Abstract
The question whether tumorigenic cancer stem cells exist in human melanomas has arisen recently1. Here we show that in melanomas, tumor stem cells (MTSC) can be isolated prospectively as a highly enriched CD271+ MTSC population using a process that maximizes viable cell transplantation1,6. In this study the tumors sampled were taken from a broad spectrum of sites and stages. High viability FACS isolated cells resuspended in a matrigel vehicle were implanted into T, B, and NK deficient Rag2−/− γc−/− mice (RG) mice. The CD271+ subset of cells was the tumor initiating population in 9/10 melanomas tested. Transplantation of isolated melanoma cells into engrafted human skin or bone in RG mice resulted in melanoma from CD271+ but not CD271− cells. We also showed that tumors transplanted by CD271+ patient cells were capable of metastasis in-vivo. Importantly, CD271+ melanoma cells lacked expression of TYR, MART and MAGE in 86%, 69% and 68% of melanoma patients respectively suggesting why T cell therapies directed at these antigens usually result in only temporary tumor shrinkage.
Cancers derive by clonal progression to appear as abnormal growths. At diagnosis, they can be at a stage ranging from low risk of metastasis and likely cure, to highly aggressive with a marked tendency for metastasis. We proposed that at early stages, the self-renewing, minority tumorigenic population can differentiate nonmalignant progeny, and at later stages the self-renewing cancer cell population may become the dominant population in a tumor2-4. Identifications of cancer stem cells (CSCs) in solid tumors3, 5-9 provided evidence that CSC appear to recapitulate the developmental program of corresponding normal tissue stem or progenitor cells, although in an incomplete and disorganized manner10. Malignant melanomas, like normal melanocytes, derive from the neural crest lineage11. The prospective isolation of mammalian neural crest stem cells was achieved by sorting for the CD271 cell subset12. Expression of CD271 has been found on a number of human neural crest derived tissues and in some human cancers including melanomas13, 14. Therefore, we searched for melanoma tumor initiating cells by testing surgical patient samples with monoclonal antibody (Mab) to CD271 as well as with Mabs to other cell surface antigens involved in maturation of melanocytes and/or melanoma development (Supplementary Table 1).
Melanomas can quickly progress from localized cutaneous disease to regional lymph node and more advanced visceral metastasis. A broad spectrum of freshly resected melanomas that included primary cutaneous lesions as well as nodal, in-transit, and cutaneous metastasis (Supplementary Table 2) were used to profile expression of CD271 and other candidate MTSC markers by FACS. After analyzing multiple samples, we found that CD271 was the most reliable cell surface molecule in distinguishing heterogeneous populations within melanomas (Supplementary Table 1). CD271 was found to be heterogeneously expressed in 9 out of 10 melanomas analyzed comprising from ~2.5% to ~41% (mean=16.7%) of the total cell population (Supplementary Fig. 1).
