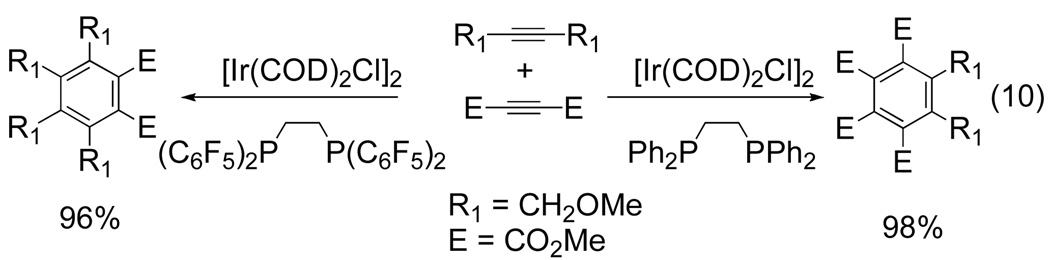Transition metal-catalyzed cycloaddition reactions allow for the rapid construction of highly functionalized molecular frameworks in one step. The use of the [2+2+2] cycloaddition has become an effective tool for the synthesis of substituted benzene ring systems.[1] There are a number of excellent procedures published that have utilized various transition metals to synthesize these targets. Recent developments in this area have focused mainly on an intermolecular approach to ring synthesis, while still maintaining the ability to control the substitution pattern of the resulting products.[1] The purpose of this Highlight is to introduce the most recent attempts to solve the lingering problem of chemoselectivity in the intermolecular [2+2+2] cycloaddition reaction in the synthesis of benzenes.
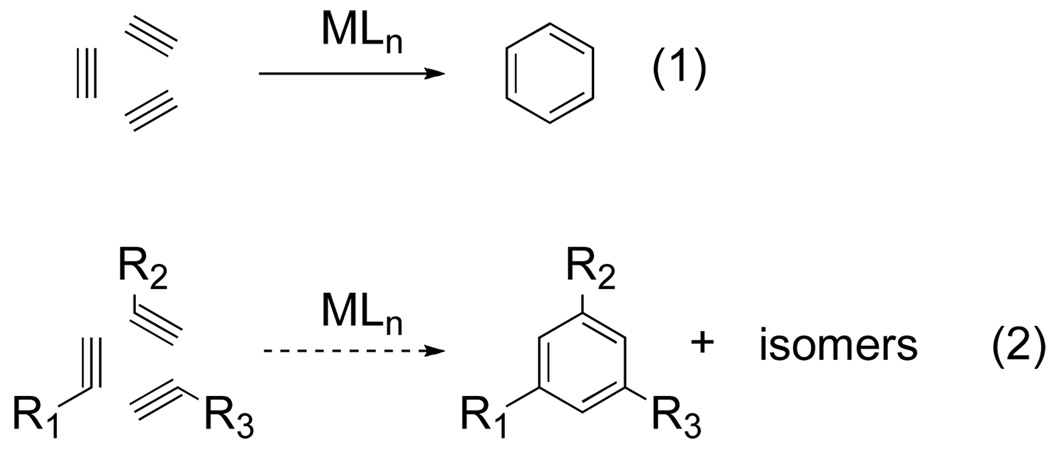
A nearly ideal synthesis of benzenoid rings involves the three component coupling of alkynes. In 1948, Reppe discovered that transition metals can catalyze the cycloaddition of alkynes into substituted benzenes (eq 1).[2] This discovery led to a paradigm shift in benzenoid ring synthesis, moving beyond Friedel-Crafts approaches of derivatizing existing aromatics.[1b] However, metal-catalyzed benzene synthesis was inherently limited to alkyne trimerization; attempts at heterotrimerization (use of two or more different alkynes) led to complex mixtures (eq 2).[1d,2]
Figure 1.
Conceptual approach to benzene synthesis
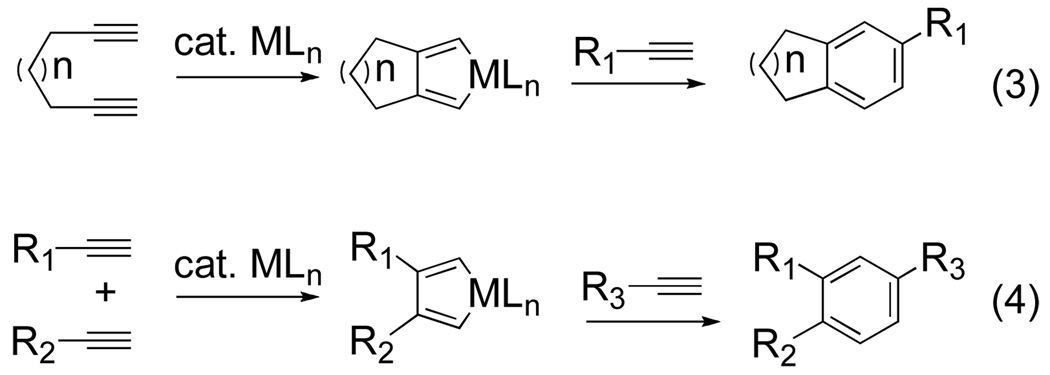
Construction of substituted benzenes is problematic due to the difficulty in controlling the chemoselectivity during the initial metallacycle formation and subsequent regioselective insertion of the third alkyne.[1c,3] The most common strategy used to overcome this limitation has relied on tethering two of the alkyne components (eq 3). Metallacycle formation may be controlled by the geometric and entropic restrictions imparted by the tether. As a result, this partially intermolecular approach has been a powerful tool in assembling polycyclic frameworks from simple unsaturated precursors. Vollhardt has had considerable success forming various benzenoid systems using CpCo(CO)2 to cyclotrimerize α,ω-diynes.[4] As a result, Co-catalyzed cycloadditions have become a versatile tool in ring synthesis of complex natural products.
