Abstract
This study investigates the effect of contaminant aging on the sorption/desorption and transport of trichloroethene in a low organic-carbon content aquifer material, comparing mass removal and long-term, low-concentration elution tailing for field-contaminated, synthetically-aged (contact times of approximately four years), and freshly-amended aquifer material. Elution of trichloroethene exhibited extensive low-concentration tailing, despite minimal retention of trichloroethene by the aquifer material. The observed nonideal transport behavior of trichloroethene is attributed primarily to rate-limited sorption/desorption, with a smaller contribution from nonlinear sorption. It is hypothesized that interaction with physically condensed carbonaceous material, comprising 61% of the aquifer material’s organic-carbon content, mediates the retention behavior of trichloroethene. The elution behavior of trichloroethene for the field-contaminated and aged treatments was essentially identical to that observed for the fresh treatments. In addition, the results of three independent mass-balance analyses, total mass eluted, solvent-extraction analysis of residual sorbed mass, and aqueous-phase concentration rebounds following stop-flow experiments, showed equivalent recoveries for the aged and fresh treatments. These results indicate that long-term contaminant aging did not significantly influence the retention and transport of trichloroethene in this low organic-carbon aquifer material.
Keywords: Contaminant aging, Trichloroethene, Low organic carbon, Kerogen
1. Introduction
Over the past three decades, many researchers have characterized sorption/desorption processes and their significant role in mediating the transport and fate of hydrophobic organic compounds in natural porous media. One phenomenon of interest is the observation that the desorption of many organic contaminants from soils and sediments is often significantly rate-limited, requiring days, months, or even years to attain equilibrium. Mechanisms potentially responsible for this recalcitrant nature of hydrophobic organic compounds include diffusion and entrapment in micropores of porous media (e.g., Steinberg et al., 1987; Ball and Roberts, 1991; Farrell and Reinhard, 1994) and/or soil organic matter (e.g., Brusseau and Rao, 1989; Pignatello, 1990; Brusseau and Rao, 1991; Brusseau et al., 1991; Deitsch and Smith, 1995; Pignatello and Xing, 1996), and chemisorption or adsorption reactions on solid surfaces or within micropores of carbonaceous geomaterials such as kerogen and black carbon (e.g., Luthy et al., 1997; Xing and Pignatello, 1997; Weber and Young, 1997; Cornelissen et al., 2005; Abu and Smith, 2006; Koelmans et al., 2006; Morelis and van Noort, 2008; Prevedouros et al., 2008). The results of prior research have shown that rate-limited desorption can constrain effective removal of contaminants from porous media (e.g., Brusseau et al., 1989; Pavlostathis and Mathavan, 1992; Deitsch and Smith, 1995; Luthy et al., 1997; Johnson et al., 2003a, b).
The mechanisms responsible for rate-limited sorption/desorption, and the properties and conditions influencing their manifestation, have been under investigation for some time (e.g., Brusseau and Rao, 1991; Brusseau et al., 1991; Pignatello and Xing, 1996; Luthy et al., 1997; Morelis et al., 2007; Chai et al., 2007, 2008). One aspect of particular interest is the potential impact of contaminant residence time, or aging, on sorption/desorption phenomena. The impact of aging on sorption/desorption behavior of organic contaminants has been investigated extensively for natural porous media with moderate or greater organic-carbon contents (greater than approximately 0.1%) (e.g., Steinberg et al., 1987; Pignatello, 1990, 1991; Pavlostathis and Mathavan, 1992; Connaughton et al., 1993; Deitsch and Smith, 1995; Hatzinger and Alexander, 1995; Cornelissen et al., 1997; Chung and Alexander, 1998; Abu and Smith, 2006). For example, Chung and Alexander (1995) investigated the impact of contaminant aging on the bioavailability of phenanthrene and atrazine for 16 soils. All of the 16 soils tested exhibited aging effects wherein the extent of contaminant mineralization was less for the 200-day aged treatment versus the unaged treatment. The smallest impacts of aging were observed for those soils with the lowest organic-carbon contents (ranging between 0.3% to 0.62%). Similarly, in a study conducted by Pignatello (1990), the smallest recalcitrant fractions of aged trichloroethene were associated with the lowest organic-carbon content medium (0.19%).
The examples noted above suggest that aging effects may often be more significant for porous media with greater organic-carbon contents, as discussed by Alexander (2000), and concomitantly that aging effects may be of lesser importance for lower organic-carbon content media such as aquifer materials. However, the weathered state (e.g., geological decomposition associated with long-term, direct contact with water) of geologically older aquifer materials may yield different sorption/desorption phenomena. First, the generally lower fractions of total organic carbon may result in multiple components (e.g., organic matter plus mineral fractions) contributing to the sorption uptake of hydrophobic organic compounds. For example, Stauffer et al. (1989) reported that the uptake of naphthalene and 1,2-dichlorobenzene by aquifer materials with low organic-carbon contents (foc < 0.10%) correlated well with organic carbon, silt, cation exchange capacity, and 1:1 clay content. Second, as a result of geological maturation, chemically-reduced, physically-condensed carbonaceous material (e.g., kerogen, unburned coal, and soot) may become more prominent and thus play an even more significant role in the uptake of hydrophobic organic compounds for these low organic-carbon-content aquifer materials.
A review of the literature shows that the impact of aging on contaminant sorption/desorption and transport has been minimally investigated for low organic-carbon-content aquifer sediments. This study was designed to investigate the effect of long-term contaminant aging on the sorption/desorption and transport of trichloroethene, one of the most predominant groundwater contaminants, in a natural porous media with low organic-carbon content. This was done by comparing the mass removal and long-term, low-concentration tailing of trichloroethene for three systems: field-contaminated, synthetically-aged (contact times of approximately four years), and freshly-amended aquifer material. Miscible-displacement experiments were conducted wherein trichloroethene elution was monitored until it was no longer detectable in the aqueous effluent samples, with corresponding concentration changes spanning five to seven orders of magnitude. The Acomplete@ removal of the contaminants from the aquifer material was simulated using a mathematical model explicitly accounting for nonlinear, rate-limited sorption/desorption, and pore/grain scale sorption/desorption heterogeneity.
2. Materials and Methods
The characterization of long-term, low-concentration elution tailing behavior requires a thorough investigation of potential interferences associated with the experimental apparatus. The experimental apparatus employed herein was constructed entirely of stainless steel to prevent adsorption. To elucidate potential retention and tailing associated with the apparatus, experiments were conducted wherein aqueous solutions saturated with trichloroethene were pumped into and eluted from a column containing no aquifer material. In addition, non-reactive tracer tests were conducted in the same manner to delineate the magnitude of apparatus-induced dispersion.
