Linear regression analysis showed that both measurements correlated highly in a linear fashion (R=0.94). The relationship between the two methods could be described by: blood volumespectrophotometric = 2.844 + (0.665 × blood volumedigital). Hemorrhage volumes determined from either method were near the known injected volume (Fig 2B). These values were somewhat lower than the injected volume of blood due to loss from seepage of injected blood into the subdural and/or subarachnoid space, and remaining blood in the needle. The values derived from the spectrophotometric method were also somewhat lower than that using the digital method. The reason for this is not entirely clear, but could stem from our not measuring blood hemoglobin from each rat. Nevertheless, the general relationship was similar for both methods. Further, we had two independent observers (XT & AB) measure hemorrhages from the same brain sections and found very good inter rater reliability with a kappa score of 0.83.
Figure 2. The digitally calculated hemorrhage volume is highly correlated to hemorrhage volume determined by hemoglobin spectrometry.
A: A Pearson correlation showed that the brain blood volume estimated from a spectrophotometric hemoglobin assay was highly correlated to the calculated hemorrhage volume using a digital, Photoshop/ImageJ based method (R= 0.94, P <0.001). A linear regression analysis indicated that the relationship is linear. B: Comparisons of known volumes of injected blood to digitally measured or hemoglobin spectrometry derived volumes. Black circle: digitally measured hemorrhage volume; white circle: blood volume based on spectrophotometric hemoglobin assay; C: Estimating brain hemorrhage volume by either method could distinguish between animals receiving 50 μl blood and 100 μl (T-test, *P<0.01)
Since blood volumes of 50–100 μl are typically studied in rodent brain hemorrhage models, it was important to establish that these methods could reliably distinguish between these amounts of blood. As shown in figure 2C, both methods could distinguish between these amounts of blood present in the brain. Further, the variability of the measurements was similar for both methods with standard deviations of 30% and 45% for the digital and spectrophotometric methods, respectively.
We show that this method is highly correlated to the hemoglobin assay. An obvious advantage is that the brain sections can be saved for other assays, circumventing the need to study additional animals. This method can potentially minimize any error introduced by cerebral edema or variations in ischemic lesion size inherent to many brain ischemia models. The same brain sections can be used to evaluate edema or infarct size for comparison to the amount of hemorrhage observed, rather than having to study a separate cohort of animals. This method could also be adapted to other similar measurements where colorimetric data are revealed in gradations of the same color family, such as estimates of blood brain barrier permeability by measuring Evans blue dye extravasation or IgG accumulation (Tang et al., 2008; Tang et al., 2007). Appropriate validation would need to be performed for each new measurement applied, including the assessment of brain hemorrhage at time points other than those studied here. Hemoglobin degradation can lead to color changes that may not be accurately captured by this method. Regardless, this method can also be easily implemented with equipment that is not particularly specialized or expensive.
Supplementary Material
Acknowledgments
This work was supported by DoD grant DAMD17-03-1-0532 (MAY), NIH NINDS grant R01 NS40516 (MAY), P50 NS014543 (MAY, RAS), and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardera A, Boada I, Feixas M, Remollo S, Blasco G, Silva Y, Pedraza S. Semi-automated method for brain hematoma and edema quantification using computed tomography. Comput Med Imaging Graph. 2009;33:304–11. doi: 10.1016/j.compmedimag.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr., Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Liu B, Yuan Q, Liu Z, Li X, Yin X. Automatic segmentation of intracranial hematoma and volume measurement. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1214–7. doi: 10.1109/IEMBS.2008.4649381. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Madden KP, Clark WM, Sasse KC, Zivin JA. Incidence of cerebral hemorrhage after treatment with tissue plasminogen activator or streptokinase following embolic stroke in rabbits [corrected] Stroke. 1990;21:1589–93. doi: 10.1161/01.str.21.11.1589. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Ringer TM, Sun GH, Yenari MA, Moseley ME. MRI of subacute hemorrhagic transformation in the rat suture occlusion model. Neuroreport. 2001;12:309–11. doi: 10.1097/00001756-200102120-00025. [DOI] [PubMed] [Google Scholar]
- Okauchi M, Hua Y, Keep RF, Morgenstern LB, Xi G. Effects of deferoxamine on intracerebral hemorrhage-induced brain injury in aged rats. Stroke. 2009;40:1858–63. doi: 10.1161/STROKEAHA.108.535765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–62. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XN, Wang Q, Koike MA, Cheng D, Goris ML, Blankenberg FG, Yenari MA. Monitoring the protective effects of minocycline treatment with radiolabeled annexin V in an experimental model of focal cerebral ischemia. J Nucl Med. 2007;48:1822–8. doi: 10.2967/jnumed.107.041335. [DOI] [PubMed] [Google Scholar]
- Thomas GR, Thibodeaux H, Bennett WF, Refino CJ, Badillo JM, Errett CJ, Zivin JA. Optimized thrombolysis of cerebral clots with tissue-type plasminogen activator in a rabbit model of embolic stroke. J Pharmacol Exp Ther. 1993;264:67–73. [PubMed] [Google Scholar]
- Williamson CS, Ets HN. The effect of age on the hemoglobin of the rat. American Journal of Physiology. 1926;77:408–82. [Google Scholar]
- Yenari MA, Beaulieu C, Steinberg GK, Moseley ME. Diffusion-weighted magnetic resonance imaging characteristics of hemorrhagic transformation in experimental embolic stroke. J Neuroimaging. 1997a;7:227–31. doi: 10.1111/jon199774227. [DOI] [PubMed] [Google Scholar]
- Yenari MA, de Crespigny A, Palmer JT, Roberts S, Schrier SL, Albers GW, Moseley ME, Steinberg GK. Improved perfusion with rt-PA and hirulog in a rabbit model of embolic stroke. J Cereb Blood Flow Metab. 1997b;17:401–11. doi: 10.1097/00004647-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Lee LK, Beaulieu C, Sun GH, Kunis D, Chang D, Albers GW, Moseley ME, Steinberg GK. Thrombolysis with reteplase, an unglycosylated plasminogen activator variant, in experimental embolic stroke. J Stroke Cerebrovasc Dis. 1998;7:179–86. doi: 10.1016/s1052-3057(98)80004-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.