Figure 2.
Example of an intra- and intermolecular metal-catalyzed [2+2+2] cycloaddition
An inherent limitation of this strategy, however, is the presence of a secondary fused ring system in the resultant benzene. In order to develop a more general approach to substituted aromatics, a fundamentally different strategy needs to be advanced, one that completely eliminates the tether (eq 4).
Over the last several years, promising new approaches to substituted benzene molecules using transition-metal catalyzed [2+2+2] cycloadditions have been reported. The selective trimerization of three different alkyne components has recently been reported using stoichiometric transition-metals such as zirconium[3] and titanium.[4,5] The stoichiometric approach avoids the potential pitfall of generating multiple different metallacycles by having the third alkyne added at the end, a solution that is impractical in a catalytic system.
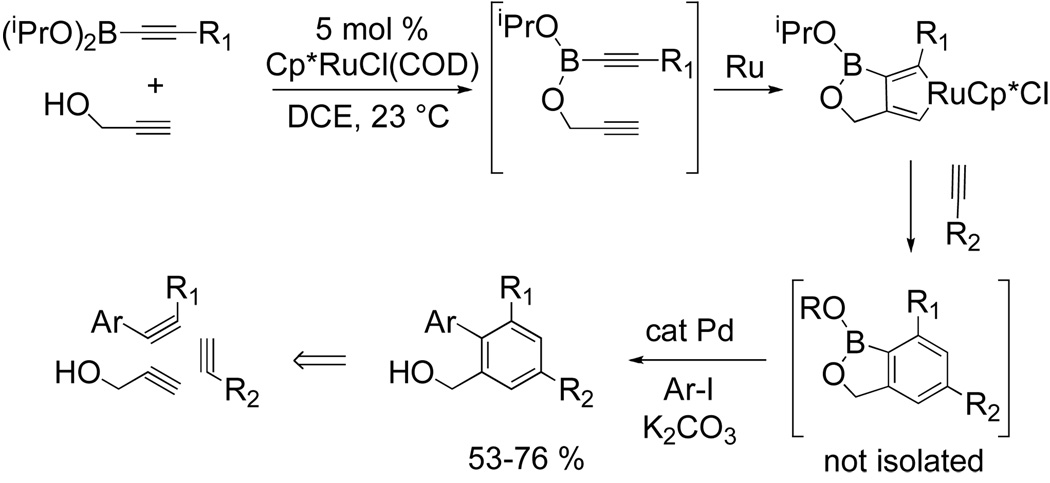
Rendering the intermolecular [2+2+2] cycloaddition reaction catalytic has been the focus in more recent literature. A unique one-pot approach for the construction of polysubstituted aromatics from three unsymmetrical alkyne components catalyzed by Cp*RuCl(COD) has been realized by Yamamoto and Itoh using a temporary boron tethering strategy.[6] This innovative, although partially intramolecular, procedure preforms a diyne intermediate in situ by reacting an alkynylboronate with propargyl alcohol which then undergoes metallacycle formation in the presence of Cp*RuCl(COD). This ruthenacyle regioselectively inserts a terminal alkyne to yield an arylboronate (Fig 3).
Figure 3.
Ru-catalyzed cyclotrimerization using a temporary boron tether
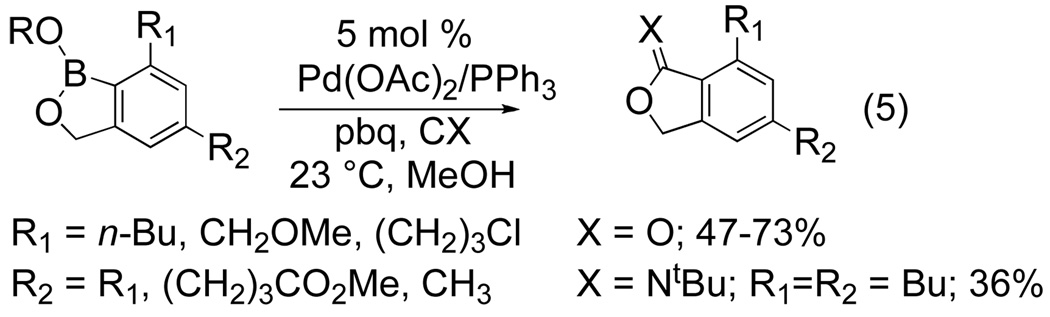
The arylboroante, though not isolable, is further functionalized using Suzuki-Miyara cross coupling to yield the biphenyl product in satisfactory yields. Importantly, this one-pot two-step protocol achieves the equivalent of a three-component [2+2+2] cycloaddition (Fig 3). A variety of terminal alkynes are well tolerated under the reaction conditions. Hetero-biaryls are also accessible when 2-iodopyridine or 2-iodothiothene is used as the coupling partner. Further elaboration of this methodology by Yamamoto and Itoh has broadened the synthetic utility of the reaction as the arylboronate is a versatile intermediate for other organic transformations. The arylboronate participates in Pd-catalyzed carbonlylation to form phthalides in varying yields in the presence of Pd(OAc)2/PPh3, p-benzoquinone, and CO (eq 5).[6b] Imidates can also be synthesized when an isocyanide is substituted for carbon monoxide. When the arylboronate derived from butynylboronate and propargyl alcohol is treated with t-butylisocyanide under the reaction conditions the imidate is isolated in low yield (eq 5).