2.1. Materials
The porous medium is a sandy aquifer material collected during well installation at a Superfund site in Tucson, AZ, wherein several thousand kg of solvent is estimated to reside in the saturated zone. A pump and treat system has been in operation at the site for more than 20 years. Concentrations of trichloroethene have remained relatively constant for the past several years, exhibiting extensive elution tailing, with a fraction of a pore volume flushed per year under the induced gradients at the site (see Zhang and Brusseau (1999) and Brusseau et al. (2007) for a complete site description). The aquifer material used in this study was collected between 44.2 m to 45.7 m below ground surface. A portion of core material was immediately packed and hereafter referred to as the Afield-contaminated aquifer material@.
Average sand, silt, and clay fractions for the individual subsamples of the aquifer material used in the column studies are included in Table 1. The d50, the diameter for which 50% of the porous medium by mass is finer, is 0.33 mm, and the uniformity coefficient (U = d60/d10) is 4.55. Mineralogical analysis was conducted for the clay fraction of the aquifer material (<2 µm). X-ray diffraction analysis was performed using a Phillips XRG-3000 generator with vertical goniometer and Cu-K radiation. The aquifer material’s clay-size fraction is primarily composed of smectite with moderate amounts of mica and kaolinite.
Table 1.
Summary of miscible-displacement experiments.
| Column | Experiment | Sand % |
Silt % |
Clay % |
Electrolyte Solution |
ρba g cm−3 |
θwb |
v cm h−1 |
TCE Co, mg L−1 |
Toc | Slope of Low- Concentration Elution Taild |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Aged | 95.7 | 1.8 | 2.5 | Synthetic GW |
1.72 | 0.34 | 22.4 | 930 | 17 | −0.008 |
| Fresh | 22.2 | 880 | 17 | −0.008 | |||||||
| B | Aged | 99.0 | 0.0 | 1.1 | 0.01 M KNO3 |
1.56 | 0.37 | 23.3 | 13.0 | 33 | −0.003 |
| Fresh | 20.4 | 12.0 | 27 | −0.003 | |||||||
| C | Aged | 97.5 | 1.3 | 1.3 | Synthetic GW |
1.81 | 0.32 | 24.2 | 790 | 18 | −0.008 |
| D | Fresh | 96.3 | 1.8 | 2.0 | Synthetic GW |
1.80 | 0.33 | 23.4 | 920 | 19 | −0.02 |
| E | Field-Contami nated |
NMf | NM | NM | Synthetic GW |
~1.73 | ~0.37 | ~25.0 | NAe | NA | −0.007 |
Bulk density (ρb).
Saturated water content (θw) determined gravimetrically by comparing saturated to dry-packed column weights.
Pulse width (To) equal to the volume of input solution divided by the pore volume of the packed column.
Calculated for the low-concentration elution tail.
Not applicable
Not measured.
Average total carbon and organic-carbon contents for the bulk media were 0.06% (∀0.01%) and 0.03% (∀0.009%), respectively. Experiments designed to further characterize the organic matter composition of this aquifer material were conducted following procedures similar to those outlined in Jeong and Werth (2005). Specifically, a chemical treatment method was used to obtain fractions of the aquifer material’s organic matter enriched in one or more type of organic carbon. Those treatment steps included phosphoric acid (10%) to remove carbonates, followed by 0.1M sodium hydroxide to remove the humic and fulvic acid fraction, and finally, treatment by acid dichromate (0.1M potassium dichromate and 2M sulfuric acid) to remove kerogen. The fraction of organic carbon in the bulk media was found to be composed of approximately 61% hard carbon (e.g., approximately 38% kerogen and 23% black carbon).
The majority of experiments were conducted using Asynthetic groundwater@. This synthetic groundwater solution was created based on the identification and quantification of major ionic species in groundwater collected from the field site. The major cations in this synthetic groundwater (and average concentration, mg L−1) were Na+1 (50), Ca+2 (36), Mg+2 (25), and major anions were NO3−1 (6), Cl−1 (60), CO3 −2/HCO3 −1 (133), and SO4 −2 (99). Trichloroethene (99.5+% purity) was obtained from Aldrich Chemical Co. Ethanol (C0 ~ 330 mg L−1), obtained from Quantum Chemical Co., or pentafluorobenzoic acid (C0 ~ 100 mg L−1) was used as a nonreactive tracer.
2.2. General Methods
Aquifer material used in this study was oven dried for 24 h (to aid in removing potentially-resident trichloroethene from the field samples) at 105 EC, sieved to exclude the >2 mm size fraction, and well mixed prior to use. Subsamples of this bulk media were stored in jars containing either trichloroethene-free synthetic groundwater (representing a Ablank@ with non-trichloroethene-amended aquifer material) or an aqueous solution of trichloroethene at approximately 900 mg L−1 or 10 mg L−1 (representing the Ahigh-concentration@ and Alow-concentration@ synthetically-aged aquifer materials, respectively) until used for miscible-displacement experiments. Analysis of subsamples of the aqueous solution collected from the Ablank@ jars yielded non-detectable trichloroethene concentrations (<0.1 µg L−1), indicating efficient removal of potentially-resident trichloroethene from the oven-dried field samples. For the high-concentration system, the jars contained a stainless-steel mesh platform wherein the aquifer material was stored to prevent direct contact with the separate pure-phase trichloroethene that was placed at the bottom of the reservoir. For the low-concentration system, subsamples of the aqueous solutions were analyzed periodically, and additional aqueous trichloroethene solution was added to the jars when necessary to maintain the target concentration. Contaminant contact times equaled approximately four years for the synthetically-aged media.
The columns, 7 cm long and 2.1 cm i.d., were packed vertically in increments with wet aquifer material (either Ablank@ or Asynthetically-aged@). The aqueous trichloroethene solutions for the miscible-displacement experiments were pumped through the column using an ISCO (Model 500D) syringe pump to minimize loss to volatilization. Trichloroethene-free synthetic groundwater was provided with an Acuflow Series II single-piston pump. The use of separate pumps minimized potential cross-contamination problems. Prior to each experiment, water was flushed though the apparatus (including the pump, tubing, and sampling ports), and analyzed to ensure no trichloroethene was detected. A separate experiment was conducted with the field-contaminated aquifer material wherein media collected immediately after coring was packed as described above in a glass column (15 cm long and 2.5 cm i.d.) with Teflon ends and bed supports. Trichloroethene-free synthetic groundwater was flushed through the field-contaminated aquifer material with an Acuflow Series II single-piston pump. For all experiments, effluent samples were collected manually using a glass, gas-tight syringe and analyzed immediately to minimize losses to volatilization.
