Abstract
Spondyloarthritis (SpA), a family of inflammatory back diseases including ankylosing spondylitis, is an important and underrecognized cause of chronic back pain in younger patients who are likely to participate in sports and athletic activities. These diseases are characterized by the presence of inflammatory back pain – lumbar or buttock/hip pain lasting longer than 3 months associated with improvement with activity, worsening with rest, relief with NSAIDs, and morning stiffness lasting longer than 30 minutes. There are also characteristic radiographic findings involving the sacroiliac joints, vertebrae, and in certain disease the peripheral joints. Exercise has long been recognized as a key component of the therapy of SpA; yielding benefits in mobility, pain, stiffness, functionality and depression. Sports also pose a risk to patients with SpA as these patients are at high risk of spinal fracture and spinal cord injury.
Low back pain is one of the most common complaints for which patients present to physicians, particularly those who participate in sports. An important and often under recognized cause of low back pain is spondyloarthritis (SpA), a family of chronic, inflammatory back diseases. This includes ankylosing spondylitis (AS), psoriatic arthritis (PsA), reactive arthritis (ReA), the arthritis associated with inflammatory bowel disease (IBD), and undifferentiated spondyloarthritis (uSpA) (12). The SpA have an estimated prevalence of up to 1.2%, with AS being the most common, affecting approximately 0.5% of the U.S. population and 5% of patients with chronic low back pain (13, 24, 26). The diagnosis of SpA is often delayed by 8-11 years from symptom onset due to the insidious onset and delayed appearance of radiographic changes. This paper will review the clinical presentation and key diagnostic features of SpA with a focus on ankylosing spondylitis, the prototype of these diseases. The important role of exercise and sports will be discussed with particular focus on benefits and special risks of sports.
Clinical presentation
The hallmark of SpA is inflammatory back pain (Table 1). This pain is classically characterized as a dull, low back or buttock/hip pain lasting longer than three months with an insidious onset. Inflammatory back pain is associated with morning stiffness lasting 30 minutes or longer, which responds readily to non-steroidal anti-inflammatory drugs (NSAIDs), is relieved with activity and worsened with rest (17, 22, 23, 26). A recent study found the presence of back pain for at least three months with two of the following four clinical features had a sensitivity and specificity of 70.3% and 81.2%, respectively, for inflammatory back pain versus mechanical (noninflammatory) back pain, including morning stiffness lasting at least thirty minutes, improvement with exercise but not rest (asked as one question), alternating buttock pain and awakening because of back pain during the second half of the night (22).
Table 1. Characteristics of Inflammatory Back Pain.
| Age of onset < 35 years |
| Insidious onset |
| Morning stiffness lasting greater than 30 minutes |
| Improvement with exercise but not with rest |
| Alternating buttock pain |
| Awakens in second half of night |
As the disease progresses, patients may develop limitation of spinal mobility, loss of lumbar and cervical lordosis and kyphotic deformities of the spine. This limitation of motion is initially due to axial inflammation and muscle spasm but is contributed to over time by ossification of the ligamentous structures and ultimately ankylosis of the sacroiliac joints, apophyseal joints and the outer fibers of the annulus fibrosis of the intervertebral discs (17, 26).
Spinal mobility can be assessed by specific physical examination maneuvers. Flexion can be assessed with the Schober test, which is performed by placing a mark at the level of the iliac crest and another mark 10cm cephalad from this mark with the patient standing completely upright. Then he or she is asked to bend maximally at the waist with locked knees and try to touch the toes. The distance is then remeasured, with a change of greater than 5 cm considered normal spinal mobility. Chest wall expansion can be assessed by placing a tape measure around the chest at the level of the xiphosternal junction with the arms over the head and asking the patient to maximally inhale and exhale. The difference is the chest circumference at maximum inhalation and exhalation is greater than 2.5 cm in a patient with normal chest expansion. Spinal extension is assessed with occiput-to-wall measurement. The patient is asked to place his or her heels and back against a wall and then asked to touch his or her head to the wall while maintaining a normal chin position. The patient with normal extension will be able to touch the wall (i.e., occiput-to-wall distance of 0cm.) Lateral flexion can be measured by having the patient place his or her hand against the leg and slide the hand down the leg while bending to the side without bending the knees. The distance from the floor to the 3rd digit is measured at the start and stop positions and should the difference should be greater than 10cm in patients with normal lateral flexion (17, 22, 23).
