Abstract
Background
The etiology of Kawasaki Disease (KD) is enigmatic, although an infectious cause is suspected. Polymorphisms in CC chemokine receptor 5 (CCR5) and/or its potent ligand CCL3L1 influence KD susceptibility in US, European and Korean populations. However, the influence of these variations on KD susceptibility, coronary artery lesions (CAL) and response to intravenous immunoglobulin (IVIG) in Japanese children, who have the highest incidence of KD, is unknown.
Methodology/Principal Findings
We used unconditional logistic regression analyses to determine the associations of the copy number of the CCL3L1 gene-containing duplication and CCR2-CCR5 haplotypes in 133 Japanese KD cases [33 with CAL and 25 with resistance to IVIG] and 312 Japanese controls without a history of KD. We observed that the deviation from the population average of four CCL3L1 copies (i.e., < or > four copies) was associated with an increased risk of KD and IVIG resistance (adjusted odds ratio (OR) = 2.25, p = 0.004 and OR = 6.26, p = 0.089, respectively). Heterozygosity for the CCR5 HHF*2 haplotype was associated with a reduced risk of both IVIG resistance (OR = 0.21, p = 0.026) and CAL development (OR = 0.44, p = 0.071).
Conclusions/Significance
The CCL3L1-CCR5 axis may play an important role in KD pathogenesis. In addition to clinical and laboratory parameters, genetic markers may also predict risk of CAL and resistance to IVIG.
Introduction
Kawasaki disease (KD) is an acute, self-limiting systemic vasculitis of infants and children [1], [2]. The most serious complication of KD is the development of coronary artery lesions (CAL) that range from transient dilatation to destruction of the vessel wall architecture resulting in aneurysms [3]. Indeed, the primary goal of KD treatment is to prevent this complication [1], [2]. There is significant inter-individual variation in KD susceptibility as well as CAL development. Moreover, although administration of a combination of a high dose intravenous immunoglobulin (IVIG) and aspirin is the standard therapy for acute KD, 15–30% of KD patients have persistent or recurrent fever after IVIG treatment [4], [5], [6], [7], [8], [9], [10]. Also, such patients are at increased risk of developing CAL [11]. Thus, identification of host factors that influence KD susceptibility, CAL development and resistance to IVIG treatment may provide new insights into KD pathogenesis, novel means for prognostication of clinical outcome, and therapeutic targets.
According to a current paradigm, KD is thought to be triggered by an infectious agent that elicits an inflammatory response directed at cardiovascular tissues in genetically susceptible hosts [1], [12], [13]. Polymorphisms in various genes have been shown to influence KD susceptibility in different populations [14], [15], [16], [17], [18], [19], [20], [21], [22]. Similarly, variations in the genes encoding CD14 [23], matrix metalloproteinase (MMP)-3 [24], vascular endothelial growth factor (VEGF) and its receptor kinase insert domain receptor (KDR) [21] have been implicated in CAL development in KD. With respect to response to IVIG, several studies have reported laboratory and demographic predictors associated with IVIG failure [6], [7], [8]. However, the generalization of scoring systems based on such predictors to multiethnic U.S. populations has not been successful [10]. The genetic basis of IVIG resistance in the setting of KD or other inflammatory, autoimmune and infectious diseases in which IVIG has been empirically used (e.g. Idiopathic thrombocytopenic purpura), including pediatric HIV and post-infectious complications [25], has not been fully elucidated.
There is evidence to suggest that recruitment of inflammatory cells in KD may be mediated through CC chemokine receptor 5 (CCR5) [15], [19], [26]. Chemotactic gradients for homing of CCR5+ cells are provided by a variety of chemokines, the most potent of which is its ligand - CC ligand 3 like 1 (CCL3L1) [27]. The genes encoding CCR5 and CCL3L1 demonstrate two distinct types of polymorphisms: single nucleotide polymorphisms in CCR5 [28] and copy number variation (CNV) in the CCL3L1-gene containing segmental duplication [29]. There is a growing interest in understanding the contribution of CNV in disease pathogenesis since it is recognized that 12% of the human genome may have undergone segmental duplications [30], [31]. We previously found that variations in CCR5 and CCL3L1 affect susceptibility to KD in parent-child trios from the United States [15].
However, there is significant variation in the prevalence of KD as well as the frequency of CCR5 genotypes and CCL3L1 copy number in different populations [15], [27], [32]. Consequently, whether the observations made in US trios can be generalized to Japanese children is unknown. To address this, we conducted a case-control study in subjects from Japan, a geographic region where the prevalence of KD is at least 10 times higher than the Western world [1], [2]. We tested the hypothesis that CCR5 haplotypes and CCL3L1 copy number influence KD susceptibility and two disease-related outcomes: development of CAL and IVIG resistance.