Trichloroethene was analyzed by headspace gas chromatography (Tekmar-Dohrmann 7050 coupled with a Shimadzu GC-17A) using either a flame ionization (FID) or electron capture (ECD) detector depending on sample concentration. Chromatographic analysis was done using a glass capillary column (SPB-624, 30-m length, 5-µm film thickness) with oven temperature programming. Specifically, analysis began with oven temperature set for two minutes at 40 EC, ramped to 150 EC at a rate of 10 EC per minute, and held for two minutes. The GC=s injection port temperature was set at 180 EC with the detectors at 180 EC and 210 EC for the FID and ECD, respectively. Aqueous-phase standards were analyzed every 48 h with check standards and blanks analyzed for quality assurance every 10 to 15 samples. The quantifiable detection limit for trichloroethene using headspace GC/ECD was 0.1 µg L−1. Ethanol was analyzed by headspace GC/FID under these same operating temperatures with a lower detection limit of 0.06 mg L−1.
2.3. Miscible-displacement Experiments
Miscible-displacement experiments were conducted using one of two influent trichloroethene concentrations of approximately 900 mg L−1 or 10 mg L−1. After wet-packing the column with either “blank” or “synthetically-aged” aquifer materials, a pulse of trichloroethene solution (approximately 18 and 30 pore volumes for the 900 mg L−1 and 10 mg L−1 experiments, respectively) was injected into the vertically positioned column to ensure saturated conditions at the start of the elution experiment. Given the small retardation factors for trichloroethene in this system (Johnson et al., 2003a, b), these pulse lengths should be sufficient to obtain robust results with regard to parameter optimization (e.g., Young and Ball, 1995). The experiments were conducted using a volumetric flowrate of 0.5 mL min−1, equivalent to an average pore water velocity (v) of approximately 23 cm h−1. The relatively rapid pore water velocity used in the miscible-displacement experiments is typical of flow within the source-zone areas of the well field employed for pump-and-treat remediation at the Superfund site from which the aquifer material was collected (Zhang and Brusseau, 1999; Brusseau et al., 2007).
A series of miscible-displacement experiments for the Ablank@ and Asynthetically-aged@ systems were conducted using four separate columns. Columns A and B comprised sequential synthetically-aged and freshly-amended miscible-displacement experiments. The sequence of miscible-displacements experiments for each of these columns is as follows: after wet-packing Column A, for example, with high-concentration, synthetically-aged aquifer material, a pulse of trichloroethene solution (17 pore volumes at 930 mg L−1) was injected into the column to ensure saturated conditions at the start of the elution experiment. Following Acomplete@ removal of trichloroethene from this high-concentration, synthetically-aged aquifer material, a second, sequential pulse of trichloroethene solution (17 pore volumes at 880 mg L−1) was injected into the same column thereby comparing the effects of synthetically-aged versus freshly-amended trichloroethene in the same subsample of aquifer material (representing contact times of approximately four years versus <8 hours). Similar sequential miscible-displacement experiments were conducted for Column B wet-packed with low-concentration, synthetically-aged aquifer material (Table 1). Columns C and D represent independent miscible-displacement experiments wherein the effect of aged versus freshly-amended trichloroethene was investigated. The Column C experiment involved elution of trichloroethene from a high-concentration aged system (contact time of approximately four years). Conversely, the Column D experiment involved elution of trichloroethene from a high-concentration freshly-amended (contact time <8 hours) system conducted using the Ablank@ aquifer material (wherein the aquifer material was stored in trichloroethene-free synthetic groundwater for four years). For all of these experiments, once the pulses of trichloroethene solution were injected, the columns were flushed with electrolyte solution until the effluent concentration fell below the quantifiable detection limit. The Column E experiment was conducted with the field-contaminated aquifer material wherein trichloroethene-free synthetic groundwater was flushed through the field-contaminated aquifer material.
At the conclusion of experiments for Column C and Column D, the aquifer material was unpacked and subjected to solvent extractions to determine final solids-phase trichloroethene concentrations. After placing approximately 8 to 10 g of the aquifer material into a 22 mL crimp-top vial, 1,2-dichloromethane was added to volume, minimizing headspace in the vials. The samples were placed on an end-over-end tumbler for approximately 72 h, centrifuged, and subsampled for analysis by GC-mass spectroscopy, with a quantifiable detection limit of approximate l y 0 . 0 3 µ g g−1. Additional investigation of rate-limited mass transfer was accomplished using the flow-interruption method (Brusseau et al., 1989).
2.4. Data Analysis
The total eluted trichloroethene mass was determined through analysis of the area under the concentration versus time curve. The retardation factor, R, and apparent distribution coefficient, Kd (L3 M−1), for trichloroethene were determined through moment analysis (e.g., Valocchi, 1985) of the trichloroethene elution curves obtained from the miscible-displacement experiments.
Rate-limited sorption/desorption has generally been incorporated into mathematical models by use of the two-domain approach, wherein the medium is divided into two sorption domains. However, this approach has been shown to be inadequate for simulating the extensive, low-concentration elution tailing often associated with long-term flushing of contaminated porous media. Such observations have led to the use of transport models that incorporate a continuous distribution of domains and associated sorption/desorption rate coefficients (e.g., Connaughton et al., 1993; Chen and Wagenet, 1995, 1997; Culver et al., 1997; Haggerty and Gorelick, 1998; Li and Brusseau, 2000; Saiers and Tao, 2000; Johnson et al., 2003b). Such a model, hereafter referred to as the continuous-distribution model, was used herein to represent nonlinear, rate-limited sorption/desorption. With this model, the impact of pore/grain-scale heterogeneity on sorption/desorption is accounted for with a continuous distribution of retention domains and associated mass-transfer rate coefficients. Parameters, calibration, and optimization procedures used for the modeling have been presented elsewhere (Johnson et al., 2003b).