Arthritis of the hips (and less commonly the shoulders) occurs in approximately 50% of patients with AS. This may be manifested clinically as flexion deformities or joint destruction of the hip with joint space loss, osteoporosis, and ankylosis (26). Hip involvement is of particularly concern as it is associated with a markedly greater increase in risk of more severe disease overall (26). Shoulder involvement can manifest as chronic rotator cuff tears of the shoulder. Patients can also have arthritis of the joints of the hands, feet, wrists and ankles, however joint involvement in SpA is more commonly an asymmetric oligoarthritis of the lower extremities and particularly the knees (16, 17, 19, 27). Patients with psoriatic arthritis frequently have peripheral arthritis as the dominant manifestation. Early psoriatic arthritis may manifest as only peripheral arthritis and ranges from oligoarthritis to a polyarthritis. More aggressive disease and a worse prognosis is associated with peripheral polyarthritis at diagnosis (16). Inflammatory arthritis of the DIP joint is found in 20%-30% of patients with early psoriatic arthritis and may be helpful in initial diagnosis, as few other inflammatory arthritides affect the DIP joint (16).
Enthesitis, inflammation of tendonous or ligamentous insertions onto bone, is one of the most characteristic findings of the SpA. The most common sites of inflammation include the Achilles tendon and the plantar fascial insertions, although involvement of the ligamentous and tendinous insertions onto the pelvic bones is also encountered. Dactylitis or “sausage digits”, which is fusiform swelling of the entire digit due to inflammation and swelling of the flexor tenosynovium, is another typical finding of the SpA (16, 17, 19, 24,27,29).
Skin involvement also occurs in SpA. Psoriasis is a sine qua non of psoriatic arthritis, and can occur after the onset of arthritis in up to 20% of patients. A careful surveillance of the skin should be performed including examination of the pinna, behind the ear, the scalp, gluteal cleft, and areas of friction such as intertrigenous spaces. Nail changes, consisting of pitting, ridging, hyperkeratosis and onycholysis occurs in 60%-80% of patients with psoriatic arthritis, particularly when there is underlying inflammation of the DIP joint (17, 16). Patients with reactive arthritis may have keratoderma blennorrhagicum (pustular lesions of the palms and soles of the feet) or circinate balanitis (coalescent plaques with a winding appearance) both of which histopathologically resembles psoriasis. Erythema nodosum is also seen in patients with enteropathic arthritis (and rarely reactive arthritis) (17, 19, 27,29).
SpA also affects the GI tract, eye, heart and lungs. Inflammatory bowel disease (Crohn's disease and ulcerative colitis) and AS likely represent a spectrum of disease with purely bowel disease at one end through enteropathic spondylitis to subclinical bowel inflammation, which is seen in up to half of patients with AS. Flares of enteropathic peripheral arthritis associated with IBD tend to occur with aggravation of the bowel disease, whereas the axial disease tends to occur (and flare) independent of activity of intestinal inflammation (17). Anterior uveitis, presenting as a painful red eye with photophobia and blurred vision, is common to all the SpA (16, 17, 19, 24,26,27,29). Cardiac manifestations, including conduction abnormalities and aortic valvular insufficiency, occasionally are seen in patients with SpA, especially AS (17). The spectrum of pulmonary involvement in AS ranges from a commonly seen restrictive ventilatory disease due to limitation in chest wall movement from costovertebral joint arthritis and fusion to an upper lobe predominant interstitial lung disease, and is encountered in approximately 1% of patients with AS. Other complications of AS include osteoporosis, spinal fracture, often accompanied by neurologic compromise, atlanto-axial subluxation, cauda equina syndrome, secondary amyloidosis, sleep disturbance and depression (17, 26).
