SUMMARY
The clarity of categorizing skeletal muscle fibers in individual motor units into phenotypes based on quantitative single fiber enzyme activities and as a function of neuromuscular activity level was examined. Neuromuscular activity was eliminated in adult cat hindlimb muscles by spinal cord isolation (SI), i.e. complete spinal cord transection at a low thoracic and a high sacral level with bilateral dorsal rhizotomy between the transection sites. One motor unit was isolated via ventral root teasing procedures from the tibialis anterior (TA) muscle of each hindlimb in control and SI cats, and physiologically tested and glycogen depleted through repetitive stimulation; fibers comprising each motor unit were visualized through glycogen staining. Each motor unit was composed of fibers of the same myosin immunohistochemical type. Myofibrillar adenosine triphosphatase, succinate dehydrogenase and α-glycerophosphate dehydrogenase activities were determined for a sample of motor unit and non-motor unit fibers, providing a measure of three enzyme activities often used to characterize fiber phenotype within a single unit. Although normal enzyme activities were altered after 6 months of inactivity, the relationships among the three enzymes were largely maintained. These data demonstrate that it is not the diversity in any single enzyme property but the profile of several metabolic pathways that underlies the significance of fiber phenotypes. These profiles must reflect a high level of coordination of expression of selected combinations of genes. Although neuromuscular activity level influences fiber phenotype, the present results demonstrate that activity-independent mechanisms remain important sources of the control of phenotype establishment in the near absence of activity.
Keywords: 3-D representations, motor unit types, succinate dehydrogenase, myofibrillar adenosine triphosphatase, α-glycerophosphate dehydrogenase
INTRODUCTION
The concept of muscle fiber types evolved rapidly upon the development of techniques to assess biochemical reactions in cross-sections of single muscle fibers, classically referred to as histochemistry, which allowed one to assess the relative activity of multiple enzymes in a single fiber (Edgerton and Simpson, 1969; Brooke and Engel, 1969; Peter et al., 1972). Using this approach one could characterize each fiber with respect to its relative enzyme activity or substrate concentration profile. The principal result from this approach was that in hindlimb skeletal muscles of several mammals there generally appeared to be approximately three identifiable populations of fibers based on myofibrillar adenosine triphosphatase (mATPase) and a series of enzymes associated with glycolytic and oxidative metabolism and the electron transport chain within the mitochondria (Peter et al., 1972).
One major difficulty with these results was the lack of quantification. Initially, the major contribution of this procedure was to give a clear impression of the types of fiber that could be identified reasonably consistently. Soon thereafter it became possible to quantify similar properties at the whole muscle level by using classical biochemical techniques, i.e. to quantify enzyme activity rates of homogenized muscle tissue in the test tube, and to measure physiological properties of the same muscle, thus providing a more direct indication of the basic mechanisms controlling the contractile properties of the muscle such as speed and fatigability (Barnard et al., 1971). Subsequently, the same approach was applied in studies of single motor units, whereby the contractile and biochemical properties of the fibers innervated by a single motoneuron could be determined (Bodine et al., 1987; Burke et al., 1973; Edstrom and Kugelberg, 1968). The same basic concept and conclusion evolved from each of the approaches noted above. Specifically, it became clear that among the hindlimb mammalian skeletal muscles studied, the motor units and muscle fibers in normal control muscles generally could be categorized into the following three populations: (1) fast contracting units and fibers that were very fatigable; (2) fast contracting units and fibers that were relatively fatigue resistant; and (3) slow contracting units and fibers that were very fatigue resistant (Burke et al., 1973; Burke and Edgerton, 1975; McDonagh et al., 1980; Peter et al., 1972).
This generalized concept that mammalian skeletal muscles of the hindlimb fall into three categories has limitations. In fact, a contrasting view is that there is little validity to a generalized concept relating to muscle fiber types (Pette and Staron, 1990) because there are many more combinations of enzyme activity levels among single muscle fibers than can be accommodated by a tripartite or even a tetrapartite (McDonagh et al., 1980) categorization. A second reason for this position is that the level of plasticity of single fibers is so dynamic that it is futile to attempt to categorize them into identifiable clusters. Pette and Staron (Pette and Staron, 1990) suggested that there are at least as many fiber types as there are motor units in a muscle.
The issues of whether there are consistently identifiable types of fiber and, if there are, how many and to what extent does this categorization depend on the activity of the motor units, are long standing (McDonagh et al., 1980). In a large part, however, the views expressed by different investigators have not been based on clearly identified quantitative criteria for distinguishing fiber types. The purposes of the present paper were to address three questions related to these issues. Firstly, can statistically defined populations (types) of fibers be identified using quantitative measurements of three enzyme systems often associated with fiber phenotypes in the cat tibialis anterior (TA) muscle? Secondly, how are these muscle fiber types related to motor unit types, i.e. are muscle fiber phenotypes linked to the physiological properties of motor units in a muscle? Thirdly, does a severe perturbation in the activation of motor units, a function often proposed to be the dominant controlling factor in the expression of muscle fiber phenotypes, result in a level of plasticity such that neither muscle fiber phenotypes nor motor unit types can be identified?