Figure 4.
Pd-catalyzed carbonylation of the arylboronate intermediate
Yamamoto’s temporary-tether strategy described above is a truly important breakthrough, but it, along with other similar strategies,[7] nevertheless suffers from the limitation of requiring a covalent linkage. Efforts at overcoming this limitation have enjoyed some successes, and often require addressing the simpler problem of regioselective trimerization of alkynes before one examines two or more alkyne partners.
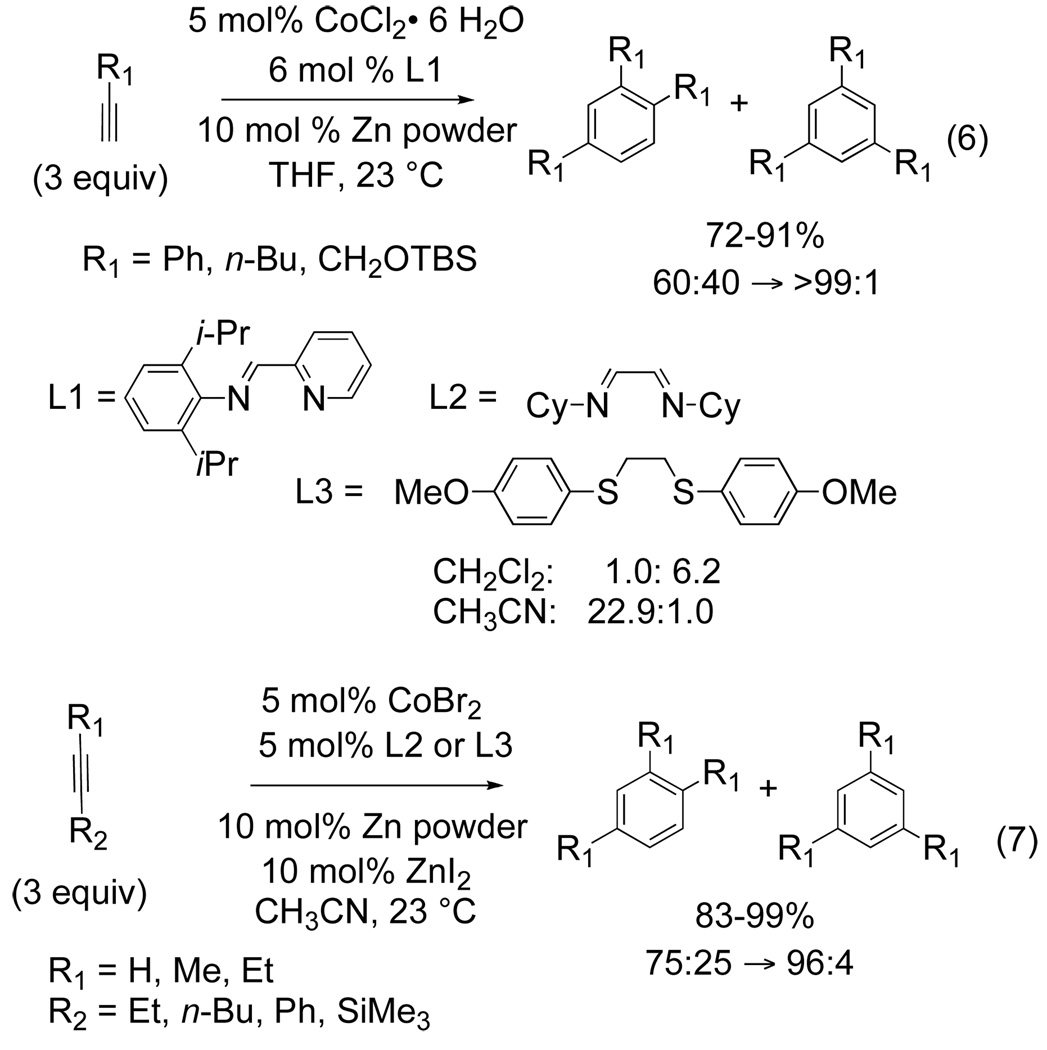
Novel catalyst systems developed by Okamoto[8] and Hilt[9] utilize cobalt salts in the presence of easily prepared or commercially available ligands to effectively cyclotimerize alkynes. Okamoto has shown that iminomethylpyridine ligands in the presence of CoCl2·6H2O/Zn catalyst system trimerize terminal alkynes to yield 1,2,4-trisubstituted benzene rings selectively in good to excellent yield (eq 6). Similar results have been achieved by Hilt when dicyclohexylimines are used as the ligand in the presence of CoBr2/Zn/ZnI2 catalyst system (eq 7).[9]
Figure 5.
Cobalt-catalyzed [2+2+2] cycloaddition
Interestingly, when 1,2-bis(4-methoxyphenyl)thioethane (L3) is used as the ligand, the choice of solvent has a profound effect on the regioselectivity of the third alkyne component.[9c] Using CH3CN as the solvent, product selectivity favors the 1,2,4-trisubstituted benzene as compared to CH2Cl2 which selectively affords the 1,3,5 isomer. It has been rationalized that the coordinating ability of the solvent influences the regioselectivity of the reaction. Although the intermolecular Co-catalyzed reactions presented are operationally simple and extremely efficient, the substrate scope appears to be limited to aliphatic and aryl-terminal alkynes. Expanding the substrate scope while maintaining the desired selectivity remains a challenge.