3. Results
The observed retardation factors and apparent distribution coefficients (Kd) obtained for trichloroethene from the miscible-displacement experiments (i.e., as a function of the initial trichloroethene aqueous-phase concentration) are presented in Table 2. These values are similar to values reported in the literature for trichloroethene (ranging from 0.01 to 0.24 mL g−1) for aquifer materials with similarly low organic-carbon contents (Lee et al., 1988; Brusseau et al., 1991; Larson et al., 1992; Benker et al., 1998; Carmichael et al., 1999: Rivett and Allen-King, 2003). Previous experimental results have shown that sorption of trichloroethene by the aquifer material is nonlinear. Specifically, the magnitude of the sorption coefficient is smaller for the experiments conducted with the higher trichloroethene input concentration. This nonlinearity was described using a Freundlich isotherm (r2 = 0.93), with a Freundlich-sorption coefficient (Kf) equal to 0.05 mg(0.17) L0.83 kg−1 and Freundlich exponent equal to 0.83 (Johnson et al., 2003a, b). Nonlinear sorption is often hypothesized to result from interactions of sorbate with so-called hard carbon, a condensed, amorphous organic matter matrix, which would be consistent with the organic-carbon properties of the aquifer material (found to be composed of approximately 61% hard carbon, e.g., kerogen and black carbon).
Table 2.
Parameter values for miscible-displacement experiments.
| Column | Experiment | TCE Co mg L−1 |
RTCEa | Kdb mL g−1 |
Fraction TCE Sorbed Massc, % |
Percent recovery |
|---|---|---|---|---|---|---|
| A | Aged | 930 | 1.003 | 0.001 | 0.3 | 99.3 |
| Fresh | 880 | 1.09 | 0.017 | 8.5 | 99.1 | |
| B | Aged | 13.0 | 1.1 | 0.026 | 11 | 98.8 |
| Fresh | 12.0 | 1.2 | 0.038 | 14 | 100.7 | |
| C | Aged | 790 | 1.07 | 0.012 | 6.6 | 99.8 |
| D | Fresh | 920 | 1.08 | 0.015 | 8.1 | 99.9 |
Retardation factor obtained through moment analysis of trichloroethene’s elution tail.
Apparent distribution coefficient as a function of the initial trichloroethene aqueous-phase concentration.
For initial condition such that total mass includes trichloroethene present in the aqueous phase.
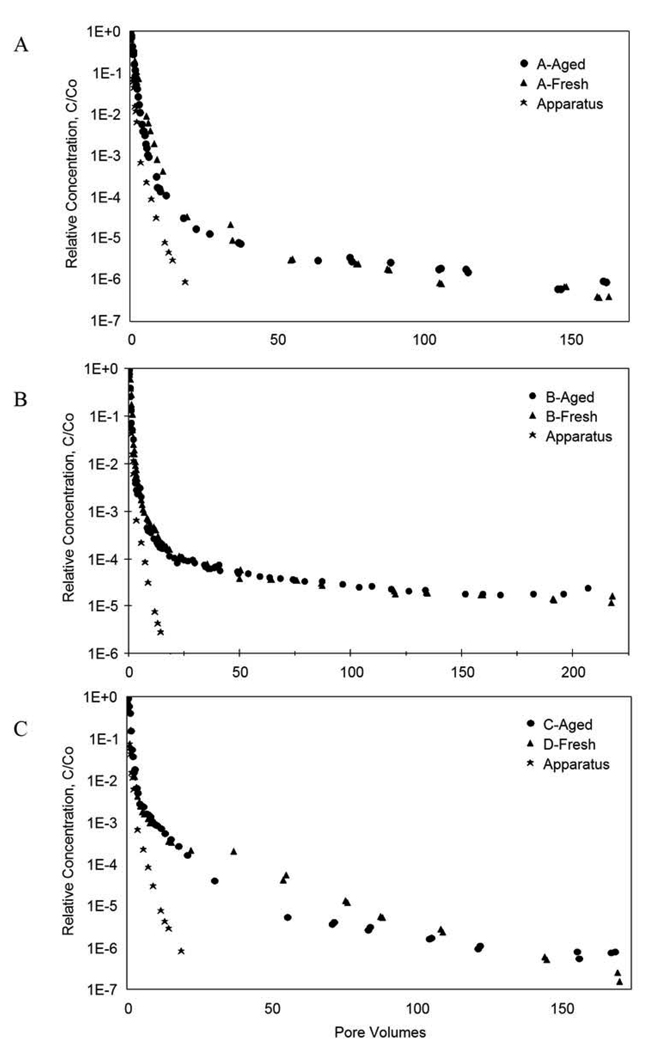
The results of experiments designed to determine the potential effects of the experimental apparatus on the transport behavior of trichloroethene are shown in Figure 1. Comparison of these elution curves indicates that there is minimal retention and elution tailing of trichloroethene associated with the experimental apparatus. These results are supported by a comparison of the nonreactive tracer results obtained for the experimental apparatus (i.e., a column containing no aquifer material) compared to those obtained for trichloroethene in the same system (results not shown).
Figure 1.
Comparison of elution tailing behavior for the long-term, synthetically-aged and freshly-amended aquifer material. Also included are results obtained for an experiment conducted using a column containing no aquifer material (Apparatus), designed to evaluate trichloroethene elution tailing associated with the experimental apparatus. (A) Column A: High-concentration trichloroethene (~900 mg L−1) sequential miscible-displacement experiments. (B) Column B: Low-concentration trichloroethene (~10 mg L−1) sequential miscible-displacement experiments. (C) Column C and Column D: Independent, high-concentration trichloroethene (~900 mg L−1) miscible-displacement experiments.