The present results indicate that three identifiable populations of fibers exist in the cat TA based on the quantification of three enzyme systems. The inter-relationships among a marker enzyme in each of three protein systems that reflect a different metabolic property, i.e. mATPase (a marker of contractile function), succinate dehydrogenase (SDH, a marker of oxidative capacity) and α-glycerophosphate dehydrogenase [GPD, an enzyme involved in the NADH/NAD exchange between the mitochondria and the cytoplasm that is highly correlated with the glycolytic capacity of a muscle (Peter et al., 1972)], clearly show three fiber-type populations when linked to the myosin isoform as shown previously in cat hindlimb muscles (Edgerton et al., 1985; Roy et al., 1996). In addition, although a 6 month period of near inactivity induced by spinal cord isolation (SI) resulted in shifts in the enzyme activities within these three systems, three populations of fibers persisted in the TA of SI cats.
MATERIALS AND METHODS
Surgical and motor unit isolation procedures
The lumbar region of the spinal cord of nine adult female cats (body weight, 2.3–3.8 kg) was isolated surgically as described by Pierotti et al. (Pierotti et al., 1991). Under deep pentobarbital anesthesia (35 mg kg−1) and aseptic conditions, a partial laminectomy was performed from T12 to S2 and a bilateral dorsal rhizotomy was performed intradurally between T12 and S2. The spinal cord was completely transected at the junctions of T12–T13 and again at L7–S1. These procedures result in near inactivity of the hindlimb muscles for prolonged periods in cats (Eldridge et al., 1981; Pierotti et al., 1991) and rats (Roy et al., 2007). The procedures for the post-surgical care of spinal-injured cats are detailed in Roy et al. (Roy et al., 1992).
Approximately 6 months following surgery, one motor unit in the TA muscle of four control and nine SI cats was successfully characterized physiologically and then the fibers belonging to the motor unit were glycogen depleted by stimulating its functionally isolated axon as described previously (Pierotti et al., 1991). All procedures were approved by the Animal Use Committee at UCLA and followed the American Physiological Society Animal Care Guidelines.
Histological procedures
At the end of the physiological testing [results reported in Pierotti et al. (Pierotti et al., 1991)], the muscles were excised and prepared for histological analyses. Each muscle was cut transversely into ~5 mm blocks over its entire length, and the blocks were mounted on cork and rapidly frozen in isopentane cooled with liquid nitrogen. To assess the glycogen content of the muscle fibers, cross-sections (20 μm thick) were cut and stained for the periodic acid–Schiff reaction (Pearse, 1961). Briefly, the sections were placed in solution (8.0 ml 100% ethanol, 1.5 ml chloroform and 0.5 ml glacial acetic acid, pre-cooled in a cryostat at −20°C for 30 min), left in the cryostat for 5 min, and then moved to room temperature for 10 min. The sections were rinsed in distilled H2O and transferred into a 0.5% periodic acid solution for 5 min. The sections then were rinsed with distilled H2O and incubated in Schiff's reagent (Sigma-Aldrich Corp., St Louis, MO, USA) at 37°C for 10 min. The sections were then air dried overnight and mounted with aquamount. An image-processing computer system was used to determine the optical density of glycogen staining for each of the outlined fibers (Cope et al., 1986). The very low optical density level of glycogen staining identified the muscle fibers belonging to an isolated motor unit. Quantitative measures of SDH and GPD were determined histochemically in a sample of motor unit fibers and a sample of non-motor unit fibers (fibers not depleted of glycogen and thus exhibiting relatively high levels of glycogen staining) located within the motor unit territory (Martin et al., 1988). In addition, a modification of the histochemical method of Weisberg et al. (Weisberg et al., 1982) was used to measure the mATPase activity (Jiang et al., 1991) in the same fibers.
Fiber enzyme activity levels were determined from digitized images of the muscle cross-sections that were stored as gray-level pictures. An image-processing system determined the light transmittance for each pixel, which was subsequently converted to an optical density. The optical density reading per fiber was calculated as an average of all pixels within the fiber boundaries. The SDH, GPD and mATPase reactions were terminated while the reactions were still linear. Thus the enzymatic activities were expressed as steady-state rates, i.e. optical density min−1. The activity of each analyzed fiber was expressed as the mean of three sections incubated with substrate minus the mean of two sections incubated without substrate. This procedure corrected for any non-specific staining that may have occurred during the reaction. These procedures have been used routinely in our laboratory (Edgerton et al., 1990; Graham et al., 1992; Jiang et al., 1991; Martin et al., 1988; Pierotti et al., 1994; Rivero et al., 1999).
Immunohistochemical procedures
Individual fibers were typed using monoclonal antibodies specific for myosin heavy chain isoforms. Antibodies BF-F8, BF-13 and BF-35 (kindly provided by Dr S. Schiaffino, University of Padova, Italy) were used in this study and their specificity in rat (Schiaffino et al., 1989) and cat (Talmadge et al., 1996) muscles has been described previously. Three fiber types are present in cat hindlimb muscles: type I (positive for BF-F8 and BF-35 and negative for BF-13), type IIa (negative for BF-F8 and positive for BF-35 and BF-13) and type IIx (negative for BF-F8 and BF-35 and positive for BF-13). Frozen sections (10 μm thick) were incubated for 30 min in a blocking solution containing 1% bovine serum albumin in phosphate-buffered saline (PBS) and then overnight at 4°C with the monoclonal antibodies. A Vectastain ABC kit (Vector Labs, Burlingame, CA, USA) was utilized to amplify the antigen–antibody complex. After a 20 min wash in PBS, secondary antigen–antibody complexes were detected by incubation for 30 min with a biotinylated antibody to mouse immunoglobulins. Each tissue section was washed for 20 min with PBS. The antigen–antibody complex was visualized by treatment with diaminobenzidine and hydrogen peroxide for 10 min. The sections were dehydrated in 70, 95 and 100% ethanol, followed by xylene and mounted with permount. In the present study almost all fibers could be classified as type I, IIa or IIx fibers. Less than 1% of the sampled fibers were classified as ‘hybrid’ fibers (all non-motor unit fibers) and these were excluded from the study. In addition, there was a rare occurrence of fibers showing signs of degeneration (fragmentation, central nuclei, etc.) in some muscles of each group, including the control group. None of these were motor unit fibers and they were excluded from the study.