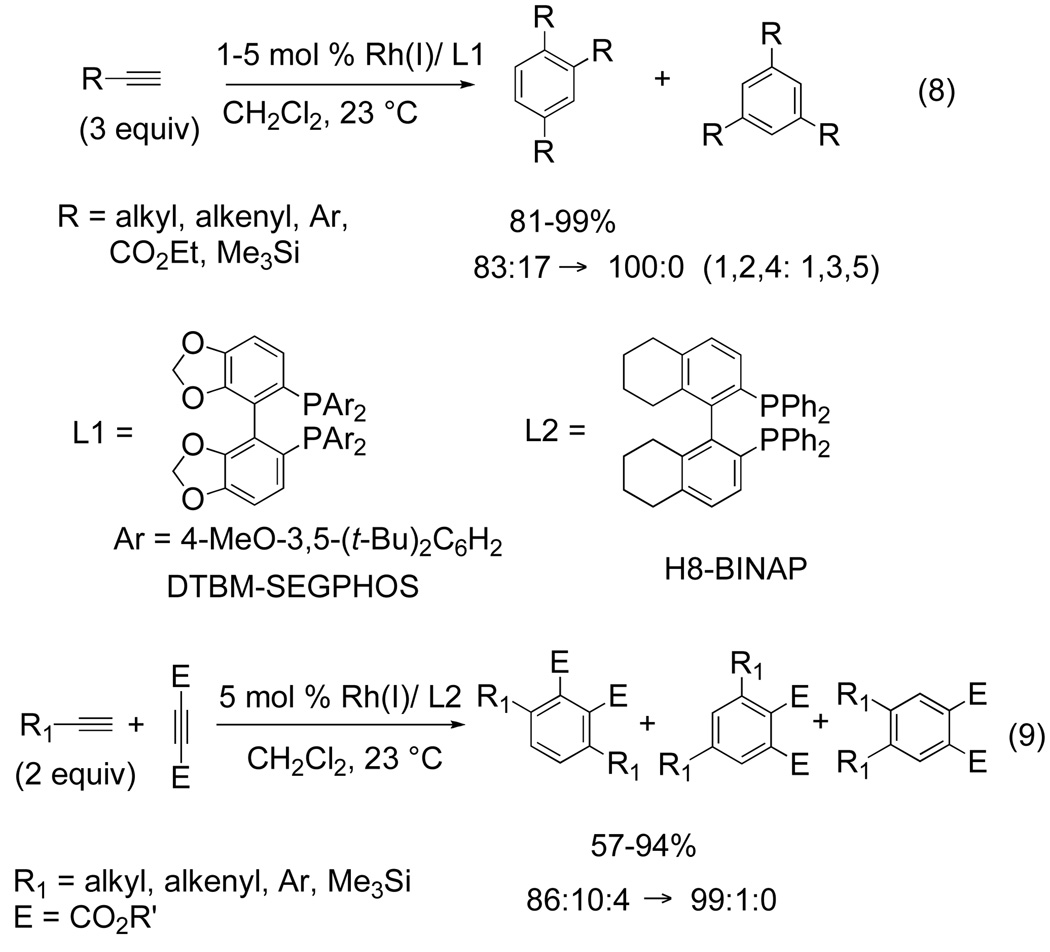
In 2003, Tanaka reported a highly regioselective intermolecular [2+2+2] homotrimerization of terminal alkynes catalyzed by a cationic Rh(I)-biaryldiphosphine complex.[1d,10] Using 5 mol % [Rh(COD)2]BF4 and DTBM-SEGPHOS as a ligand, the cyclotrimerization of 1-dodecyne or cyclohexenyl acetylene was achieved in high yield giving predominantly the 1,2,4-substituted benzene over the 1,3,5 isomer (eq 8).[10] The use of neutral rhodium(I) or cationic iridium(I) failed to yield significant amount of product. Tanaka’s attempts at facilitating an intermolecular heterotrimerization between two different alkynes relied on extreme electronic differentiation of the π-components to control initial metallacycle formation. Upon screening two different alkynes, it was found that the cationic rhodium(I)/H8-BINAP is effective in the chemo- and regioselective cycloaddition of terminal alkynes with acetylene dicarboxylates giving the 1,2,3,4-substituted benzenes in excellent yield (eq 9).
Figure 6.
Rh-catalyzed intermolecular [2+2+2] cycloaddition
It is also important to note that the reaction is tolerant of many functional groups including alkyl halides, ethers and alkenes. The selectivity of the reactions above (eqs 8 and 9) contrasts that of the work reported by Patrick in which a titanium complex, supporting a p-tert-butylcalix[4]arene ligand, yields primarily the 1,2,4-isomer in 95% isolated yield when terminal arylacetylenes or trimethylsilylacetylenes are used.[11]
Although cationic Ir(I) was not successful in the cycloadditions performed by Tanaka,[10] Takeuchi has shown that neutral iridium(I) systems are capable of facilitating the cyclization of dimethylacetylene dicarboxylate (DMAD) with terminal alkynes.[12] The choice of ligand was shown to have a profound effect on the chemoselectivity of this reaction. For example, when 1,2-bis(diphenylphosphino)ethane (dppe) is used as the ligand, two molecules of DMAD are incorporated into the product (eq 10). However, when the perfluoroaryl derivative of dppe is used as the ligand, one molecule of DMAD reacts with two molecules of the acetylene.