Diagnosis
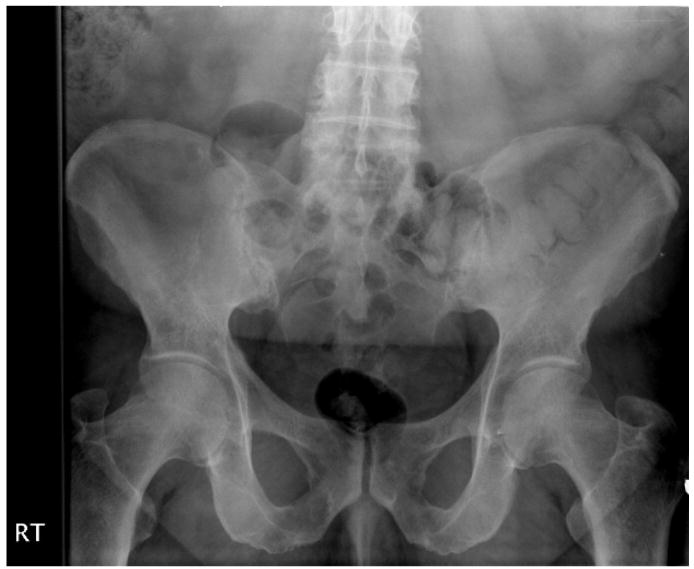
Imaging studies play an important role in the diagnosis of the SpA. The most characteristic radiographic finding is erosion, ankylosis and sclerosis of the sacroiliac (SI) joints (Figure 1). The earliest changes are asymmetric blurring of the cortical margins followed by irregular erosion and sclerosis of the joint margin. Pseudowidening of the joint space then develops with fibrosis and bony ankylosis appearing in advanced disease. Enthesitis of the ligamentous attachments to the iliac tuberosity produces a whiskering appearance. The pubic symphysis also can be affected with erosions and eventual fusion.
Figure 1. Bilateral Grade III sacroiliitis with sclerosis, erosions and joint space narrowing of bilateral SI joints.
Plain radiographs of the spine show Romanus lesions (“shiny corners”) and squaring of the vertebral body due to erosions at the attachments of the spinal ligaments early in disease followed by syndesmophyte formation due to ossification of the outer layer of the annulus fibrosis and eventual ankylosis of the spine, producing a “bamboo spine” appearance. Syndesmophyte formation and ankylosis tends to progress throughout the spine, although women often have cervical disease occurring independently of lumbar involvement (17, 24, 25, 26).
Plain radiographs of the hands and feet are also helpful in the evaluation of the patient with psoriatic arthritis or reactive arthritis. Radiographic changes of the peripheral joints, including soft tissue swelling, erosions, periarticular osteopenia, periostitis and joint space narrowing, are seen in 75% of patients with psoriatic arthritis. The erosions of psoriatic arthritis can be aggressive leading to destruction of the articular surface of proximal bone of the joint and “pencil in cup” appearance. These radiographic findings are similar to rheumatoid arthritis but tend to be asymmetric and may involve the DIP joints unlike rheumatoid arthritis. Reactive arthritis affects the peripheral joints, predominantly in the lower extremities, whereas psoriatic arthritis equally affects lower and upper extremities (16, 25).
Abnormalities on standard radiographs typically are not seen until up to 8-11 years after disease onset leading to a significant delay in diagnosis and initiation of therapy. Earlier radiographic changes can be detected with MRI and although with less specificity, nuclear scintigraphy. Sacroiliitis can be detected by MRI earlier than plain radiographs. Findings in early disease include bone marrow edema adjacent to the inflamed SI joint, contrast enhancement, sclerosis and eventually erosions of the joint. MRI is limited by expense and availability and currently there are no validated criteria for interpretation or staging (though such are under development) (4, 14, 20). Scintigraphy can show increased radiotracer uptake in inflamed joints including the SI joints but has poor sensitivity and specificity for SpA (23, 24, 25, 26). Ultrasonography is a developing technology which has been validated for detection of synovitis and enthesitis in established psoriatic arthritis, though concerns persist about interobserver variability (16).