Statistical analyses
To make comparisons among muscle fibers across animals, the absolute enzyme activities were normalized (z-scores) to the mean for each muscle. Since three enzyme activities were measured in each fiber, a multivariate analysis of variance (MANOVA) was used to test whether (1) fibers from the experimental group differed from fibers in the control group, (2) fiber types across experimental groups differed from one another, and (3) there was an interaction between group and fiber type. The most appropriate procedure for determining whether the data from the three distinct fiber types differed significantly was MANOVA. Rather than performing three separate ANOVAs, one on each of the variables (mATPase, SDH and GPD), MANOVA provided a single test of whether the three fiber types differed in the multivariate sense (capitalizing on the covariance structure of the data). Separate ANOVAs would have much less statistical power. In effect, MANOVA can allow the null hypothesis to be rejected under conditions in which the separate ANOVAs cannot. A Hotelling procedure was used to compare data from control and SI rats within fiber type. Statistical significance was set at P⩽0.05 for all comparisons.
The MANOVA was used to determine, for each fiber type, a set of weights on all three of the variables that maximally discriminated between the fiber types, i.e. discriminant functions. In addition to allowing for an omnibus test for a difference between the fiber types, MANOVA provided a manner whereby individual fibers could be categorized as type I, IIa or IIx solely on the basis of their mATPase, SDH and GPD values.
Given three distinct sets of multivariate data, a graphical depiction was desired to show a multivariate confidence region for each set. This required the reconstruction of a boundary such that (1) data within the boundary probably belonged to that set, and (2) data outside of the boundary were far enough removed from the centroid that they were regarded as outliers. Rather than focusing on one variable at a time, a probability density measure was used to take into account the covariance structure of the data within each set. The sets of data were generated identifying three muscle fiber types based on three variables (mATPase, SDH and GPD). For each set of data (all muscle fibers of a given type), it was possible to establish confidence regions for values on each variable separately by calculating z-scores. In this case, the distance of an item from the mean of one variable was expressed in terms of the standard deviation units for that variable. Setting the criterion at two standard deviation units, it was possible to judge an item to be an outlier if it was removed more than two units from the mean of each of the three variables. This procedure was considered appropriate since it ignored the covariance between the three variables.
To better characterize the distance of a given item (fiber) from the three means for its fiber type (which, taken together, are termed the centroid), a calculation of a deviation vector for that item (fiber) was needed to measure its distance from the centroid. Rather than dividing by the three standard deviation values, the deviation vector was transformed by the inverse of the covariance matrix for that fiber type, forming what is termed the Mahalanobis distance. To test whether the item was an outlier or not, this distance value was transformed into an F-value with degrees of freedom determined by the number of variables (M), and the number of items (fibers) in the fiber type (N). The probability of incorrect rejection of an extreme point was determined by looking up F(M, N–M) in an appropriate statistical table. To determine the probability density function itself, a chi-squared table was consulted with M degrees of freedom.
RESULTS
A percentage range of enzyme activity was calculated for each motor unit for each of the three enzymes to examine the range of quantitative activities displayed within a given motor unit. Table 1 lists the mean (±s.d.) and the range for each normalized enzyme measurement for the motor unit fibers relative to the range of normalized (z-score) values measured for all (>800) muscle fibers in the TA of control and SI rats. These values were calculated as the range for fibers of a single motor unit divided by the total range for a sample of motor unit and non-motor unit fibers of all types within the same muscle, and then expressed as a percentage. This measurement provides a measure of the overlap in the enzyme activities for each motor unit with respect to the whole muscle. For each enzyme, the mean range for a motor unit was ~10 to 50% of the total range for all fibers for both control and SI muscles. Overall, the enzyme activity of fibers within a motor unit occupied ~25% of the total range that was observed among all fibers in both control and SI muscles (Pierotti et al., 1994).
Table 1.
Mean and range in enzyme properties of single motor unit fibers relative to the range across all fibers
| Control |
SI |
|||||
|---|---|---|---|---|---|---|
| mATPase | SDH | GPD | mATPase | SDH | GPD | |
| Mean ± s.d. (%) | 25±6 | 21±12 | 26±13 | 31±7 | 27±13 | 26±6 |
| Range (%) | 18–31 | 10–36 | 8–40 | 22–42 | 8–54 | 14–38 |
mATPase, myofibrillar adenosine triphosphatase; SDH, succinate dehydrogenase; GDP, α-glycerophosphate dehydrogenase; SI, spinal cord isolation.