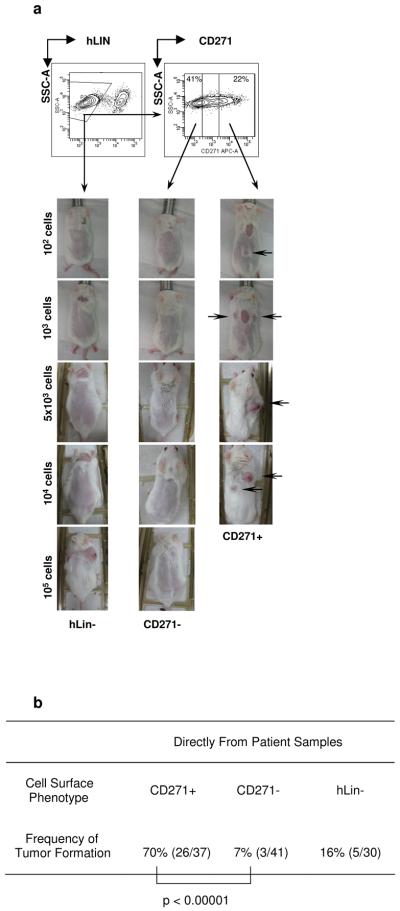
In order to assess the presence of MTSC and to avoid factors which could allow selection of the most aggressive tumor subsets during passaging, our cells were isolated directly from surgical patient samples and transplanted using the recently reported melanoma in-vivo transplantation assay1 (see Methods and Supplemental Section). Strikingly, we found that the CD271+ cell population isolated directly from six different patients transplanted melanomas in RG mice at a dramatically higher rate as compared to CD271− or Lin- (bulk population) cells obtained from the same tumor (Fig. 1), (Supplementary Fig. 2-4; Supplementary Table 3). In doses ranging from 10 to 105 cells, CD271+ cells engrafted in 70% (26/37) of the transplants compared to 7% (3/41) of CD271− (p < 0.0001) and 16% (5/30) of Lin- cells (p < 0.0001). Some melanomas were too small for direct analysis, and in these cases pieces of freshly resected tumors were transplanted subcutaneously onto the back of RG mice (Xeno P0, n=3). Alternatively, xenografts were established from cells amplified in-vitro for 1-2 passages (Xeno Pi, n=2) after being isolated from patient tumors. CD271+ expression in xenografted tumors varied from 6.4% to 75.3% (mean=26.3%) of the total cell population (Supplementary Fig. 5). The CD271+ population isolated from xenografted tumors engrafted growing melanomas in 72% of CD271+ cell injections (26/36) compared to 20% tumor engraftment from CD271− cells (5/25) (p=0.0001) (Table 1; Supplementary Fig. 6a, 7-9).
Figure 1. Isolation of melanoma tumor stem cells (MTSC) expressing CD271P75(NGFR) from melanoma patients.
a, Representative contour plot FACS gating sequence leading to purification of live, hLin- (CD45−/CD2−/CD3−/ CD31−), CD271+ and C271− cells from Mel114 patient sample; CD271+ but not CD271− melanoma cells induce tumors upon intradermal injection in 30% matrigel into B-, T- and NK cell deficient Rag2−/− γc−/− (RG) mice after 28-32 weeks. b, Summary Table of tumor formation frequencies by CD271+ and CD271− human melanoma cells isolated from all patients.
Table 1.
Summary of engraftments from xenografted tumors
| Xenografted Samples (P0, Pi) | ||
|---|---|---|
| Cell Surface Phenotype | CD271+ | CD271− |
| Frequency of Tumor Formation | 72% (26/36) | 20% (5/25) |
|
| ||
| p = 0.0001 | ||
RG mice were injected with live, mLin− (H2−Kd−/mCD45−/mTer119−) CD271+ and CD271− melanoma cells isolated by FACS and mixed with matrigel. Numbers indicate ratio of tumor incidence relative to the number of injections.
Further, we wished to determine whether newly identified MTSCs were capable of self-renewal and differentiation in-vivo. First, we analyzed engrafted melanomas derived from purified CD271+ cells. All but one tumor redeveloped both CD271+ and CD271− cell populations with very similar proportions of positive and negative cells as compared to the cancer samples from which they were initially purified (Supplementary Fig. 10). We tested whether the CD271+ and/or the CD271− cells from xenografts representing two different patients were able to secondarily transplant melanoma in-vivo; 72.2% (13/18) of the samples of CD271+ cells engrafted growing melanomas, compared to 27.7% (5/18) for CD271− cells (p=0.009), in cell doses ranging from 10 to 5×103 injected cells (Supplementary Fig. 6b). Altogether CD271+ melanoma cells isolated from xenografted samples engrafted at 72% (39/54) compared to 23% (10/43) for CD271− cells (p<0.0001) (Table 3) that required higher cell doses and longer latency.