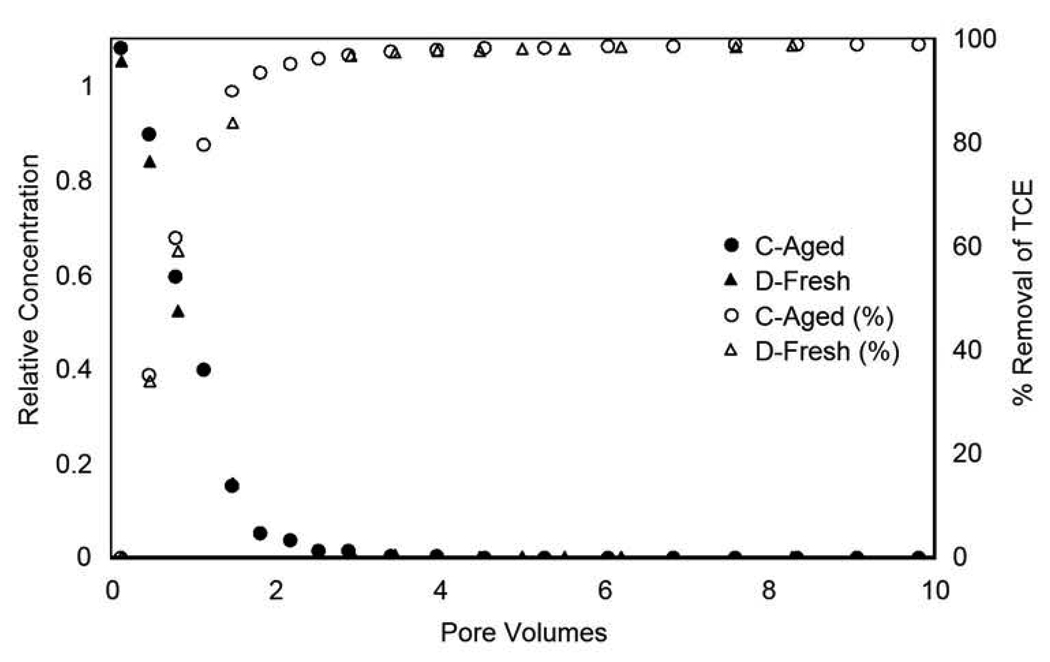
The elution behavior of trichloroethene observed for the long-term, synthetically-aged treatments is compared to that observed for the fresh treatments in Figure 1. The overall elution behavior of trichloroethene, specifically the rapid concentration decrease and the long tail, is essentially identical for the two treatments. The elution of trichloroethene to the detection limit required greater than 200 pore volumes of flushing for the low-Co experiments and greater than 150 pore volumes of flushing for the high-Co experiments, despite the minimal retention of trichloroethene by the aquifer material. This nonideal behavior indicates that significant mass-transfer constraints are influencing transport of trichloroethene in the system. For example, approximately 98% of the total trichloroethene mass (including resident aqueous-phase mass) was removed in the first four pore volumes for Column C, whereas the remaining trichloroethene mass removal required greater than 150 pore volumes of additional flushing (see Figure 2). This 2% of total mass represents approximately 29% of the initial sorbed mass prior to elution. The similarity in elution behavior observed for the two treatments suggests minimal impact of aging.
Figure 2.
Representative mass removal curves for the long-term aged and freshly-amended aquifer material.
The data obtained from the miscible-displacement experiments were analyzed by moment analysis to determine mass recoveries, which ranged from 98.8% to 100.7% (Table 2). No effect of contaminant aging on removal efficiencies was observed. Following the complete elution of trichloroethene (i.e., effluent concentrations below the detection limit), solvent extractions of the aquifer material were performed for Columns C and D. The concentration of trichloroethene was below detectable levels (corresponding to <0.1% of the initial trichloroethene mass) for both cases, independent of contaminant aging, which is in agreement with the elution mass recovery values reported in Table 2. The efficiency of the solvent extraction using dichloromethane may be somewhat constrained for systems comprising condensed, carbonaceous organic material (e.g., Jonker and Koelmans, 2002a). However, as noted previously, the mass balance is consistent between the solvent-extraction and elution-recovery analyses. In addition, the results are consistent with those of the flow-interruption experiments described below.
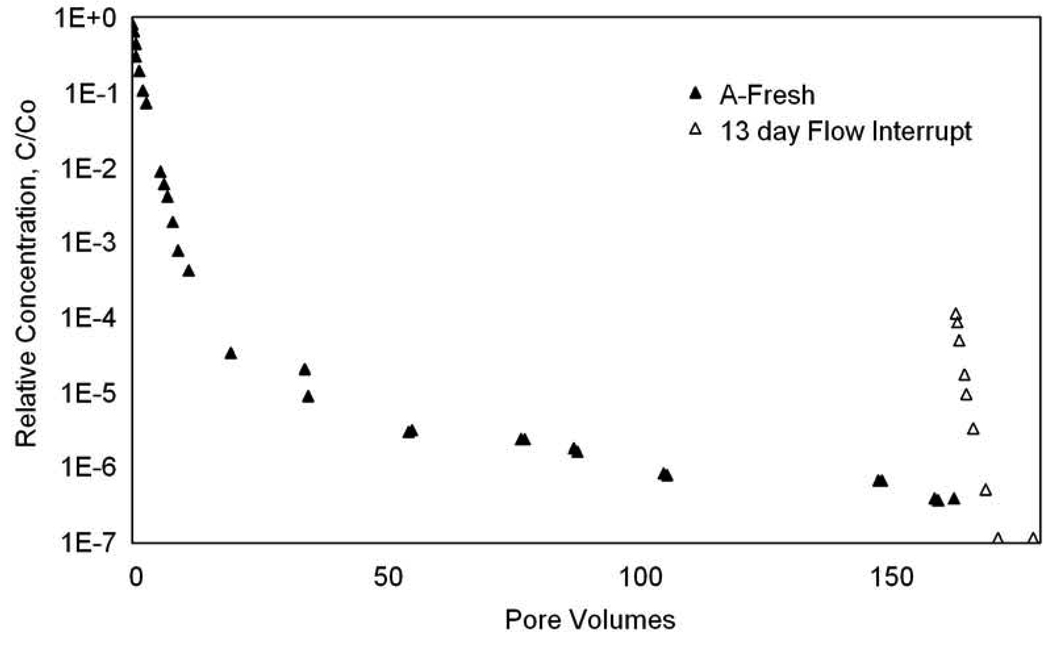
Flow-interruption experiments were performed at the end of selected trichloroethene elution experiments wherein flow was interrupted for 13 days. The aqueous-phase concentrations increased after flow was resumed (Figure 3), indicating the existence of nonuniform concentration distributions and associated rate-limited mass transfer (i.e., nonequilibrium conditions) in the system. Additionally, moment analysis of these flow-interruption experiments yielded nearly identical mass recoveries (1.0 µg and 0.93 µg, for Aged-A and Fresh-A, respectively) for the two treatments, i.e., independent of trichloroethene=s contact time with the aquifer material. This is consistent with the observations reported above.
Figure 3.
Representative trichloroethene elution tailing behavior for the flow-interruption experiments. Flow was interrupted during the elution front for 13 days.