Laboratory abnormalities in SpA are nonspecific and not as useful as the clinical presentation for diagnosis of a specific disease. Patients often have nonspecific markers of inflammation including elevated C reactive protein, erythrocyte sedimentation rate, and normochromic normocytic anemia. These inflammatory markers do not correlate well with disease activity, although are used in clinical trials. Nevertheless, better biomarkers are needed. Patients with psoriatic arthritis have a low titer rheumatoid factor in up to 10% of cases. This complicates diagnosis, as psoriatic arthritis can appear in some cases clinically and radiographically similar to rheumatoid arthritis. Expert evaluation is necessary for discrimination of these diseases (16, 17).
Human leukocyte antigen (HLA) testing is the most useful laboratory study in appropriately selected patients. HLA-B27 is involved in antigen presentation in the immune system and is thought to be the key to the pathogenesis of the SpA through abnormalities in antigen presentation (the “arthritogenic peptide” theory) and/or possibly misfolding of the molecule, impaired intracellular bacterial killing. HLA-B27 is found in 90% of patients of European ancestry with AS, 60% with psoriatic arthritis, 70% with reactive arthritis, and 70% with the spondylitis associated with IBD (21). Family members of patients with AS who are HLA-B27 positive have a 16 fold increase in the risk of developing AS themselves if they are also HLA-B27 positive compared to HLA-B27 positive individuals in the general population (26). Only 5% of individuals with HLA-B27 in the general population will develop AS, thus the presence of HLA-B27 alone does not correlate well with occurrence of SpA. Therefore interpretation of HLA-B27 testing must be done with consideration of the disease prevalence in a given patient population.
As stated above, the prevalence of the SpA in the general population ranges up to 1.2%. This increases to 5% when considering patients with chronic low back pain. The likelihood of a patient having an axial SpA increases further to 14% if the patient has inflammatory back pain, compared to 2% of the patient does not have inflammatory back. The finding of various clinical, radiographic and laboratory findings further alters the probability of a patient having a SpA. Patients with inflammatory back pain and 2-3 of these findings will have a probability of at least 90% of having an axial spondyloarthritis. The most powerful findings are HLA-B27 positivity, characteristic MRI findings, anterior uveitis, and family history of a SpA, psoriasis, IBD or anterior uveitis (22, 23).
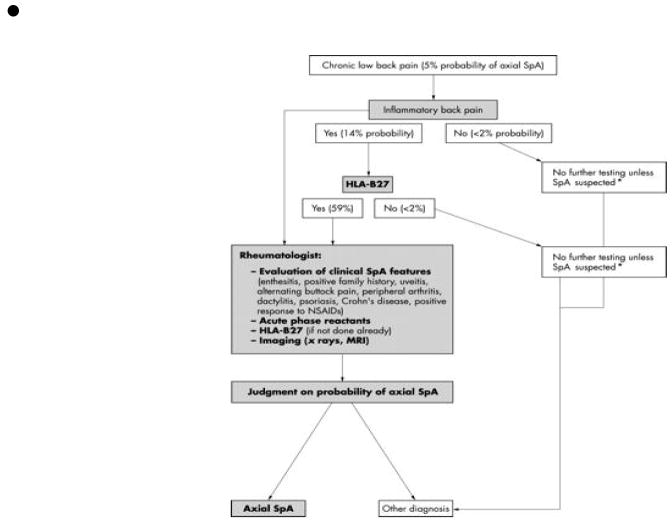
A diagnostic algorithm has been proposed for general physicians to facilitate early diagnosis and referral of patients with axial SpA (Figure 2). A patient with back pain lasting greater than 3 months and meeting criteria for inflammatory back pain should undergo HLA-B27 testing. If the patient is HLA-B27 positive, he or she should be referred to a rheumatologist as the probability of the patient having SpA is approximately 59%. If the patient has back pain lasting less than 3 months, does not meet the criteria for inflammatory back pain, or has a negative HLA-B27 test, no further testing should be done beyond each respective step unless compelling evidence of SpA being present as the likelihood is less than 2%. This diagnostic algorithm is not applicable to non-white patients due to the racial disparities in HLA-B27 frequency (22, 23). This approach was recently validated in a prospective study of 350 referrals from orthopedic surgeons and primary care physicians. Using this algorithm, a definitive diagnosis of axial SpA was made in 45.4% with an additional 9.1% diagnosed with possible SpA (2).