In summary, our tumorigenic assays with surgical and xeno transplanted melanomas described above provide strong evidence that in most patients MTSCs reside in the CD271+ fraction, which are able not only to induce tumors but also to re-establish the original CD271 expression heterogeneity of the primary tumor. It is important to note however, that there was an increase in combined engraftment efficiency of CD271− cells isolated from xenopassaged melanomas compared to the combined engraftment frequency of CD271− cells isolated from surgical samples (Table 3). In addition, in our experiments with established melanoma cell lines we observed that unfractionated cells were able to engraft at 100% frequencies from as little as 10 cells (Supplementary Fig. 11). These results, suggest that melanoma cells kept for prolonged periods of time in-vitro as cell cultures or in-vivo as passaged xenografts continue to undergo malignant progression, such that successful subclones emerge, in some samples independent of their cell surface immunophenotype. Additional experiments would be required to understand precise molecular mechanisms underlying tumorigenic evolutions of cells during their passaging in-vitro or in-vivo, and their relationship to the patient's tumor.
Further we wished to determine whether MTSCs can be identified in the context of more physiologically relevant human tissue micro-environment. We therefore created humanized mice by grafting fragments of normal human skin or bone onto the back of RG and NSG mice (see supplementary section). CD271+ but not CD271− cells isolated from two independent primary dermal melanomas (Mel43 and Mel826) induced tumors in the human skin grafts (Fig. 2a-b). Similarly, CD271+ cells but not CD271− cells isolated from melanoma adjacent to the patella (Mel 210) formed a tumor in NSG mouse grafted with human bone fragment (Fig. 2c).
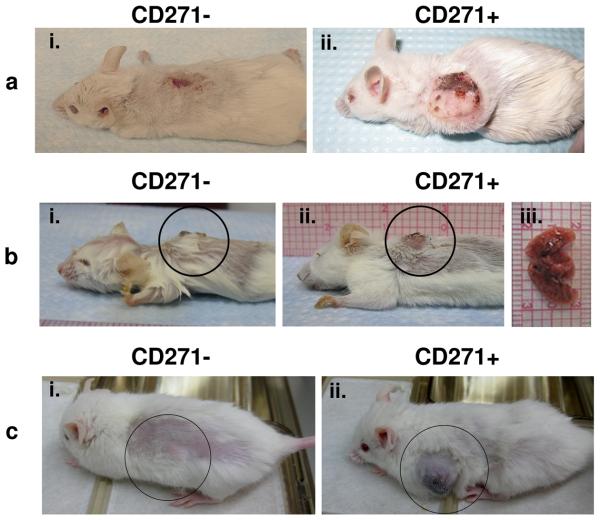
Figure 2. MTSCs induce tumors in humanized mouse models.
a, Humanized RG mice that contained skin grafts from the same healthy donor were used to assess the tumorigenic potential of CD271+ cells. Dermal melanoma xenograft (P0) from a primary patient (Mel43) was used to purify live, mLin-, CD271+ and CD271− melanoma cells by FACS and injected into separate human skin grafts on RG mice from the same healthy donor. (i) human skin graft of RG mice 28 weeks after injection of 2×104 CD271− cells and (ii) melanoma formation in human skin graft of RG mice 28 weeks after injection of 2×104 of CD271+ cells; b, Surgically resected primary melanoma from mel826 patient was used to isolate live, hLin-, CD271+ and CD271− cells by FACS and injected into separate human skin grafts on RG mice from the same healthy donor; (i) human skin graft 16 weeks after injection of 2×104 CD271− cells; (ii)-(iii) melanoma tumor formation and lung metastasis 16 weeks after injection of 6×103 CD271+ cells; c, Surgically resected melanoma adjacent to the patella from the patient mel210 was used to purify live, hLin-, CD271+ and CD271− melanoma cells by FACS; 103 cells of each highly purified fraction were injected separately into two NSG mice subcutaneously near the grafted human bone fragment (i) human bone graft of NSG mice 20 weeks after injection of 103 CD271− cells and (ii) 103 CD271+ cells.