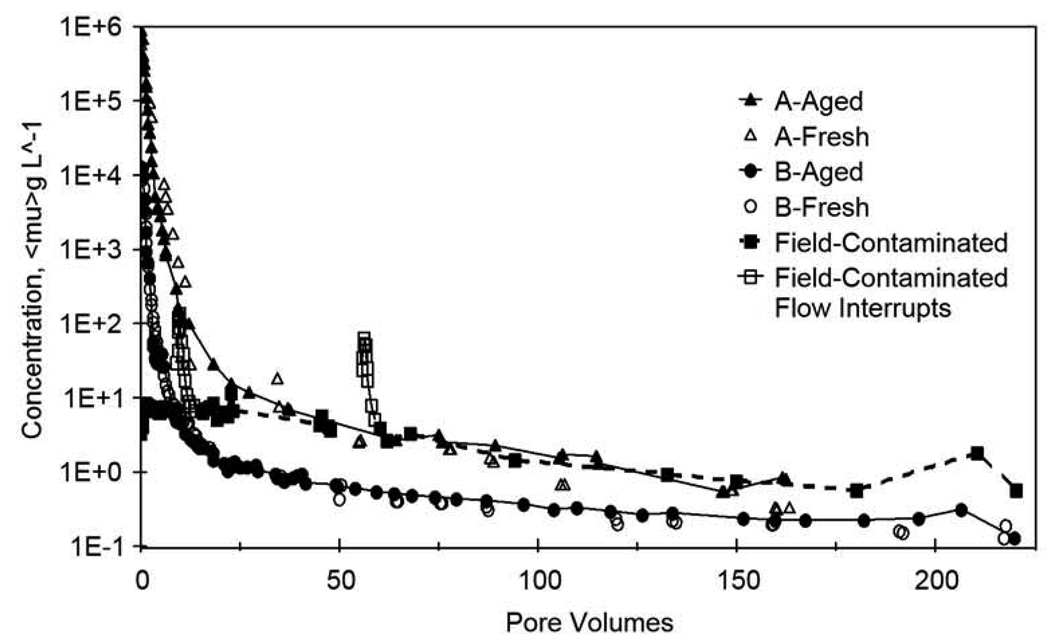
A comparison of the elution tailing behavior of the field-contaminated, synthetically-aged, and freshly-amended aquifer material is shown in Figure 4. While some uncertainty is associated with the elution curve for the field-contaminated aquifer material due to the flow-interruptions, very similar low-concentration elution tailing is observed for all cases, independent of contaminant aging. Furthermore, the slopes of the elution tails (log-concentration versus pore volumes) for the field-contaminated (−0.007), Aged-A (−0.008), and Fresh-A (−0.008), for example, are very similar (see Table 1). This analysis indicates similar low-concentration elution behavior for the field-contaminated, synthetically-aged, and freshly-amended aquifer material (representing contact times of >40 years, ~4 years, and <8 hours, respectively), supporting the finding mentioned previously that the general nonideal behavior of trichloroethene is independent of contaminant aging.
Figure 4.
Comparison of long-term, elution tailing behavior for the field-contaminated, synthetically-aged, and freshly-amended aquifer material. Column A: High-concentration trichloroethene (~900 mg L−1) sequential miscible-displacement experiments. Column B: Low-concentration trichloroethene (~10 mg L−1) sequential miscible-displacement experiments.
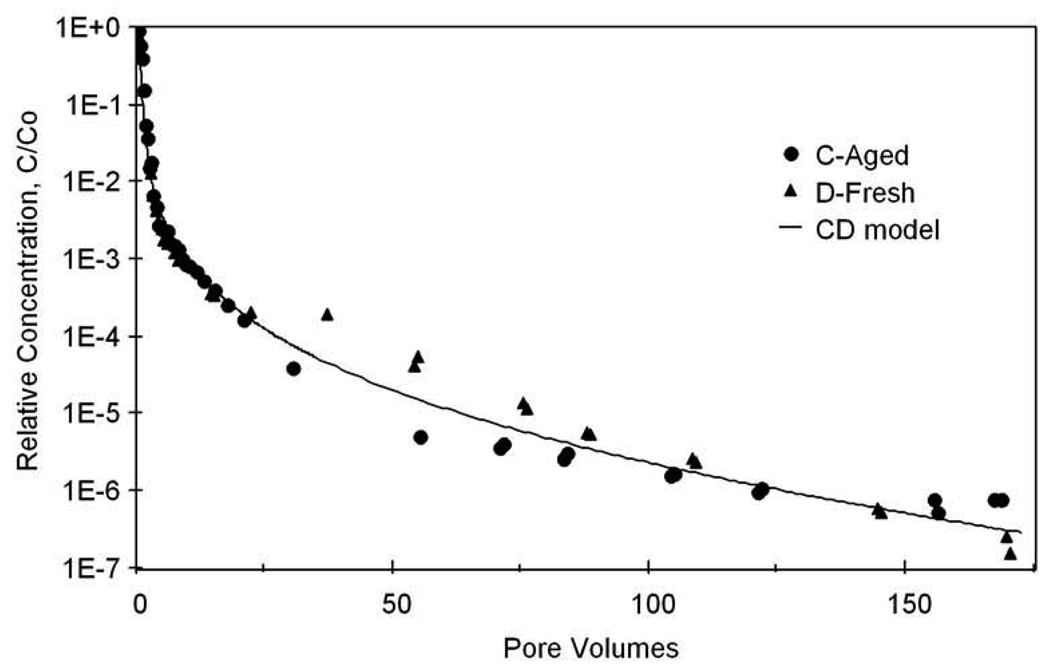
A representative simulation of trichloroethene transport and elution, obtained using the continuous-distribution model, is compared in Figure 5 to the measured data obtained for the miscible-displacement experiments. The model provides a good fit to the full elution curve, including the rapid decrease during the initial stage of flushing and the low-concentration elution tail. Conversely, the nonideal trichloroethene elution behavior observed for these experiments could not be accurately simulated using the widely used two-domain approach (results not shown). The mean (k2) and the variance of the mass-transfer rate coefficient (σk2) determined from the miscible-displacements experiments (averaging 3.4 (∀1.3) h−1 and 9.8 (∀4.2), respectively) yielded essentially identical parameter values for all of the miscible-displacement experiments independent of trichloroethene contact time. Additional simulations were conducted to investigate the relative importance of rate-limited versus nonlinear sorption, with the results indicating that rate-limited sorption had a much greater impact on the low-concentration tailing behavior of trichloroethene.
Figure 5.
Representative measured and simulated trichloroethene elution tailing. The simulation is produced using a model incorporating a continuous distribution of retention domains to represent nonlinear, rate-limited sorption/desorption.
4. Conclusions
Elution of trichloroethene from a low organic-carbon-content aquifer material exhibited extensive low-concentration tailing, despite minimal retention of trichloroethene by the aquifer material. The observed nonideal behavior clearly indicates that significant mass-transfer constraints influence trichloroethene transport in this aquifer material. The elution behavior of trichloroethene from field-contaminated aquifer material was essentially identical to that for the synthetically-aged treatment (approximately four years) and for the freshly-amended treatment (contact time <8 hours). In addition, the results of three independent mass-balance analyses, total mass eluted, solvent-extraction analysis of residual sorbed mass, and flow-interruption rebound showed equivalent recoveries for the aged and fresh treatments. These results indicate that long-term contaminant aging did not influence sorption/desorption and transport of trichloroethene in this low organic-carbon aquifer material.