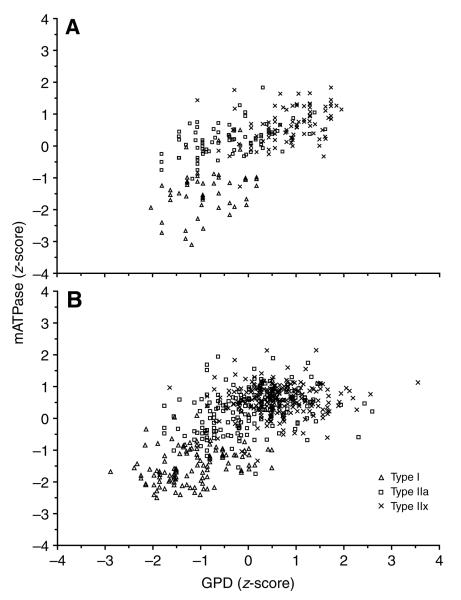
The relationship between mATPase and SDH activities within motor unit fibers for the normalized data from the control (Fig. 1A) and SI (Fig. 1B) cats is plotted by myosin heavy chain type. Note the same general pattern for the three fiber types for the control and SI data. While there is clear overlap between the three fiber-type groups when comparing two enzyme activities with a known myosin phenotype, there are also apparent clusters of fibers in both graphs. Type I and IIx fibers comprise the most distinct groups with respect to the mATPase/SDH ratio. The relationship between mATPase and GPD activities for motor unit fibers in control and SI muscles is shown in Fig. 2A and B, respectively. Again the type I and IIx fibers comprise the most distinct groups with respect to the mATPase/GPD ratio in both groups, with overlap in either group largely due to type IIa fibers.
Fig. 1.
The relationship between normalized succinate dehydrogenase (SDH) and normalized myofibrillar adenosine triphosphatase (mATPase) activity for the motor unit fibers in the tibialis anterior (TA) of all control (A) and all spinal cord isolated (SI, B) cats. Note the clusters for each fiber type, particularly for the type I and type IIx fibers.
Fig. 2.
The relationship between normalized α-glycerophosphate dehydrogenase (GPD) and normalized mATPase activity for the motor unit fibers in the TA of all control (A) and all SI (B) cats. Again, note the clusters for each fiber type, particularly for the type I and type IIx fibers. Abbreviations as in Fig. 1.
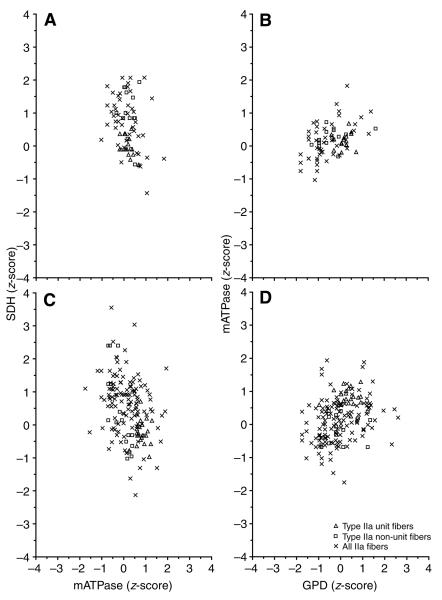
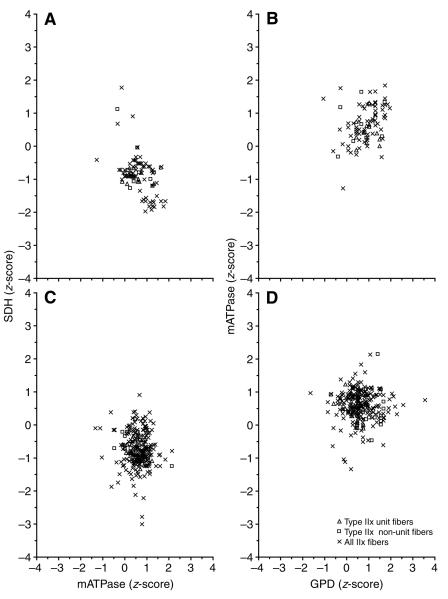
These data were further sub-divided by motor unit type to determine whether relationships similar to those observed in the entire muscle fiber population existed at the motor unit level. Fig. 3 depicts the SDH vs mATPase (A,C) and mATPase vs GPD (B,D) ranges for motor unit and non-motor unit type I fibers from a control (A,B) and a SI (C,D) slow motor unit. In addition, all of the type I fibers analyzed across all cats were included for comparative purposes. Note that although the motor unit fibers have a smaller variability than the non-motor unit fibers within and across motor units, they still exhibit a relatively wide range of enzyme activities. The enzyme ranges for a control and SI motor unit composed of type IIa (Fig. 4) and type IIx (Fig. 5) fibers show a pattern similar to that observed for the type I fibers.
Fig. 3.
The relationship between normalized SDH and mATPase (A,C) and normalized mATPase and GPD (B,D) activity for type I motor unit fibers (glycogen depleted) and type I non-motor unit fibers (not glycogen depleted, located within the territory of the motor unit fibers) in a slow TA motor unit from a control (A,B) and a SI (C,D) cat is shown. In addition, data for all type I fibers across all cats are included for comparative purposes. Note that the ranges in the enzyme activities of motor unit fibers generally overlap those of non-motor unit fibers in both the control and SI cats. Abbreviations as in Figs 1 and 2.
Fig. 4.
The relationship between normalized SDH and mATPase (A,C) and normalized mATPase and GPD (B,D) activity for type IIa motor unit fibers (glycogen depleted) and type IIa non-motor unit fibers (not glycogen depleted, located within the territory of the motor unit fibers) in a fast TA motor unit from a control (A,B) and a SI (C,D) cat is shown. In addition, data for all type IIa fibers across all cats are included for comparative purposes. Note that the ranges in the enzyme activities of motor unit fibers generally overlap those of non-motor unit fibers in both the control and SI cats. Abbreviations as in Figs 1 and 2.
Fig. 5.