The poor survival rate of melanoma patients diagnosed with advanced stages is due to the high metastatic potential of this cancer that rapidly invades lungs, liver, brain and other organs. Organ analysis of the humanized mouse injected intradermaly with Mel826 CD271+ patient's cells revealed that these cells had metastasized into the lungs and caused formation of metastatic nodules (Fig. 2b). In-vivo intradermal tumorigenic assays of melanomas surgically removed from two additional patients Mel1119 and Mel213 have demonstrated that tumors formed by CD271+ cells had the ability to form metastases in the liver (Supplementary Fig. 2b) and lung (Supplementary Fig. 4a-b), while no tumors nor metastases were observed in matching mice injected with CD271− cells isolated from the same patients. These results show that CD271+ MTSCs give rise to cell population in the tumor capable of metastasis. Expression of CD271 in primary melanomas has been previously associated with perineural invasivion15; in addition, other groups have shown that CD271 and its ligand NGF can regulate invasive properties of metastatic melanoma cell lines in-vitro16, 17. Further studies will be required to elucidate precise molecular mechanisms driving metastatic progression of CD271+ cells in-vivo.
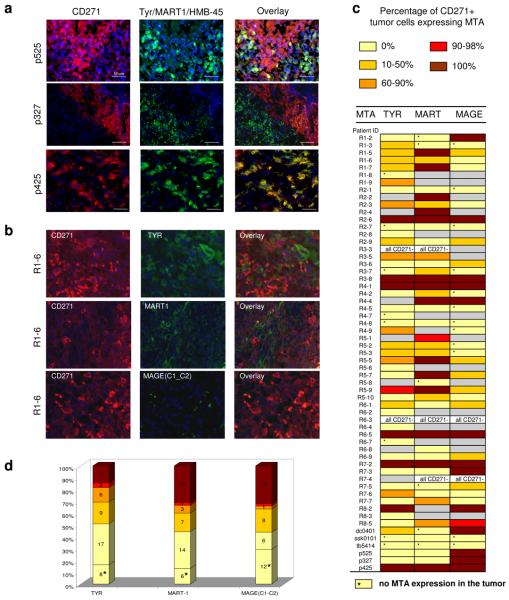
Pioneering studies of T cell immunity to melanoma antigens18, 19 have led to immunotherapy trials20. Multiple immunotherapies based on the well defined melanoma tumor antigens (MTAs) such as Tyrosinase, MART/MELAN-A and other have had limited success in melanoma patients21, 22. Our IHC expression analysis of Mel327, Mel525 and Mel425 tumor sections with CD271 Mab and with MTA Mab cocktail (Tyr/MART/HMB-45) revealed that the CD271+ cells driving melanoma progression lack expression of those markers (Fig. 3a). At the same time MAGE family proteins (MAGE C1, C2) were found to be expressed on a higher fraction of CD271+ cells (Supplementary Fig. 14) of the same patients. Next, we used melanoma tissue arrays to individually assess expression of MTAs (TYR, MART1, MAGE (C1-C2)) and CD271 in multiple melanoma patients. In 86% (42/49), 69% (31/45) and 68% (28/41) of melanoma patients CD271+ tumor cells either completely or partially lacked expression of TYR, MART1 and MAGE(C1-C2) respectively (Fig. 3b-d; Supplementary Fig. 15-27). These results uncover possible limitations of existing melanoma immunotherapies, and can be used to argue in favour of new approaches that will be directed at all MTSCs in a tumor, as well as their progeny.
Figure 3. Most melanomas contain CD271+ tumor cells that either completely or partially lack expression of MTAs.
a. Immunofluorescent analysis of CD271, Tyr/MART1/HMB-45 expression in tissue sections of melanoma patients Mel525, Mel327 and Mel 425; b, Representative images of immunofluorescent analysis of CD271, TYR, MART1 and MAGE (C1-C2) expression in tissue cores of melanoma patient from tissue array. c, Table indicating expression of melanoma tumor antigens (MTAs) TYR, MART1 and MAGE C1-C2 in CD271+ cells of each patient's tumor. Colour bars indicate percentage range of CD271+ cells that expressed MTA; grey bars indicate that expression of CD271 and MTA was not detected during analysis of the tumor core. d, Stacked bar graph indicating proportion of melanoma patients with ranges of MTA positivity of CD271+ cells.