Figure 2. A Diagnostic Approach to the Patient with Low Back Pain.
Treatment
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as indomethacin have long been known to be effective in treating the spondyloarthritides. They have been shown to decrease pain, joint tenderness, and improve measures of spinal mobility (26). Conventional disease modifying antirheumatic drugs (DMARDs) including antimalarials, gold, D-penicillamine, azathioprine have not been found to be effective for the treatment of AS (26). Sulfasalazine and methotrexate are two exceptions. Sulfasalazine has been found to be effective for the treatment of peripheral arthritis but not the axial disease (6). The efficacy of methotrexate for AS is more controversial; a recent Cochrane review found insufficient evidence to support a benefit of methotrexate treatment (5). Biologic agents targeting tumor necrosis factor alpha (TNF) have emerged as potent additions to the therapeutic armamentarium. Etanercept, infliximab, and adalimumab significantly improve a patient's quality of life, spinal pain, and functionality that have been maintained during long-term follow-up (3, 7, 31).
While TNF agents and NSAIDs can produce significant improvements in pain and functionality, exercise continues to hold a central role in the treatment of AS. A collection of exercises is available for patients from the Spondylitis Association of America (www.spondylitis.org). (Table 2) In one study, patients with AS who performed these exercises for eight weeks had significantly improved functional capacity and decreased pain and depression scores (3). Patients who exercised at least 200 minutes per week and at least five days per week were found to have modest but significant decrease in pain and stiffness and less functional disability than those who exercised less. These patients did a variety of exercises including back exercises, swimming, weight lifting and walking (18). Patients who tried a different exercise protocol including back stretching and aerobic exercises also had improvements in spinal mobility and exercise capacity after 12 weeks (30).
Table 2.
Exercises for Spondyloarthritis – Adapted from “Back in Action” by Spondylitis Association of America
| Chest Expansion | Single Knee to Chest | Leg Slides Out |
| Chin Tuck | Double Knee to Chest | Leg Rotation |
| Neck Rotation | Trunk Rotation | Back Extension |
| Neck Side Bending | Shoulder Stretch | Trunk Side Bending |
| Hamstring Stretch | Shoulde Blade Stretch | “Cat & Camel” |
| Pelvic Tilt | Bridging | Kneeling Stretch |
| Hip Flexor Stretch | Trunk Side Bending | Trunk Rotation |
| Calf Stretch | Wall Calf Stretch | Corner Stretch |
Alternatives to standard home-based exercises have been evaluated. Patients taught an exercise protocol based on Global Positional Re-education (GPR) had improved functional capacity and mobility compared to patients taught standard stretching exercises (15). This improvement diminished but was better maintained in the GPR group after 12 months. The GPR method favors stretching muscle groups based on function and gravitation forces as opposed to stretching individual muscles (15). Group physiotherapy has shown small benefits in mobility and global assessment but no change in pain, stiffness, or function compared to home-based exercises (10).
Currently, recommendations are for all patients with ankylosing spondylitis to perform home-based unsupervised exercises similar to those available through the Spondylitis Association of America. (Level of evidence 1b) Patients should try to maintain proper posture and avoid stooping or bending if possible.(Level of evidence 5) Patients with significant functional decline but are still independent and ambulatory should be referred for intensive, focused physical therapy. (Level of evidence 1b)(10). These recommendations should be extended to all patients with axial involvement.