Figure 7.
Ligand controlled product formation of an Ir-catalyzed [2+2+2] cycloaddition
Polysubstituted benzenes are produced in high yields (up to 98%) when a variety of substituted terminal and internal alkynes are used with the dppe ligand. Regioselectivity decreases when terminal aliphatic alkynes are used with the perfluoroaryl derivative. For example, 1-hexyne gives a mixture of 1,2,4,5- and 1,2,3,5- substituted benzenes in a 64:36 ratio and 95% yield. Chemoselectivity was rationalized based on the electronics of the metal center. An electron rich iridium(I) center, arising from the coordination of dppe, would lead to more effective binding of an electron deficient alkyne such as DMAD. The use of an electron withdrawing ligand, such as the perfluoroaryl dppe, coordination of the acetylene over DMAD is preferred.
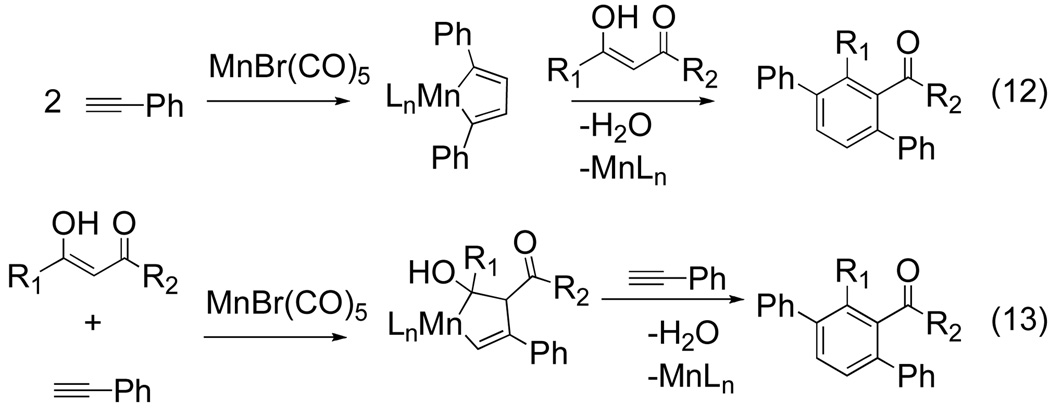
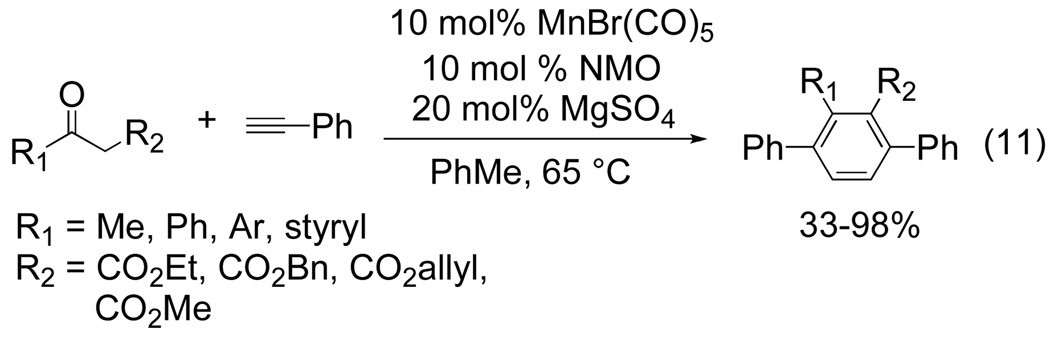
The electronic differentiation strategy between alkynes fails in cases where similar substituents are desired. For that reason, a complementary approach has recently been advanced which relies on the use of alkyne surrogates as one of the π components. In 2008, a coupling reaction of β-keto esters with terminal alkynes was reported by Nakamura and Tsuji as well as Takai and Kuninobu.[13,14] Both groups have reported the manganese-catalyzed dehydrative [2+2+2] coupling of 1,3-dicarbonyl compounds with arylacetylenes. The enol form of the β-ketoester mimics the role of an alkyne and undergoes a cycloaddition followed by dehydration to yield p-terphenyl derivatives. Using 10 mol % of MnBr(CO)5, 10 mol % NMO, and 20 mol% of MgSO4, Nakamura and Tsuji were able to successfully couple 1,3-dicarbonyls with phenylacetylene (eq 12).[13]
Figure 9.
Proposed pathway of Mn-catalyzed [2+2+2] cycloaddition of 1,3-dicarbonyls with terminal acetylenes13
The Mn-catalyzed [2+2+2] cycloaddition reaction proved to be sensitive to the steric and electronic properties of the R1 group of the 1,3-dicarbonyl. Substrates bearing a bulky substituent such as cyclohexyl or t-butyl at the R1 position failed to give product. This is most likely due to the inability of a sterically encumbering enol to coordinate to an already congested metal center. Likewise, electrondonating groups at the R1 position, such as p-methoxyphenyl, produced product in slightly lower yields. Electron-rich acetylenes were found to accelerate the rate of reaction compared to electron deficient alkynes. A reasonable argument is that electron rich alkynes coordinate more readily to the electron deficient Mn-center. Although the reaction is tolerant of acetylene functionalization, internal alkynes and o-methyl substituted phenylacetylene gave no desired product. Substrate scope of this reaction appears to be limited to aromatic terminal alkynes.