In this study, the neural crest stem cell marker, CD271, was implicated as a cancer stem cell marker, allowing identification and prospective isolation of melanoma cancer stem cells. Previous studies on human melanoma have found several candidate markers that unequivocally identify within the tumor a cancer stem cell population23, 24. A recent report1 using principally metastatic melanoma cells isolated either from patients lymph nodes or from tumors passaged by serial xenografts in mice revealed frequencies of ~1 in 2 to ~1 in 8 cells that are tumor initiating. That study has been popularly characterized as showing that in melanoma there are no cancer stem cells and that in fact, all cells in a cancer may be equally tumorigenic25-27. Our data based on transplantation of a broad spectrum of both primary and advanced stage melanomas contradicts those conclusions. Importantly, differences in MTSC frequencies are not due to the mouse strains used (RG vs. NSG) as revealed in our direct comparison of their tumor cell engraftment sensitivity (Supplementary Fig. 13). In the studies of hematopoietic cancers we and others found that a continuous selection of more malignant cell subsets occurs28, 29 and that it might be triggered by oncogenic mutations affecting early stages of HSC differentiation30. This opens the possibility that during cancer progression the entire tumor may be made up of less and of more aggressive malignant clones. The extreme of this view is that some subclones may emerge (either as a result of passaging or disease progression) that largely or completely fail to differentiate non-tumorigenic subsets. This view could reconcile the data presented here with a previous report1. Melanomas are intrinsically extremely aggressive tumors and can quickly undergo tumorigenic evolution towards more malignant stages. Our data is consistent with the possibility that some metastatic melanomas may have very high frequencies of tumorigenic cells (for example in Mel415 Supplementary Fig. 12) similar to the rare outlier cases in other cancers3. The most crucial test of the TSC hypothesis is that markers or pathways restricted to TSCs can be targets for curative therapies in the patient, which has not yet been done. Conversely, therapies targeting markers on the progeny of TSCs, but not on TSCs themselves, should be less efficient. MTAs (TYR, MART-1, MAGE C1-C2) used during induced or adoptive T cell immune responses are expressed at high frequencies on CD271− cells, (Supplementary Fig. 28) but are either completely or partially lacking on the CD271+ cells of the significant proportion of melanoma patients analyzed. Existing immunotherapies fail to completely eradicate melanomas19, 20, perhaps because all MTSCs also need to be eliminated. Identification of MTSCs by their cell surface immunophenotype may allow patient by patient selection for more efficient immunotherapies and/or drugs to be tested in clinical trials.
Methods Summary
Tumor tissues were digested into single cell suspension as previously described6, 7, 9. Tumor cell suspensions were stained with anti human CD271 antibodies directly conjugated to Biotin (557195 BD Pharmingen) or Alexa Fluor 647 (560326 BD Pharmingen) and a human lineage cocktail of Mabs directly or custom conjugated to pacific blue: anti-CD45 (4528 Invitrogen), CD2 (555324 BD Pharmingen), CD3 555329 BD Pharmingen), CD31 (303114 Biolegend); in case of xenografted tumors the following antibodies to mouse lineage markers were used: H2Kd (553565 BD PharMingen), CD45.2 (109820 Biolegend) and mTer119 (557915 BD PharMingen) directly conjugated to pacific blue or FITC. All antibodies were used in a 1:50 dilution except H2Kd (1:100) to allow separation of mouse cells. Propidium iodide was used to exclude nonviable cells. Flow cytometry analysis and cell sorting was performed on a BD FACSAria (Becton Dickinson) under 20 psi with a 100-μm nozzle.
During FACS isolation of candidate MTSC populations we adopted the strategy of setting up negative and positive sort gates at least one log apart to prevent cross-contamination between negative and positive cell fractions that might occur when low expressing positive cells cannot be distinguished from negative cells with a high degree of probability. FACS-sorted tumor cells were counted in hemocytometer (Hausser Scientific, Horsham, PA) and graded numbers of cells were suspended in a volume of 50 μl of media containing 30% Standard Matrigel (354234 BD Pharmingen, Franklin Lakes, NJ). Suspension was then injected by 31-gauge insulin syringes (Becton Dickinson) intradermaly on the flank of B-, T- and NK cell deficient 4 to 8 weeks old RG mice anesthetized with isoflurane-O2.