Materials and Methods
Ethics Statement
This study was approved by the institutional review boards of Yamaguchi and Kurume University Hospitals in Japan and the University of California San Diego and the University of Texas Health Science Center in San Antonio in the U.S. and written informed consent was given by the parents of all KD subjects and controls.
Study subjects
We conducted an unmatched case-control study of 133 cases of KD and 312 controls collected between January 2002 and April 2005. The KD patients were recruited from three sites: the Department of Pediatrics, Yamaguchi University Hospital; Oita Children's Hospital; and Kurume University Hospitals, Japan. All patients met the Japanese criteria for the diagnosis of KD [33]. CAL was defined as a luminal diameter >3 mm for patients <4 years or >4 mm for patients >5 years of age, or an internal diameter of one or more segments at least 1.5 times larger than the adjacent segment [34]. IVIG-resistant subjects were defined as KD patients who had persistent fever (≥38.0°C) for at least 36 hours after completion of the IVIG infusion and who received secondary treatment after the initial treatment with IVIG. KD patients who did not receive secondary treatment were considered to have responded to the initial IVIG treatment. The initial IVIG was administered as a single infusion of 2 g/kg/day. All KD patients also received oral aspirin (30 mg/kg/d). Controls were Japanese adults without a history of KD recruited from three centers: San Diego, CA, and Yamaguchi University and RIKEN in Tokyo, Japan. Most of the controls of Japanese origin (28% from Yamaguchi University, 60% from Riken, and 12% from San Diego) were healthy adults and some had common diseases of adulthood unrelated to KD.
Genotyping
The methods for genotyping CCR5 polymorphisms are described elsewhere [15], [27], [32]. The variations in CCR5 were categorized into haplotypes as described previously and were designated as CCR5 human haplogroups A (HHA), HHB, HHC, HHD, HHE, HHF*1, HHF*2, HHG*1, and HHG*2 [35]. The CCR5 haplotypes that bear the CCR5-Δ32 or CCR2-64I polymorphisms are designated as the CCR5 HHG*2 and HHF*2 haplotypes, respectively [32], [35] Copy number of the CCL3L1 gene-containing segmental duplication was estimated as described previously [27]. The assay used to genotype CCL3L1 copy number captures three separate CCL3L genes (CCL3L1, CCL3L2 and CCL3L3) as described previously [27].
Statistical analysis
Allele frequency and Hardy-Weinberg equilibrium for all the CCR5 haplotypes was estimated using the PowerMarker software [36]. We used unconditional multiple logistic regression analysis to evaluate the association of CCR5 haplotypes and CCL3L1 copy number with KD-related outcomes. The median number of CCL3L1 copies in the study population was 4 and for this reason the study subjects were trichotomized into those possessing <4, 4 and >4 CCL3L1 copies. In these regression analyses, we included CCR5 haplotypes (HHA, HHC, HHE, HHF*1, HHF*2 and HHG1) and CCL3L1 copy number (less than 4 and greater than 4) in the same regression model. To determine whether CCL3L1 gene copy number modified the KD-influencing effects of CCR5 haplotypes, we used the Mantel-Haenszel test of homogeneity. We used Stata 10.0 (Stata Corp, College Station, Texas) for the statistical analysis.
Results
Among the cases there were 55 (41.35%) females and 78 (58.65%) males whereas in the control group there were 190 (60.90%) females and 122 (39.10%) males. KD patients with available echocardiographic data were categorized into 2 groups according to the presence of CAL. There were 33 (27.5%) and 87 (72.5%) patients with and without CAL, respectively. Mean age of disease onset was 43.5 months (range 2–270 months). Of the 95 cases who were treated with IVIG within the first 10 days of onset of fever, 25 (26.32%) were resistant to treatment.
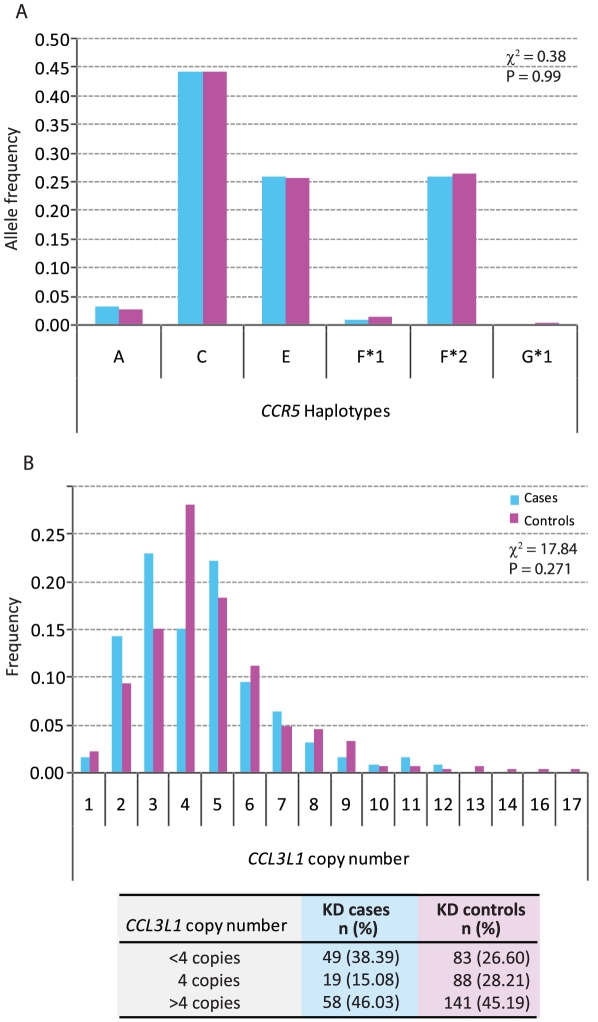
The most common CCR5 haplotype was CCR5 HHC, followed by HHF*2 and HHE (Fig. 1A). In the Japanese population the HHG*2 haplotype bearing the CCR5-Δ32 mutation is very rare. The CCR5 locus was in Hardy-Weinberg equilibrium (Exact P = 0.9808 in controls and 0.5624 in cases). The median CCL3L1 copy number in both cases and controls was four (Fig. 1B).