The relationship between normalized SDH and mATPase (A,C) and normalized mATPase and GPD (B,D) activity for type IIx motor unit fibers (glycogen depleted) and type IIx non-motor unit fibers (not glycogen depleted, located within the territory of the motor unit fibers) in a fast TA motor unit from a control (A,B) and a SI (C,D) cat is shown. In addition, data for all type IIx fibers across all cats are included for comparative purposes. Note that the ranges in the enzyme activities of motor unit fibers generally overlap those of non-motor unit fibers in both the control and SI cats. Abbreviations as in Figs 1 and 2.
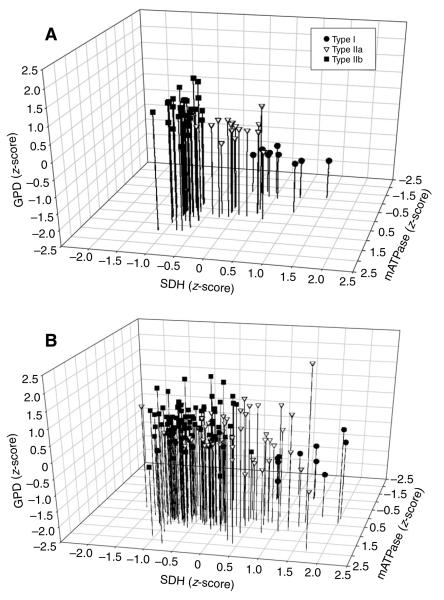
Enzyme activities of fibers from the three motor units depicted in Figs 3-5 were plotted using the normalized centroid value for each of the three variables. For either the control (Fig. 6A) or SI (Fig. 6B) motor units there was clustering of fibers reflecting three muscle fiber types. These data also indicate that similar relationships among the three variables, i.e. mATPase, SDH and GPD, were present for fibers within a motor unit.
Fig. 6.
A 3-D representation of the relationship among SDH, GPD and mATPase activities in the fibers of the motor units from all control (A) and all SI (B) cats. Note the presence of three clusters of fiber types for both the control and the SI cats. Abbreviations as in Figs 1 and 2.
The omnibus test of whether the fiber types differed in the multivariate sense was based on the Wilks' lambda test. This gave F(6.1538)=315.0342 (P<0.0001). The first discriminant function accounted for 83% of the variance due to differences among fiber types. For the sake of completeness, a MANOVA was performed to test whether there was a difference between the control and SI animals, and there was no significant difference [F(3.769)=0.43, P=0.73]. Interestingly, the interaction between group (control and SI) and type was significant [F(6.1538)=8.0455, P<0.0001]. Therefore, the SI manipulation influenced the pattern of activities, i.e. the relationship among the activities of mATPase, SDH and GPD between different fibers, although it had no main effect on those activities.
The nature of this interaction was examined by looking at the distances between the centroids. All six centroids differed on the three activity measures, but these differences were reduced to a simple Euclidean distance measure for each pairwise comparison of centroids on those three dimensions. These distances then were submitted to classical metric scaling (Torgerson, 1958) to derive two new dimensions upon which to plot the six points corresponding to the factorial crossing of two groups with three types. The six points were plotted to illustrate how a large interaction could occur when there was no group effect (Fig. 7). Inactivity had little effect on type I fibers, reduced type IIa fibers on dimension 2, and increased type IIx fibers on both dimensions 1 and 2. The contrary motion between the type IIa and type IIx fibers nullifies the group main effect.
Fig. 7.
Location of centroids of control (C) and SI (E) fibers in each of the three fiber types (type I, 1; type IIa, 2; and type IIx, 3). Metric re-scaling (see text in Results section) produced a new coordinate system (dimension 1 and dimension 2) in which the six centroids had maximum separation.
DISCUSSION
Although fibers can be classified into overlapping groups based on myosin type and enzyme properties, and motor units may be characterized based largely on sag and fatigue properties (Burke et al., 1973), the overlapping regions between groups has led to one viewpoint: that there is little significance to the concept of muscle fiber types. The expression of multiple protein isoforms within a single fiber (Caiozzo et al., 2003; Roy et al., 1997; Staron and Pette, 1986; Staron and Pette, 1987; Talmadge et al., 1999; Stephenson, 2001) clearly shows a greater diversity than once was apparent among mammalian fibers. This diversity raises the issue of the biological significance of the concept of muscle fiber types (Pette and Staron, 1990). As noted earlier, the question is: are there as many fiber types as there are motor units in a muscle?
The present data set is unique in that we examined the combination of three protein systems within single muscle fibers of physiologically defined motor unit types. These three protein systems represent those around which gene expression in mammalian muscles is proposed to be organized (Burke and Edgerton, 1975; Edgerton and Simpson, 1969). We also asked the question whether the dynamic plasticity of muscle fibers makes classification of muscle fibers and motor units into types essentially irrelevant. If the neuron plays an important role in modulating the proteins within a single muscle fiber as well as within a single motor unit, then modulation of neuromuscular activity should also affect the identification of specific fiber types at the level of organization of the motor unit. The issues specifically addressed in the present study were whether a muscle fiber could be categorized as statistically unique based on the measurements of the three metabolic enzymes studied, whether these metabolic markers are unique to a motor unit type and, furthermore, whether these levels of organized states would still be applicable following 6 months of electrical silence.
Our results indicate that the activity levels of markers of three enzyme systems can be used to identify different populations of fibers if the myosin phenotype is known. Although a detailed characterization of other parameters may further enhance the differences among the clusters (fiber types), the protein systems studied in the present paper may represent those with which many other protein systems are co-regulated. The multivariate analysis allowed us to examine the covariance characteristics among fibers as a function of motor unit identification. The relationship of one variable to another defines a very strict limit on the group in which a fiber may be placed, in much the same way that the relationships among variables dictate the functional limits of the fibers in question.