Methods
Tumor specimens were provided by SU hospital after obtaining the informed consent. Experiments were conducted on Rag2−/γc− DKO mice in accordance with guidelines established by the SU Administrative Panels for Lab Animal Care.
Primary Tumor Implantation
RG mice were anesthetized with isoflurane-O2 and small pieces (<2 mm) of fresh tumor were implanted on both sides of the flank. The incision was sealed with surgical staple.
Tumor Digestion
Tumors were minced with a razor blade, and then placed in a solution of liberase blendzymes 2 and 4 (Roche) in Media 199 (Invitrogen) at 37°C for up to 3h to allow complete cell dissociation with pipetting every 30 min. Cells were filtered through 40μm nylon mesh, treated with LCK buffer to eliminate erythrocytes and washed twice with HBSS/2% Heat Inactivated Calf Serum (HICS). Cells were stained for flow cytometry or injected into mice as whole-tumor single-cell suspensions.
Analysis and Cell Separation by Flow Cytometry
The single-cell suspensions were washed in HBSS/2% HICS and counted and then resuspended in 100 μl per 106 cells of HBSS and incubated with 1 mg/ml Sandoglobin for 10 min. The suspensions were then washed with HBSS/2% HICS, resuspended in 100 μl per 106 cells of HBSS, and stained with antibodies. Anti-CD271 (biotin-, or Alexa Fluor647 -conjugated, BD Pharmingen), at a 1:50 dilution; lineage markers diluted at 1:50 anti-CD45, CD2, CD3, CD31 directly or custom conjugated to pacific blue were used to allow identification of contaminating nontumor cells from patient samples. Tumors that had been passaged in the mouse were incubated with anti-H2kd (diluted 1:100; BD Pharmingen) anti-mCD45.2 and mTer119. Antibodies were directly conjugated to Pacific Blue or FITC, Stained cells were washed and resuspended at 0.5 ml per 106 cells with Hoechst 33342 or propidium iodide to allow exclusion of nonviable cells. Flow cytometry analysis and cell sorting was performed on a BD FACSAria (Becton Dickinson) under 20 psi with a 100-μm nozzle.
Single-Cell Suspension Injections
FACS-sorted tumor cells were counted in hemocytometer (Hausser Scientific, Horsham, PA) and graded numbers of cells were suspended in a volume of 50 μl of media containing 30% standard Matrigel (354234 BD Pharmingen, Franklin Lakes, NJ). Suspension was then injected by 31-gauge insulin syringes (Becton Dickinson) intradermaly on the flank of B-, T- and NK cell deficient 4 to 8 weeks old RG mice anesthetized with isoflurane-O2.
Humanized Mice Models
Generation of RG mice with grafted human skin: briefly, full thickness human skin sample was obtained under signed consent from patients undergoing breast reduction, abdominoplasty or face lift. Adult mice were anesthetized by inhalation of 2% isoflurane in 100% oxygen at a flow rate of 2L per minute. The dorsum of the mouse was shaved with an electric clipper and then treated with a depilatory agent to completely remove the hair. Following sterile prep, a rectangular area of approximately 1 – 1.5 cm by 1 – 1.5 cm of skin was resected leaving the panniculus carnosus intact. Human skin graft was sutured in place with 6-0 prolene and covered with a non-adhesive dry dressing. Mice are then allowed to recover from anesthesia in a warm chamber then housed individually in separate cages.
Generation of NSG mice with grafted human bone: briefly, human abortuses' bones were obtained from Advanced Bioscience Resources, Inc. Adult mice are anesthetized by inhalation of isoflurane. 2% isoflurane in 100% oxygen at a flow rate of 2L per minute will be used. A small patch of fur is shaved, and a small (2mm) incision is made in the skin. A pair of blunt forceps is used to generate a small pocket underneath the skin and a small (5 mm) piece of aborted long bone is inserted into the pocket. The incision is then closed with a single wound clip. A 10 microliter volume of 2% Iidocaine:1/100000 epinephrine is applied to the wound site, which provides analgesic for a period of 2 hours. Mice are then allowed to recover from anesthesia in a warm chamber then housed individually in separated cage.