Clearance Considerations
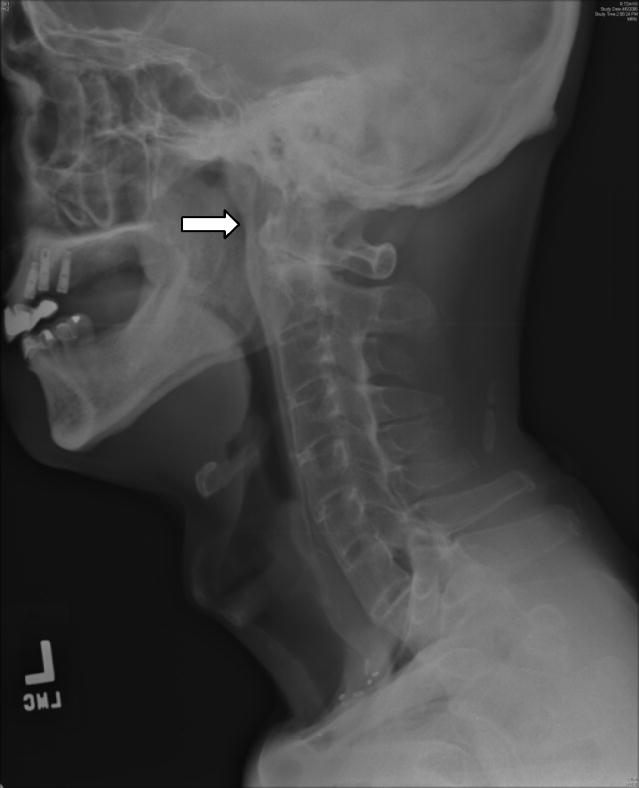
Special considerations for patients with SpA who participate in sports include cardiac disease and spinal fractures. There are currently no evidence based guidelines for cardiac screening of patients with SpA. Our recommendation is to obtain a baseline EKG in all patients to screen for conduction abnormalities. Further cardiac evaluation should be guided by the history and physical examination with attention to signs and symptoms of ischemic heart disease, heart failure or valvular heart disease, with prompt echocardiographic evaluation in case of the latter. Patients with spondylitis should be cautioned when participating in sports activity due to increased risk of spinal fracture, particularly of the cervical spine, due to increased rigidity and osteoporosis of the spine, which can occur even after trivial trauma (8, 17, 26, 28). In one study, fractures occurred in 4.6% of patients with ankylosing spondylitis at an average of 24.6 years after disease onset, and the frequency of fracture increased as disease duration increased (28). Patients with ankylosing spondylitis are at increased risk of fracture of the intervertebral disc due to hyperextension injuries. Fractures occur at the dens, lower cervical vertebra and the cervico-thoracic junction placing the patient at risk of a catastrophic spinal cord injury (Figure 3). In the event of a neck injury, careful radiographic evaluation of the cervical spine with visualization of the lower cervical spine should be performed. If the cervical spine is not adequately visualized, a CT of the neck should be obtained before “clearing” the patient.
Figure 3. Fusion of C2-C7 with fracture of Dens (arrow).
Care must also be taken during transfer and imaging as extension of the neck with normalization of kyphosis can produce a wedge osteotomy effect and spinal cord injury with resultant quadriplegia. Similar concerns underlie intubation, where hyperextension of the neck practiced during standard intubation can have catastrophic results. The patient with should consider wearing a medical alert bracelet to alert Emergency Medical Technicians to this in the event of an accident where the patient may be unconscious. Otherwise the best guide for positioning is the patient. The head should be supported such that the patient is comfortable and has preservation of the field of vision as it was before injury (8). All patients with AS should be cautioned to avoid sports at high risk for spinal injury such as football, ice hockey, wrestling, diving, skiing, snowboarding, rugby, cheerleading or baseball (Level 5)(9).