Several researchers have reported significant effects of aging on the sorption/desorption behavior of hydrophobic organic contaminants in natural porous media. However, most of these studies have involved soils or sediments with moderate or greater organic-carbon contents (greater than approximately 0.1%), and few have been conducted using media with low organic-carbon contents. In the present study, organic-carbon content of the aquifer material was 0.03% (∀0.009%). While the objectives of this study did not include a detailed analysis of mechanisms responsible for the sorption/desorption of trichloroethene, a few preliminary conclusions can be drawn from the results. First, the fact that the elution behavior of trichloroethene was essentially identical between the three treatments, irrespective of the contact time, indicates that the attainment of sorption equilibrium was relatively rapid. Second, trichloroethene=s nonlinear sorption suggests the presence of variable and heterogeneous sorption energies, the existence of which is often associated with condensed, carbonaceous material, consistent with the organic-carbon properties of the aquifer material (found to be composed of approximately 61% hard carbon, e.g., kerogen and black carbon). Previous research has shown that that sorption of organic compounds by condensed, carbonaceous materials is often nonlinear and can be relatively rapid. For example, rapid equilibration times for the sorption of polyaromatic hydrocarbons to small particles of soot have been reported by several researchers (e.g., Bucheli and Gustafsson, 2000; Jonker and Koelmans, 2002b). These observations suggest that the interaction of trichloroethene with hard-carbon components of the aquifer material mediates the transport and fate behavior of trichloroethene for this system. The observed minimal impact of aging may be related at least in part to the weathered state of this geologically older aquifer material.
Acknowledgments
This work was supported by grants provided by the Superfund Basic Research Program of the National Institute of Environmental Health Sciences (NIEHS), grant number ES04940, and the U.S. DOD through the Strategic Environmental Research & Development Program. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the U.S. DOD or the NIEHS.
References
- Abu A, Smith S. Mechanistic characterization of adsorption and slow desorption of phenanthrene aged in soils. Environ. Sci. Technol. 2006;40:5409–5414. doi: 10.1021/es060489h. [DOI] [PubMed] [Google Scholar]
- Alexander M. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ. Sci. Technol. 2000;34:4259–4265. [Google Scholar]
- Ball WP, Roberts PV. Long-term sorption of halogenated organic chemicals by aquifer material: 2. Intraparticle diffusion. Environ. Sci. Technol. 1991;25:1237–1249. [Google Scholar]
- Benker E, Davis GB, Barry DA. Estimating the retardation coefficient of trichloroethene for a sand aquifer low in sediment organic carbon - a comparison of methods. J. Contam. Hydrol. 1998;30:157–178. [Google Scholar]
- Brusseau ML, Rao PSC. Sorption nonideality during organic contaminant transport in porous media. Crit. Rev. Environ. Control. 1989;19:33–99. [Google Scholar]
- Brusseau ML, Rao PSC. Influence of sorbate structure on nonequilibrium sorption of organic compounds. Environ. Sci. Technol. 1991;25:1501–1506. [Google Scholar]
- Brusseau ML, Rao PSC, Jessup RE, Davidson JM. Flow Interruption: A method for investigating sorption nonequilibrium. J. Contam. Hydrol. 1989;4:223–240. [Google Scholar]
- Brusseau ML, Jessup RE, Rao PSC. Nonequilibrium sorption of organic chemicals: Elucidation of rate-limiting processes. Environ. Sci. Technol. 1991;25:134–142. [Google Scholar]
- Brusseau ML, Nelson NT, Zhang Z, Blue JE, Rohrer J, Allen T. Source-zone characterization of a chlorinated-solvent contaminated Superfund site in Tucson, AZ. J. Contam. Hydrol. 2007;90:21–40. doi: 10.1016/j.jconhyd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Bucheli TD, Gustafsson Ö. Quantification of the soot-water distribution coefficient of PAHs provides mechanistic basis for enhanced sorption observations. Environ. Sci. Technol. 2000;34:5144–5151. [Google Scholar]
- Carmichael LM, Smith TG, Pardieck DL. Organic compounds in the environment: Site-specific sorption values for mixtures of volatile and semivolatile organic compounds in sandy soils. J. Environ. Qual. 1999;28:888–897. [Google Scholar]
- Chai Y, Qiu X, Davis JW, Budinsky RA, Jr, Bartels MJ, Saghir SA. Effects of black carbon and montmorillonite clay on multiphasic hexachlorobenzene desorption from sediments. Chemosphere. 2007;69:1204–1212. doi: 10.1016/j.chemosphere.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Chai Y, Davis JW, Saghir SA, Qiu X, Budinsky RA, Jr, Bartels MJ. Effects of aging and sediment composition on hexachlorobenzene desorption resistance compared to oral bioavailability in rats. Chemosphere. 2008;72:432–441. doi: 10.1016/j.chemosphere.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Chen W, Wagenet RJ. Solute transport in porous media with sorption-site heterogeneity. Environ. Sci. Technol. 1995;29:2725–2734. doi: 10.1021/es00011a005. [DOI] [PubMed] [Google Scholar]
- Chen W, Wagenet RJ. Description of atrazine transport in soil with heterogeneous nonequilibrium sorption. Soil Sci.. Soc. Am. J. 1997;61:360–371. [Google Scholar]
- Chung N, Alexander M. Differences in sequestration and bioavailability of organic compounds aged in dissimilar soils. Environ. Sci. Technol. 1998;32:855–860. [Google Scholar]
- Connaughton DF, Stedinger JR, Lion LW, Shuler ML. Description of time-varying desorption kinetics: Release of naphthalene from contaminated soils. Environ. Sci. Technol. 1993;27:2397–2403. [Google Scholar]
- Cornelissen G, van Noort PCM, Govers HAJ. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: Sediment extraction with Tenax7 and effects of contact time and solute hydrophobicity. Environ. Toxicol. Chem. 1997;16:1351–1357. [Google Scholar]
- Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM. Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ. Sci. Technol. 2005;39:6881–6895. doi: 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- Culver TB, Hallisey SP, Sahoo D, Deitsch JJ, Smith JA. Modeling the desorption of organic contaminants from long-term contaminated soil using distributed mass transfer rates. Environ. Sci. Technol. 1997;31:1581–1588. [Google Scholar]