Tissue Immunofluorescence
A small piece of tumor specimen was kept aside and frozen in optimal cutting temperature (OCT) embedding media. Seven-micron sections were cut, fixed in ice-cold acetone for 4 min, and air-dried. Slides were then rinsed in PBS, and blocked in PBS with 1% BSA, 5% goat serum (for extracellular antigens) and 1% BSA, 5% goat serum and 0.01% triton-X100 (for intracellular antigens) for 30 min. Primary antibodies used: rat anti- human CD271 (27005 Abcam), Pan Melanoma cocktail (mixture of mouse anti- human Tyrosinase, -MART1 and -HMB-45 antibodies) (CM165B Biocare Medical) and -MAGE(C1-C2) a kind gift of Leonard Cohen Lab LICR New York Branch. Sections were incubated with the primary antibody diluted in blocking solution overnight at +4°C, washed in PBS, followed by secondary antibodies (goat anti-rat AlexaFluor 594 and goat anti-mouse AlexaFluor 488 (Molecular Probes)) incubation for 1h at RT. Slides were again washed, incubated with Hoechst 33342 (Invitrogen, Carlsbad, CA) for 3 min, rinsed in PBS, and coverslipped with Fluoromount G (Southern Biotech, Birmingham, AL). Staining was analyzed under LeicaDM4000B microscope. Pictures were taken under 40x objective and scale bars are equal to 50um
Melanoma Tissue Array Immunostaining
Paraffin melanoma tissue array slides (US Biomax Inc) containing 80 1.5mm tissue cores from primary and metastatic melanoma patients were deparaffinized through three changes of xylene, incubating 10 min in each change. Slides were hydrated to water by dipping them 20-30 times in each of two changes of 100% ethanol, two changes of 95% ethanol, one of 80% ethanol, one of 70 % ethanol, and two changes of distilled water. Slides were placed in microwave, covered with 50mM TRIS/20mM EDTA pH 9.0 buffer and microwaved for 15 minutes. Slides were cooled for 30 minutes, rinsed in distilled water twice and once in PBS for 5 minutes. Three tissue array slides were stained each with the following antibody combination: CD271 (abcam ab3125 R5) 1:100 & TYR (novacastra #NCL-L-TYROS) 1:20; CD271 1:100 & Mart-1 (biocare #CM077) 1:400; CD271 1:100 & MAGE (abcam ab60049) 1:50 for 45 minutes at RT. Slides were washed for 15 minutes in PBS. The following fluorescent secondary antibodies at 1:100 were used to visualize each antigen: goat anti mouse IgG1_AF594 for CD271, goat anti mouse IgG2a_AF647 for TYR, goat anti rabbit_AF647 for MAGE, goat anti mouse IgG2b_AF647 for MART1. Slides were incubated in the dark for 45 minutes. Slides were washed for 15 minutes in PBS and mounted in fluorescent mounting medium containing DAPI (ProLong antifade reagent with DAPI, Invitrogen cat#P36935). Slides were scanned and photographed at 20X using Zeiss AxioImager motorized upright fluorescent microscope using appropriate filters for each fluorochrome.
Supplementary Material
Table 2.