Conclusions
Spondyloarthritis is an important cause of low back pain. These diseases are initially easily and frequently overlooked but can be identified early with careful consideration of the history, particularly when the symptoms are consistent with inflammatory back pain. Further evaluation with conventional radiographs, MRI and judicious testing for the HLA-B27 serotype allows early identification and initiation of treatments with the potential to relieve symptoms and limit disability. Exercise is an essential part of the treatment of SpA and improves mobility, lessens pain, and improves functionality; however, patients with a SpA are at higher risk of spinal fracture and should avoid sports associated with spinal injuries.
References
- 1.Boden B, Jarvis C. Spinal Injury in Sports. Neurol Clinic. 2006;26:63–78. doi: 10.1016/j.ncl.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Brandt HC, et al. Performance of referral recommendations in patients with chronic back pain and suspected axial spondyloarhritis. Ann Rheum Dis. 2007;66:1479–1484. doi: 10.1136/ard.2006.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, et al. Efficacy and Safety of Infliximab in Patients With Ankylosing Spondylitis Over a Two-Year Period. Arthritis & Rheumatism. 2008;59(9):1270–1278. doi: 10.1002/art.24001. [DOI] [PubMed] [Google Scholar]
- 4.Bredella M, et al. MRI of the Sacroiliac Joints in Patients with Moderate to Severe Ankylosing Spondylitis. Am J Roentgenol. 2006;187(6):1420–6. doi: 10.2214/AJR.05.1423. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Sys Rev. 2006;4 doi: 10.1002/14651858.CD004524.pub3. CD004524. [DOI] [PubMed] [Google Scholar]
- 6.Clegg DO, Reda DJ, Abdellatif M. Comparison of Sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondyloarthropathies. Arthritis & Rheumatism. 1999;42(11):2325–2329. doi: 10.1002/1529-0131(199911)42:11<2325::AID-ANR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Davis JC, et al. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis. 2008;67:346–352. doi: 10.1136/ard.2007.078139. [DOI] [PubMed] [Google Scholar]
- 8.Elgan M, Khan M. Does physical therapy still have a place in the treatment of ankylosing spondylitis? Curr Opin Rheum. 2008;20:282–286. doi: 10.1097/BOR.0b013e3282fa13c9. [DOI] [PubMed] [Google Scholar]
- 9.Feldtkaller E, Vosse D, Geusens P, van der Linden S. Prevalence and Annual Incidence of Vertebral Fractures in patients with Ankylosing Spondylitis. Rheum Int. 2006;26:234–239. doi: 10.1007/s00296-004-0556-8. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-de-las-Peñas F, Alonso-Blanco C, Alguacil-Diego I, Miangolarra-Page J. One year Follow-up of Two Exercise Interventions for the Management of Patients with Ankylosing Spondylitis: A Randomized Controlled Trial. Am J Phys Med Rehabil. 2006;85:559–567. doi: 10.1097/01.phm.0000223358.25983.df. [DOI] [PubMed] [Google Scholar]
- 11.Gladman D, et al. International Spondyloarthritis Interobserver Reliability Exercise – The INSPIRE Study: I. Assessment of Spinal Measures. J Rheumatol. 2007;34:1733–1739. [PubMed] [Google Scholar]
- 12.Healy P, Helliwell P. Classification of the SpA. Curr Op Rheum. 2005;17:395–399. doi: 10.1097/01.bor.0000167753.01168.bd. [DOI] [PubMed] [Google Scholar]
- 13.Helmick, et al. Estimates of the Prevalence of Arthritis and Other Rheumatic Conditions in the United States: Part 1. Arthritis & Rheumatism. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 14.Heuft-Dorenbosch L, et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann Rheum Dis. 2006;65:804–808. doi: 10.1136/ard.2005.044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ince G, Sarpel T, Purgun B, Erdogen S. Effects of a Multimodal Exercise Program for People with Ankylosing Spondylitis. Physical Therapy. 2006;86(7):924–935. [PubMed] [Google Scholar]