Takai and Kuninobu have also shown that the cycloaddition between β-keto esters and terminal alkynes is facile under neat reaction conditions at 80 °C in the presence of molecular sieves (4Å).[14] While the mechanism is not well understood, Takai and Kuninobu have suggested that it proceeds via one of two metallacycle intermediates (Figure 9).
In the top case (eq 12), two equivalents of alkyne undergo a cycloaddition to from manganacyclopentadiene which subsequently intercepts the enol form of the β-keto ester and upon reductive elimination and dehydration gives the desired product. Alternately, manganacyclopentadiene formation could occur via an oxidative cyclization between the 1,3-dicarbonyl and an alkyne (eq 13). A second equivalent of alkyne inserts to give the benzene adduct.
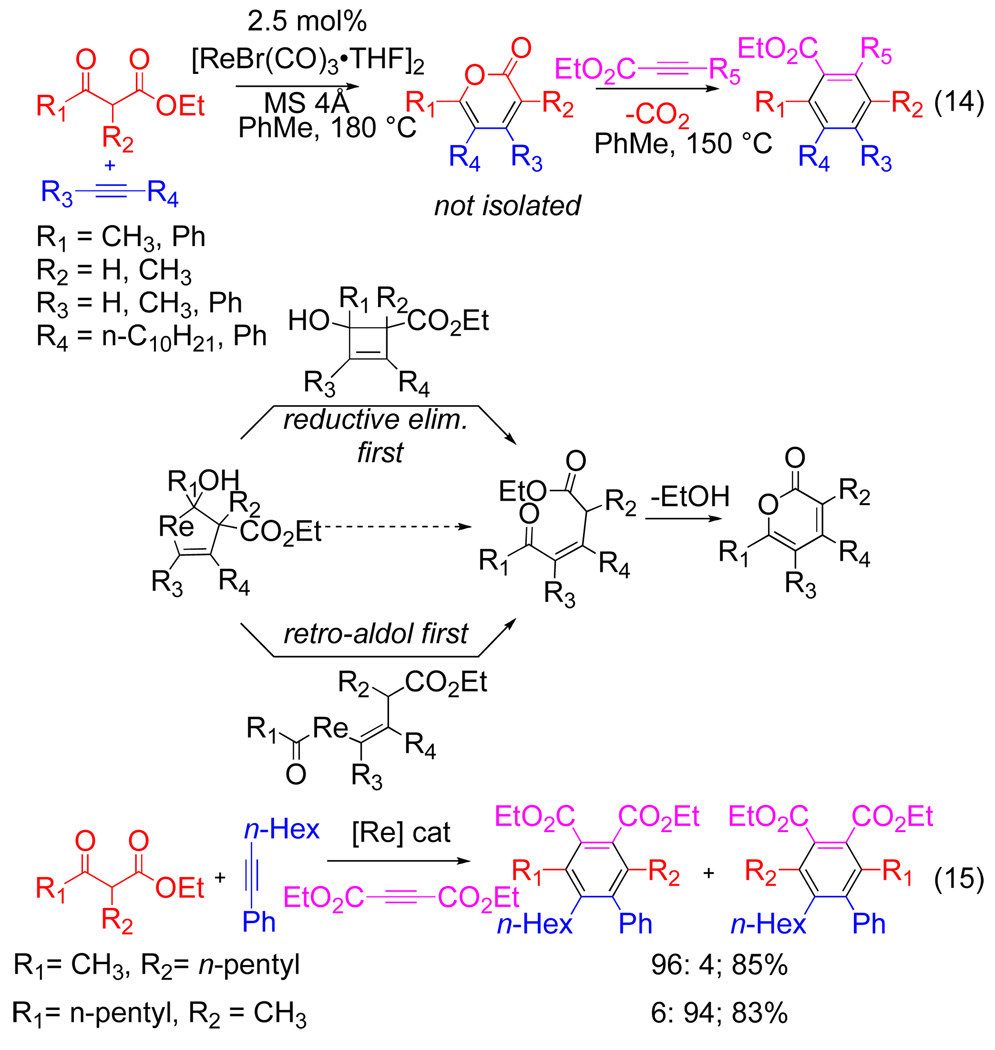
Intriguingly, the use of a rhenium catalyst in lieu of manganese with 1,3-dicarbonyls and alkynes results in the formation of pyrone adducts instead of benzenes, as recently reported by Kuninobu and Takai.[15] A subsequent addition of electron-deficient alkyne results in a [4+2]-retro-[4+2] sequence to form benzenoid rings. The overall strategy, while involving two distinct steps, introduces the equivalent of three different cycloaddition components and affords product selectively. Manipulating the alkyl substituents on the ketoester results in complementary access to either substitution pattern (eq 15).
Figure 10.