Comparative analysis of melanoma initiation
| Tumor Cell Source | Frequency of Tumor Formation | ||
|---|---|---|---|
| CD271+ | CD271− | ||
| Patient samples | 70% (26/37) | 7% (3/41) | p = 0.04 |
| Xenografted samples (P0,Pi,Ps) |
74% (39/53) | 23% (10/43) | |
Comparison summary table of CD271+ and CD271− melanoma cells tumor engraftment frequencies isolated directly from clinical patient samples and from xenografted tumors. p value refers to CD271− population in patient vs. xenograft samples
Acknowledgments
We thank Janet Bueno and Susan Hicks for obtaining patient consent and specimens; Kelli Montgomery for immunohistochemistry analyses; Dr. Dongkyoon Kim for NSG bone grafted mice; Drs. Keith Chan, Laurie Ailles, Chris Park for advise and help in FACS and tumor transplantation assays; Dr. Leonard Cohen for MAGE antibodies; Libuse Jerabek, Theresa Storm, and Adriane Mosley for laboratory and mouse management; Patricia Lovelace for FACS management; Dr. Andrew Olsen for help with microscopy; all members of Weissman Laboratory for experimental suggestions; Dr. Michael Clarke and members of his laboratory for critical discussions and reading. This research was initially supported in part by American Cancer Society fellowship and in part by NIH F32 CA126252 NRSA fellowship to A.D.B.; the Virginia & D.K. Ludwig Fund for Cancer Research to I.L.W.; NIH/NCRR CTSA grant UL1 RR025744 to Stanford Spectrum M.v.R.; The Oak Foundation and NIH 1RC2 DE02077-01 to M.T.L.; the Oak Foundation and Ellenburg Faculty Scholar Endowment to G.P.Y.
Footnotes
Conflict of interest statement: I.L.W. was a member of the scientific advisory board of Amgen and owns significant Amgen stock; he cofounded and consulted for Systemix; he is a cofounder and director of Stem Cells, Inc.; and cofounded Cellerant, Inc. None of these companies are in the cancer stem cell field, at least while IW was an advisor or held the stock.
References
- 1.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman I. Stem cell research: paths to cancer therapies and regenerative medicine. Jama. 2005;294:1359–66. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 5.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 6.Chan KS, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–21. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 11.Baroffio A, Dupin E, Le Douarin NM. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci U S A. 1988;85:5325–9. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–49. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 13.Chesa PG, Rettig WJ, Thomson TM, Old LJ, Melamed MR. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. J Histochem Cytochem. 1988;36:383–9. doi: 10.1177/36.4.2831267. [DOI] [PubMed] [Google Scholar]
- 14.Pietra G, et al. Natural killer cells kill human melanoma cells with characteristics of cancer stem cells. Int Immunol. 2009;21:793–801. doi: 10.1093/intimm/dxp047. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto S, Odland PB, Piepkorn M, Bothwell M. Evidence that the p75 neurotrophin receptor mediates perineural spread of desmoplastic melanoma. J Am Acad Dermatol. 1996;35:725–31. doi: 10.1016/s0190-9622(96)90728-8. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti D, Menter D, Jin L, Nakajima M, Nicolson GL. Nerve growth factor effects on human and mouse melanoma cell invasion and heparanase production. Int J Cancer. 1993;55:692–9. doi: 10.1002/ijc.2910550430. [DOI] [PubMed] [Google Scholar]
- 17.Truzzi F, et al. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J Invest Dermatol. 2008;128:2031–40. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- 18.Herin M, et al. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer. 1987;39:390–6. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- 19.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA. Development of cancer immunotherapies based on identification of the genes encoding cancer regression antigens. J Natl Cancer Inst. 1996;88:1635–44. doi: 10.1093/jnci/88.22.1635. [DOI] [PubMed] [Google Scholar]
- 21.Cormier JN, et al. Enhancement of cellular immunity in melanoma patients immunized with a peptide from MART-1/Melan A. Cancer J Sci Am. 1997;3:37–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Scheibenbogen C, et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J Immunother. 2000;23:275–81. doi: 10.1097/00002371-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Schatton T, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Porta C. Cancer stem cells: lessons from melanoma. Stem Cell Rev Rep. 2009;5:61–5. doi: 10.1007/s12015-008-9048-7. [DOI] [PubMed] [Google Scholar]
- 25.Eaves CJ. Cancer stem cells: Here, there, everywhere? Nature. 2008;456:581–2. doi: 10.1038/456581a. [DOI] [PubMed] [Google Scholar]
- 26.Passegue E, Rafii S, Herlyn M. Cancer stem cells are everywhere. Nat Med. 2009;15:23. doi: 10.1038/nm0109-23. [DOI] [PubMed] [Google Scholar]
- 27.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–7. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A. 2000;97:7521–6. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akala OO, et al. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–32. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.