- Deitsch JJ, Smith JA. Effect of triton X-100 on the rate of trichloroethene desorption from soil to water. Environ. Sci. Technol. 1995;29:1069–1080. doi: 10.1021/es00004a029. [DOI] [PubMed] [Google Scholar]
- Farrell J, Reinhard M. Desorption of halogenated organics from model solids, sediments, and soil under unsaturated conditions. 2. Kinetics. Environ. Sci. Technol. 1994;28:63–72. doi: 10.1021/es00050a010. [DOI] [PubMed] [Google Scholar]
- Haggerty R, Gorelick SM. Modeling mass transfer processes in soil columns with pore-scale heterogeneity. Soil Sci. Soc. Am. J. 1998;62:62–74. [Google Scholar]
- Hatzinger PB, Alexander M. Effect of aging of chemicals in soils on their biodegradability and extractability. Environ. Sci. Technol. 1995;29:537–545. doi: 10.1021/es00002a033. [DOI] [PubMed] [Google Scholar]
- Jeong S, Werth CJ. Evaluation of methods to obtain geosorbent fractions enriched in carbonaceous materials that affect hydrophobic organic chemical sorption. Environ. Sci. Technol. 2005;39:3279–3288. doi: 10.1021/es0491836. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Gupta K, Putz DK, Hu Q, Brusseau ML. The effect of local-scale physical heterogeneity and non-linear, rate-limited sorption/desorption on contaminant transport in porous media. J. Contam. Hydrol. 2003a;64:35–58. doi: 10.1016/S0169-7722(02)00103-1. [DOI] [PubMed] [Google Scholar]
- Johnson GR, Zhang Z, Brusseau ML. Characterizing and quantifying the impact of immiscible-liquid dissolution and non-linear, rate-limited sorption/desorption on low-concentration elution tailing. Water Resour. Res. 2003b;39:1120. doi:10.1029/2002WR001435. [Google Scholar]
- Jonker MTO, Koelmans AA. Extraction of polycyclic aromatic hydrocarbons from soot and sediment: Solvent evaluation and implications for sorption mechanism. Environ. Sci. Technol. 2002a;36:4107–4113. doi: 10.1021/es0103290. [DOI] [PubMed] [Google Scholar]
- Jonker MTO, Koelmans AA. Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in aqueous environment: Mechanistic considerations. Environ. Sci. Technol. 2002b;36:3725–3734. doi: 10.1021/es020019x. [DOI] [PubMed] [Google Scholar]
- Koelmans AA, Jonker MTO, Cornelissen G, Bucheli TD, van Noort PCM, Gustafsson Ö. Black carbon: The reverse of its dark side. Chemosphere. 2006;63:365–377. doi: 10.1016/j.chemosphere.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Larson T, Kjeldsen P, Christensen TH. Sorption of hydrophobic hydrocarbons on three aquifer materials in a flow through system. Chemosphere. 1992;24:439–451. [Google Scholar]
- Lee LS, Rao PSC, Brusseau ML, Ogwada RA. Nonequilibrium sorption of organic contaminants during flow through columns of aquifer materials. Environ. Toxicol. Chem. 1988;7:779–793. [Google Scholar]
- Li Z, Brusseau ML. Nonideal transport of reactive solutes in heterogeneous porous media: 6. Microscopic and macroscopic approaches for incorporating heterogeneous rate-limited mass transfer. Water Resour. Res. 2000;36:2853–2867. [Google Scholar]
- Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhard M, Traina SJ, Weber WJ, Jr, Westall JC. Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 1997;31:3341–3347. [Google Scholar]
- Morelis S, van Noort PCM. Kinetics of phenanthrene desorption from activated carbons to water. Chemosphere. 2008;71:2044–2049. doi: 10.1016/j.chemosphere.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Morelis S, van den Heuvel H, van Noort PCM. Competition between phenanthrene, chrysene, and 2,5-dichlorobiphenyl for high-energy adsorption sites in a sediment. Chemosphere. 2007;68:2028–2032. doi: 10.1016/j.chemosphere.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Pavlostathis SG, Mathavan GN. Desorption kinetics of selected volatile organic compounds from field contaminated soils. Environ. Sci. Technol. 1992;26:532–538. [Google Scholar]
- Pignatello JJ, Xing B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 1996;30:1–11. [Google Scholar]
- Pignatello JJ. Slowly reversible sorption of aliphatic halocarbons in soils. II. Mechanistic aspects. Environ. Toxicol. Chem. 1990;9:1117–1126. [Google Scholar]
- Pignatello JJ. Desorption of tetrachloroethene and 1,2-dibromo-3-chloropropane from aquifer sediments. Environ. Toxicol. Chem. 1991;10:1399–1404. [Google Scholar]
- Pignatello JJ, Xing B. Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 1996;30:1–11. [Google Scholar]
- Prevedouros K, Palm-Cousins A, Gustafsson Ö, Cousins IT. Development of a black carbon-inclusive multi-media model: Application for PAHs in Stockholm. Chemosphere. 2008;70:607–615. doi: 10.1016/j.chemosphere.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Rivett MO, Allen-King RM. A controlled field experiment on groundwater contamination by a multicomponent DNAPL: dissolved-plume retardation. J. Contam. Hydrol. 2003;66:117–146. doi: 10.1016/S0169-7722(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Saiers JE, Tao G. Evaluation of continuous distribution models for rate-limited solute adsorption to geologic media. Water Resour. Res. 2000;36:1627–1639. [Google Scholar]
- Stauffer TB, MacIntyre WG, Wickman DC. Sorption of nonpolar organic chemicals on low-carbon-content aquifer materials. Environ. Toxicol. Chem. 1989;8:845–852. [Google Scholar]
- Steinberg SM, Pignatello JJ, Sawhney BL. Persistence of 1,2-dibromoethane in soils: Entrapment in intraparticle micropores. Environ. Sci. Technol. 1987;21:1201–1208. [Google Scholar]
- Valocchi AJ. Validity of the local equilibrium assumption for modeling sorbing solute transport through homogeneous soils. Water Resour. Res. 1985;21:808–820. [Google Scholar]
- Weber WJ, Jr, Young TM. A distributed reactivity model for sorption by soils and sediments. 6. Mechanistic implications of desorption under supercritical fluid conditions. Environ. Sci. Technol. 1997;31:1686–1691. [Google Scholar]
- Xing B, Pignatelloc JJ. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environ. Sci. Technol. 1997;31:792–799. [Google Scholar]
- Young DF, Ball WP. Effects of column conditions on the first-order rate modeling of nonequilibrium solute breakthrough. Water Resour. Res. 1995;31:2181–2192. [Google Scholar]
- Zhang ZH, Brusseau ML. Nonideal transport of reactive solutes in heterogeneous porous media. 5. Simulating regional-scale behavior of a trichloroethene plume during pump-and-treat remediation. Water Resour. Res. 1999;35:2921–2935. [Google Scholar]