Re catalyzed pyrone synthesis/[4+2]/retro-[4+2]
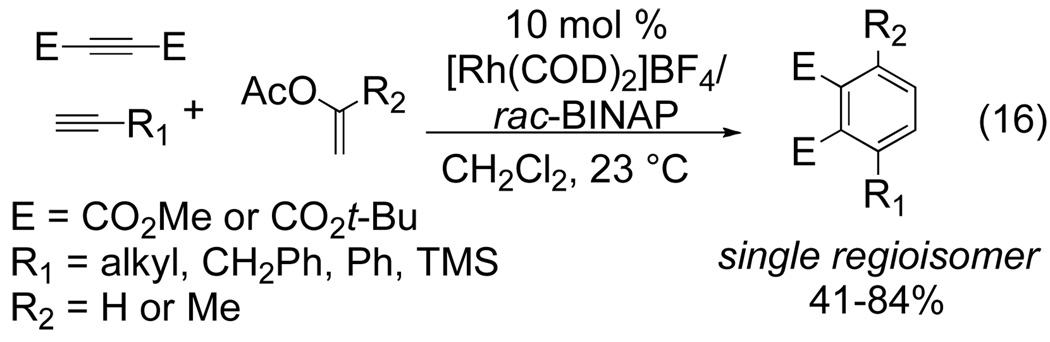
Tanaka has shown that enol acetates are competent alkyne surrogates in intermolecular rhodium-catalyzed [2+2+2] cycloadditions.[16] The use of 10 mol % of [Rh(COD)2]BF4/rac-BINAP catalyst system yields a tetrasubstituted benzene as a single regioisomer (eq 16). Importantly, Tanaka succeeds in coupling three distinct “alkynes” in this transformation.
Figure 11.
Rh-catalyzed cycloaddition using enol acetates as alkyne surrogates
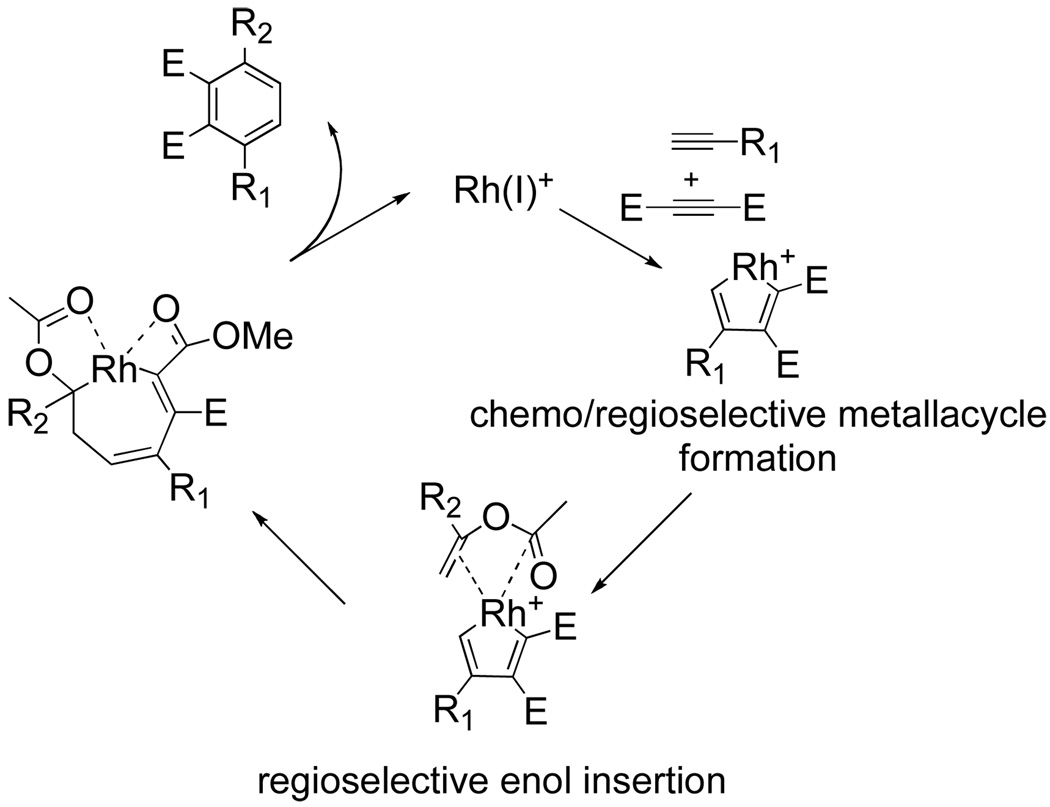
Terminal aliphatic, aryl, and silyl alkynes are well tolerated yielding benzenes in varying yields as single regioisomers. Mechanistically, it is hypothesized that the regioselectivity of the enol insertion is controlled by the coordination of the enol carbonyl moiety to the cationic Rh(I) center as illustrated in Figure 12. Insertion of the third alkyne component yields a cationic rhodacycle in which the rhodium center is stabilized by the carbonyl functionality of the enol carbonyl as well as the dicarboxylate carbonyl. Reductive elimination and aromatization by loss of acetic acid closes the catalytic cycle and affords the desired product.
Figure 12.
Cationic rhodium-catalyzed cycloaddition using enol acetate
The reports highlighted above are clear advances to more general [2+2+2] hetero-cycloadditions en route to polysubstituted benzenoid systems. That said, significant challenges still remain. Expanding the substrate scope to include more diverse alkynes and alkyne surrogates as well as solving the regioselectivity problem involving aliphatic alkynes will lead to a truly general, intermolecular [2+2+2] hetero-cyclotrimerization reaction. Although the reaction and substrate scope of each reaction presented above are still being investigated, the intermolecular approach to polysubstituted benzenes will certainly find a broader application in the synthesis of small molecules.
Figure 8.