- 16.Kane D, Pathare S. Early Psoriatic Arthritis. Rheum Dis Clin N Am. 2005;31:641–657. doi: 10.1016/j.rdc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Khan M. Update on Spondyloarthropathies. Ann Intern Med. 2002;136:896–907. doi: 10.7326/0003-4819-136-12-200206180-00011. [DOI] [PubMed] [Google Scholar]
- 18.Lim H, Moon Y, Lee M. Effects of home-based daily exercise therapy on joint mobility, daily activity, pain and depression in patients with ankylosing spondylitis. Rheum Int. 2005;25:225–229. doi: 10.1007/s00296-004-0536-z. [DOI] [PubMed] [Google Scholar]
- 19.Palm O, et al. Prevalence of Ankylosing Spondylitis and Other Spondyloarthropathies Among Patients with Inflammatory Bowel Disease: A Population Study (The IBSEN Study) J Rheumatol. 2002;29:511–5. [PubMed] [Google Scholar]
- 20.Puhakka K, et al. Magnetic resonance imaging of sacroiliitis in early seronegative Spondyloarthropathy: Abnormality correlated to clinical and laboratory findings. Rheumatology. 2004;43:234–237. doi: 10.1093/rheumatology/keh008. [DOI] [PubMed] [Google Scholar]
- 21.Reveille J, Arnett F. Spondyloarthritis: Update in pathogenesis & management. Am J of Med. 2005;118:592–603. doi: 10.1016/j.amjmed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Rudwaleit M, et al. Inflammatory Back Pain in Ankylosing Spondylitis. Arthritis & Rheumatism. 2006;54(2):569–578. doi: 10.1002/art.21619. [DOI] [PubMed] [Google Scholar]
- 23.Rudwaleit M, Khan M, Sieper J. The Challenge of Diagnosis and Classification of Ankylosing Spondylitis. Arthritis & Rheumatism. 2005;52(4):1000–1008. doi: 10.1002/art.20990. [DOI] [PubMed] [Google Scholar]
- 24.Rudwaleit M, Van der Heijde D, Khan M, Braun J, Sieper J. How to Diagnose Axial Spondyloarthropathy Early. Ann Rheum Dis. 2004;63:535–543. doi: 10.1136/ard.2003.011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomen D. Brower Seronegative spondyloarthropathies: imaging. In: Hochberg M, Silman A, Smolen J, Weinblatt M, Weisman M, editors. Rheumatology. 4th. Philadelphia: Mosby-Elsevier; 2008. pp. P1131–1141. [Google Scholar]
- 26.Sieper J, Braun J, Rudwaleit M, Boonen A, Zinch A. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61(sup iii):iii8–iii18. doi: 10.1136/ard.61.suppl_3.iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieper J, et al. Diagnosing Reactive Arthritis: Role of Clinical Setting in the Value of Serologic and Microbiologic Assays. Arthritis & Rheumatism. 2002;46(2):319–327. doi: 10.1002/art.504. [DOI] [PubMed] [Google Scholar]
- 28.Thumbikant P, et al. Spinal Cord Injury in Patients with Ankylosing Spondylitis. Spine. 2007;32(26):2989–2995. doi: 10.1097/BRS.0b013e31815cddfc. [DOI] [PubMed] [Google Scholar]
- 29.Turkcapar N, et al. The prevalence of extraintestinal manifestations and HLA association in patients with inflammatory bowel disease. Rheumatol Int. 2006;26:663–668. doi: 10.1007/s00296-005-0044-9. [DOI] [PubMed] [Google Scholar]
- 30.Uhrin Z, Kuzis S, Ward M. Exercise & Changes in Health Status in Patients with Ankylosing Spondylitis. Arch Int Med. 2000;160:2969–2975. doi: 10.1001/archinte.160.19.2969. [DOI] [PubMed] [Google Scholar]
- 31.van der Heijde D, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2008 doi: 10.1136/ard.2007.087270. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]