Based on the MANOVA analyses, we conclude that there were three groups of fibers for both control and SI populations. Furthermore, if the population of muscle fibers in the present data set is sub-divided based on myosin type and motor unit type, we conclude that statistically distinct types still can be identified. Although near-complete inactivity was imposed on the SI muscles and adaptations occurred with respect to the individual enzyme systems, the relationships among the systems were altered without blurring a striking distinction among the fiber and motor unit types. The fact that these distinctions are present at the whole muscle and motor unit levels strengthens the argument for distinct types of muscle fiber and motor unit and that this distinction persists in almost the complete absence of neuromuscular activity.
While the presence of a continuum among fibers of a given muscle for any single variable is apparent, a single variable cannot describe the functional diversity of individual muscle fibers. We propose that the biological significance of motor unit and muscle fiber types is in the coordination of the expression of genes that define the protein levels for the interactive protein systems. It is apparent that the proteins within any given system will be coordinated, e.g. enzymes that carry out glycolysis, glycogenolysis, etc. But the significance of the concept of muscle fiber types must be that there is a substantial coordination of the expression of genes within and among protein systems. There is a high level of interdependence of the expression of type I and type II myosin. If type I myosin expression is depressed in a fiber, a type II isoform will be enhanced and vice versa (Baldwin and Haddad, 2001). Although multiple isoforms are expressed in some fibers, even in muscles of control animals (Caiozzo et al., 2003; Roy et al., 1997; Staron and Pette, 1986; Staron and Pette, 1987; Talmadge et al., 1999), there is a high probability that the expression of one will dominate in a fiber adapted to a steady state. In the present study, there was a rare occurrence of hybrid fibers in the TA of control or SI cats.
It is significant that the net effect beyond this extensive coordination of genes within a fiber and among fibers of a motor unit results in the considerable diversity in function among motor units. Without this gene coordination there would be diversity in the biochemical properties, but no coordination of gene expression or function at either the fiber (and therefore no fiber types) or motor unit level. It is this coordinated diversity among fibers within a muscle that defines the potential of the motor units (and fibers) of a given muscle to produce work at a given power, duration and pattern. If a fiber expresses a slow myosin isoform, its GPD activity will be relatively low, given that the activity of this enzyme is a predictable indicator of the glycolytic and glycogenolytic pathways (Bass et al., 1969; Gregory et al., 2001; Staudte and Pette, 1972; Peter et al., 1972). The present data also suggest a high probability that the type I fiber will have a relatively high SDH activity, i.e. its potential for oxidative phosphorylation will be relatively high. It is also apparent that the coordination of protein systems within a fiber and motor unit extends beyond the three enzyme systems presently studied (Hallauer and Hastings, 2002; Hamm et al., 1988; Hood et al., 2006; Spangenburg and Booth, 2003).
There is a multitude of evidence from other enzyme systems that further supports the idea of coordinated systems within fiber types. For instance, Kong et al. (Kong et al., 1994) reported a 20-fold difference in the amount of the glucose transporter isoform GLUT4 among the three fiber types in the rabbit TA muscle. Type I fibers had the highest level followed by type IIa and then IIx. They went on to note that GLUT4 was highly correlated with the levels of malate dehydrogenase and hexokinase. Following electrical stimulation of the peroneal nerve, they reported that GLUT4 and hexokinase increased and remained coordinated. Meng et al. (Meng et al., 1993) reported that myoglobin content paralleled oxidative capacity and was correlated to fiber type in three muscles having widely varying fiber-type compositions. Type I fibers had a higher content than type IIa fibers and the lowest amount was found in type IIx fibers. Nishida et al. (Nishida et al., 1995) reported the highest levels of myoglobin in slow fibers that had high lactate dehydrogenase (H-type isoform) activities. The lowest myoglobin content was found in fast fibers with the highest lactate dehydrogenase (M-type isoform) activities. Similar correlations were found between carbonic anhydrase III and myoglobin in three fiber types from human psoas muscle (Zheng et al., 1992). Each of these studies indicates that there is not a random expression of proteins in a muscle fiber but a coordinated program for the expression of protein systems among a variety of species. The mechanisms by which this coordination of gene expression within a single fiber and among the fibers of a motor unit can occur remain unclear. One of the most obvious candidates for this coordination within a muscle unit is neuronal, with this neural influence exerted via activity-dependent and -independent means (Hyatt et al., 2003; Hyatt et al., 2006; Roy et al., 1996).
Numerous studies have reported some level of control manifested via the motoneurons that limits the diversity in the properties among fibers of a muscle unit. In the case of the cat TA, it is about 25–30% of the diversity observed among all fibers of the muscle (Pierotti et al., 1994). Although the phenotype of a fiber can be modulated by varying the amount and/or pattern of activation it receives (Pette and Vrbova, 1999; Salmons and Sreter, 1976), it is equally clear that the level of activation is not the only, and perhaps not even the dominant, control mechanism exerted by the motoneuron (Edgerton et al., 1996). The range in motor unit and muscle fiber types observed in muscles for control animals persists after 6 months of virtual electrical silence (Pierotti et al., 1991). In addition, when a change does occur in a pool of silenced motor units, it occurs at the motor unit level, i.e. some motor units change while others do not (Zhong et al., 2002).