Mn-catalyzed [2+2+2] cycloaddition of 1,3-dicarbonyls with terminal acetylenes
References
- 1.For recent reviews on the synthesis of ring systems using [2+2+2] cycloadditions see: Kotha S, Brahmachary E, Lahiri K. Eur. J. Org. Chem. 2005:4741–4767. Saito S, Yamamoto Y. Chem. Rev. 2000;100:2901–2915. doi: 10.1021/cr990281x. Chopade PR, Louie J. Adv. Synth. Catal. 2006;348:2307–2327. Tanaka K. Synlett. 2007;10:1977–1993. Shibata T, Tsuchikama K. Org. Biomol. Chem. 2008;6:1317–1323. doi: 10.1039/b720031e. Yamamoto Y. Curr. Org. Chem. 2005;9:503–1519. Heller B, Hapke M. Chem Soc. Rev. 2007;36:1085–1094. doi: 10.1039/b607877j. Varela JA, Saá C. Chem. Rev. 2003;103:3787–3801. doi: 10.1021/cr030677f. Henry GD. Tetrahedron. 2004;60:6043–6061. Agenet N, Busine O, Slowinski F, Gandon V, Aubert C, Malacria M. Org. React. 2007;68:1–302.
- 2.Reppe W, Schweckendiek WJ. Liebigs Ann. Chem. 1948;560:104–116. [Google Scholar]
- 3.Takahashi T, Xi Z, Yamazaki A, Liu Y, Nakajima K, Kotora M. J. Am. Chem. Soc. 1998;120:1672–1680. and references therein. [Google Scholar]
- 4.a) Vollhardt PC. Angew. Chem. Int. Ed. Engl. 1984;23:539–556. [Google Scholar]; b) Schore NEK. Chem. Rev. 1988;88:1081–1119. [Google Scholar]
- 5. Suzuki D, Urabe H, Sato F. J. Am. Chem. Soc. 2001;123:7925–7926. doi: 10.1021/ja0161913. For the synthesis of substituted pyridines from nitriles see: b) Suzuki D, Nobe Y, Watai Y, Tanaka R, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7474–7479. doi: 10.1021/ja0502730. Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780. doi: 10.1021/ja050261e.
- 6.a) Yamamoto Y, Ishii J, Nishiyama H, Itoh K. J. Am. Chem. Soc. 2004;126:3712–3713. doi: 10.1021/ja049673y. [DOI] [PubMed] [Google Scholar]; b) Yamamoto Y, Ishii J, Nishiyama H, Itoh K. J. Am. Chem. Soc. 2005;127:9625–9631. doi: 10.1021/ja051377d. [DOI] [PubMed] [Google Scholar]
- 7.Chang H-T, Jeganmohan M, Cheng C-H. Chem. Commun. 2005:4955. doi: 10.1039/b509731b. [DOI] [PubMed] [Google Scholar]
- 8. Saino N, Amemiya F, Tanabe E, Kase K, Okamoto S. Org. Lett. 2006;8:1439–1442. doi: 10.1021/ol0602295. Goswami A, Ito T, Okamoto S. Adv. Synth. Catal. 2007;349:2368–2374. For an intramolecular [2+2+2] cyclization utilizing Co or Fe catalysts and N-Heterocyclic carbenes as ligands see: c) Saino N, Kogure D, Okamoto S. Org. Lett. 2005;7:3065–3067. doi: 10.1021/ol051048q. For Co-catalyzed cycloaddition reactions of terminal alkynes in ionic liquids see: d) Lombardo M, Pasi F, Trombini C, Seddon K, Pitner W. Green Chem. 2007;9:321–322.
- 9.a) Hilt G, Volger T, Hess W, Galbiati F. Chem. Commun. 2005:1474–1475. doi: 10.1039/b417832g. [DOI] [PubMed] [Google Scholar]; b) Hilt G, Hess W, Volger T, Hengst C. J. Organomet. Chem. 2005;690:5170–5181. [Google Scholar]; c) Hilt G, Hengst C, Hess W. Eur. J. Org. Chem. 2008:2293–2297. [Google Scholar]
- 10.a) Tanaka K, Shirasaka K. Org. Lett. 2003;5:4697–4699. doi: 10.1021/ol035963s. [DOI] [PubMed] [Google Scholar]; b) Tanaka K, Toyoda K, Wada A, Shirasaka K, Hirano M. Chem. Eur. J. 2005;11:1145–1156. doi: 10.1002/chem.200401017. [DOI] [PubMed] [Google Scholar]
- 11.Ozerov OV, Ladipo FT, Patrick BO. J. Am. Chem. Soc. 1999;121:7941–7942. [Google Scholar]
- 12.Takeuchi R, Nakaya Y. Org. Lett. 2003;5:3659–3662. doi: 10.1021/ol035330d. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Yamagata K, Fujimoto T, Nakamura E. J. Am. Chem. Soc. 2008;130:7792–7793. doi: 10.1021/ja8015186. [DOI] [PubMed] [Google Scholar]
- 14.Kuninobu Y, Nishi M, Yudha S, Takai K. Org. Lett. 2008;10:3009–3011. doi: 10.1021/ol800969h. [DOI] [PubMed] [Google Scholar]
- 15.Kuninobu Y, Takata H, Kawata A, Takai K. Org. Lett. 2008;10:3133–3135. doi: 10.1021/ol801226t. [DOI] [PubMed] [Google Scholar]
- 16.Hara H, Hirano M, Tanaka K. Org. Lett. 2008;10:2537–2540. doi: 10.1021/ol800813g. [DOI] [PubMed] [Google Scholar]