The mechanisms through which this neuronal control occurs in the absence of neural activity remain elusive, but it is clear that muscle fiber phenotypes are not defined solely by the activity levels or patterns of the motoneurons (Edgerton et al., 1996; Hyatt et al., 2003; Hyatt et al., 2006). In addition, there is clear evidence that when the axon remains intact with the muscle as in the SI preparation, compared with denervation, the TA muscle atrophies less and myogenic genes are modulated less severely, thus illustrating the importance of a non-activity source of neural control (Hyatt et al., 2003; Hyatt et al., 2006).
Perspective
The present data clearly show that the biological significance of the diversity in the biochemical properties of muscle fibers is manifested in (1) the coordination of gene expression within and among protein systems of a single fiber, (2) the coordination of this gene expression within a single fiber with that in all fibers innervated by the same motoneuron, and (3) the range and combination of gene expression patterns that are formed and sustained by a pool of motoneurons that innervate a muscle in a way that matches the physiological demands of a given muscle. The present data also demonstrate a high level of persistence of the diversity and coordination of protein systems within and among fibers of a motor unit and across motor units of the same muscle after 6 months of electrical silence. In general, these data demonstrate that it is not the diversity in values of any single property that underlies the significance of muscle fiber phenotypes but the coordination of the expression of selected combinations of genes. The mechanisms that control this level of coordination seem to be mediated neurally as well as non-neurally. In addition, the neurally induced control can be via neural activity-or non-activity-linked mechanisms.
Acknowledgments
This work was supported in part by NIH grant NS16333.
REFERENCES
- Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J. Appl. Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- Barnard RJ, Edgerton VR, Furukawa T, Peter JB. Histochemical, biochemical, and contractile properties of red, white, and intermediate fibers. Am. J. Physiol. 1971;220:410–414. doi: 10.1152/ajplegacy.1971.220.2.410. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur. J. Biochem. 1969;10:198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J. Neurophysiol. 1987;57:1730–1745. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 1. Adult male and female. Neurology. 1969;19:221–233. doi: 10.1212/wnl.19.3.221. [DOI] [PubMed] [Google Scholar]
- Burke RE, Edgerton VR. Motor unit properties and selective involvement in movement. Exerc. Sport Sci. Rev. 1975;3:31–81. [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiozzo VJ, Baker MJ, Huang K, Chou H, Wu YZ, Baldwin KM. Single-fiber myosin heavy chain polymorphism: how many patterns and what proportions? Am. J. Physiol. 2003;285:R570–R580. doi: 10.1152/ajpregu.00646.2002. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J. Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Simpson DR. The intermediate muscle fiber of rats and guinea pigs. J. Histochem. Cytochem. 1969;17:828–838. doi: 10.1177/17.12.828. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Martin TP, Bodine SC, Roy RR. How flexible is the neural control of muscle properties? J. Exp. Biol. 1985;115:393–402. doi: 10.1242/jeb.115.1.393. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Bodine-Fowler SC, Pierotti DJ, Unguez GA, Martin TP, Jiang B, Chalmers GR. Motoneurons-muscle fiber connectivity and interdependence. In: Pette D, editor. The Dynamic State of Muscle Fibers. Walter de Gruyter; Berlin: 1990. pp. 217–231. [Google Scholar]
- Edgerton VR, Bodine-Fowler S, Roy RR, Ishihara A, Hodgson JA. Neuromuscular adaptation. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology: Section 12: Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; New York: 1996. pp. 54–88. [Google Scholar]
- Edstrom L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J. Neurol. Neurosurg. Psychiatry. 1968;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge L, Liebhold M, Steinbach JH. Alterations in cat skeletal neuromuscular junctions following prolonged inactivity. J. Physiol. 1981;313:529–545. doi: 10.1113/jphysiol.1981.sp013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SC, Roy RR, Navarro C, Jiang B, Pierotti D, Bodine-Fowler S, Edgerton VR. Enzyme and size profiles in chronically inactive cat soleus muscle fibers. Muscle Nerve. 1992;15:27–36. doi: 10.1002/mus.880150106. [DOI] [PubMed] [Google Scholar]
- Gregory CM, Vandenborne K, Dudley GA. Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve. 2001;24:387–393. doi: 10.1002/1097-4598(200103)24:3<387::aid-mus1010>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hallauer PL, Hastings KE. Coregulation of fast contractile protein transgene and glycolytic enzyme expression in mouse skeletal muscle. Am. J. Physiol. 2002;282:C113–C124. doi: 10.1152/ajpcell.00294.2001. [DOI] [PubMed] [Google Scholar]
- Hamm TM, Nemeth PM, Solanki L, Gordon DA, Reinking RM, Stuart DG. Association between biochemical and physiological properties in single motor units. Muscle Nerve. 1988;11:245–254. doi: 10.1002/mus.880110309. [DOI] [PubMed] [Google Scholar]
- Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006;209:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, Roy RR, Baldwin KM, Edgerton VR. Nerve activity-independent regulation of skeletal muscle atrophy: role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol. 2003;285:C1161–C1173. doi: 10.1152/ajpcell.00128.2003. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, Roy RR, Baldwin KM, Wernig A, Edgerton VR. Activity-unrelated neural control of myogenic factors in a slow muscle. Muscle Nerve. 2006;33:49–60. doi: 10.1002/mus.20433. [DOI] [PubMed] [Google Scholar]
- Jiang B, Roy RR, Navarro C, Nguyen Q, Pierotti D, Edgerton VR. Enzymatic responses of cat medial gastrocnemius fibers to chronic inactivity. J. Appl. Physiol. 1991;70:231–239. doi: 10.1152/jappl.1991.70.1.231. [DOI] [PubMed] [Google Scholar]
- Kong X, Manchester J, Salmons S, Lawrence JC., Jr Glucose transporters in single skeletal muscle fibers. Relationship to hexokinase and regulation by contractile activity. J. Biol. Chem. 1994;269:12963–12967. [PubMed] [Google Scholar]
- Martin TP, Bodine-Fowler S, Roy RR, Eldred E, Edgerton VR. Metabolic and fiber size properties of cat tibialis anterior motor units. Am. J. Physiol. 1988;255:C43–C50. doi: 10.1152/ajpcell.1988.255.1.C43. [DOI] [PubMed] [Google Scholar]
- McDonagh JC, Binder MD, Reinking RM, Stuart DG. Tetrapartite classification of motor units of cat tibialis posterior. J. Neurophysiol. 1980;44:696–712. doi: 10.1152/jn.1980.44.4.696. [DOI] [PubMed] [Google Scholar]
- Meng H, Bentley TB, Pittman RN. Myoglobin content of hamster skeletal muscles. J. Appl. Physiol. 1993;74:2194–2197. doi: 10.1152/jappl.1993.74.5.2194. [DOI] [PubMed] [Google Scholar]
- Nishida J, Machida NW, Tagome M, Kasugai Y. Distribution of parvalbumin in specific fibre types of chicken skeletal muscles. Br. Poult. Sci. 1995;36:585–597. doi: 10.1080/00071669508417804. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry – Theoretical and Applied. Little, Brown and Co; Boston: 1961. [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian muscle fibers. Rev. Physiol. Biochem. Pharmacol. 1990;116:2–47. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Bodine-Fowler SC, Hodgson JA, Edgerton VR. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J. Physiol. 1991;444:175–192. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Hodgson JA, Edgerton VR. Level of independence of motor unit properties from neuromuscular activity. Muscle Nerve. 1994;17:1324–1335. doi: 10.1002/mus.880171112. [DOI] [PubMed] [Google Scholar]
- Rivero JL, Talmadge RJ, Edgerton VR. Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem. Cell Biol. 1999;111:277–287. doi: 10.1007/s004180050358. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hodgson JA, Lauretz S, Pierotti DJ, Gayek RJ, Edgerton VR. Chronic spinal cord injured cats: surgical procedures and management. Lab. Anim. Sci. 1992;42:335–343. [PubMed] [Google Scholar]
- Roy RR, Eldridge L, Baldwin KM, Edgerton VR. Neural influence on slow muscle properties: inactivity with and without cross-reinnervation. Muscle Nerve. 1996;19:707–714. doi: 10.1002/(SICI)1097-4598(199606)19:6<707::AID-MUS4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Roy RR, Talmadge RJ, Fox K, Lee M, Ishihara A, Edgerton VR. Modulation of MHC isoforms in functionally overloaded and exercised rat plantaris fibers. J. Appl. Physiol. 1997;83:280–290. doi: 10.1152/jappl.1997.83.1.280. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Khalili N, Kim SJ, Higuchi N, Monti RJ, Grossman E, Hodgson JA, Edgerton VR. Is spinal cord isolation a good model of muscle disuse? Muscle Nerve. 2007;35:312–321. doi: 10.1002/mus.20706. [DOI] [PubMed] [Google Scholar]
- Salmons S, Sreter FA. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976;263:30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J. Muscle Res. Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol. Scand. 2003;178:413–424. doi: 10.1046/j.1365-201X.2003.01158.x. [DOI] [PubMed] [Google Scholar]
- Staron RS, Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry. 1986;86:19–23. doi: 10.1007/BF00492341. [DOI] [PubMed] [Google Scholar]
- Staron RS, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit tibialis anterior muscle. Biochem. J. 1987;243:695–699. doi: 10.1042/bj2430695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudte HW, Pette D. Correlations between enzymes of energy-supplying metabolism as a basic pattern of organization in muscle. Comp. Biochem. Physiol. 1972;41B:533–540. doi: 10.1016/0305-0491(72)90116-2. [DOI] [PubMed] [Google Scholar]
- Stephenson GM. Hybrid skeletal muscle fibres: a rare or common phenomenon? Clin. Exp. Pharmacol. Physiol. 2001;28:692–702. doi: 10.1046/j.1440-1681.2001.03505.x. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Edgerton VR. Myosin heavy chain profile of cat soleus following chronic reduced activity or inactivity. Muscle Nerve. 1996;19:980–988. doi: 10.1002/mus.880190802. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Edgerton VR. Persistence of hybrid fibers in rat soleus after spinal cord transection. Anat. Rec. 1999;255:188–201. doi: 10.1002/(SICI)1097-0185(19990601)255:2<188::AID-AR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Torgerson WS. Theory and Methods of Scaling. John Wiley & Sons; New York: 1958. [Google Scholar]
- Weisberg A, Winegrad S, Tucker M, McClellan G. Histochemical detection of specific isozymes of myosin in rat ventricular cells. Circ. Res. 1982;51:802–809. doi: 10.1161/01.res.51.6.802. [DOI] [PubMed] [Google Scholar]
- Zheng A, Rahkila P, Vuori J, Rasi S, Takala T, Vaananen HK. Quantification of carbonic anhydrase III and myoglobin in different fiber types of human psoas muscle. Histochemistry. 1992;97:77–81. doi: 10.1007/BF00271284. [DOI] [PubMed] [Google Scholar]
- Zhong H, Roy RR, Hodgson JA, Talmadge RJ, Grossman EJ, Edgerton VR. Activity-independent neural influences on cat soleus motor unit phenotypes. Muscle Nerve. 2002;26:252–264. doi: 10.1002/mus.10190. [DOI] [PubMed] [Google Scholar]