Figure 1. Distribution of CCR5 haplotypes and CCL3L1 copy number in cases (blue bars) and controls (purple bars).
(A) Distribution of CCR5 haplotypes and (B) Distribution of CCL3L1 copy number. The overall difference of distribution between cases and controls was tested for significance using the χ2 test. The table at the bottom of Panel B shows frequencies of CCL3L1 copy number categories in cases and controls. The categories were derived since 4 was the median copy number in the study population.
To determine whether CCR5 haplotypes or copy number of the CCL3L1 gene-containing segemental duplication was associated with an altered risk of developing KD, we first performed stepwise unconditional logistic regression analyses. We found that both possession of <4 (OR = 2.73, 95% CI = 1.49–5.03, p = 0.001) and >4 CCL3L1 copies (OR = 1.91, 95% CI = 1.06–3.41, p = 0.03) was associated with an increased risk of developing KD (Table 1, Final model). Since gender distribution in the case and control groups was different, we adjusted for this covariate, and the adjusted odds ratios indicated that possession of <4 (ORadjusted = 2.64, 95% CI = 1.42–4.88, p = 0.002) and >4 CCL3L1 copies (ORadjusted = 2.00, 95% CI = 1.11–3.61, p = 0.022) remained associated with a significantly higher risk of developing KD. Thus, departure from the population average of 4 CCL3L1 copies (i.e., either < or >4 copies) was associated with a significantly increased risk of KD before (OR = 2.21, 95% CI = 1.28–3.82, p = 0.004) and after adjustment for gender (ORadjusted = 2.25, 95% CI = 1.29–3.91, p = 0.004).
Table 1. Association of CCR5 haplotypes and CCL3L1 copy number with Kawasaki disease susceptibility.
| CCR5 haplotype/CCL3L1 copy number | OR | 95% CI | P value |
| Full Model | |||
| CCR5 HHA | 1.13 | 0.44–2.87 | 0.802 |
| CCR5 HHC | 0.70 | 0.39–1.26 | 0.236 |
| CCR5 HHE | 0.80 | 0.47–1.36 | 0.407 |
| CCR5 HHF*1 | 0.58 | 0.12–2.91 | 0.512 |
| CCR5 HHF*2 | 0.75 | 0.44–1.27 | 0.283 |
| CCL3L1 <4copies | 2.71 | 1.47–4.99 | 0.001 |
| CCL3L1 >4 copies | 1.90 | 1.06–3.42 | 0.031 |
| Final Model (Probability Criterion of 0.1) | |||
| CCL3L1 <4copies | 2.73 | 1.49–5.03 | 0.001 |
| CCL3L1 >4 copies | 1.91 | 1.06–3.41 | 0.030 |
Full model shows results from a logistic regression model including all the indicated predictors while final model indicates the results from the stepwise regression using a retention criterion of 0.1; OR, Odds Ratio; CI, Confidence Interval.
The results in Table 1 indicated that none of the CCR5 haplotypes had a significant association with the risk of KD. In previous studies, we found that the copy number of CCL3L1 modified the SLE-, Kawasaki disease-, and HIV-1-disease-influencing effects of CCR5 haplotypes ([15], [37] and data not shown). Thus, one possibility was that the association of CCR5 haplotypes with KD susceptibility is present only when it is present in the context of a specific CCL3L1 copy number. To assess this possibility, we conducted the analysis shown in Table 2. We found that CCR5 haplotypes did not influence KD susceptibility in subjects possessing <4, >4 or 4 copies of CCL3L1 (Table 2).
Table 2. Lack of a modifying influence of the CCL3L1 gene copy number on the association of five common CCR5 haplotypes found in the study population with the risk of KD.
| CCR5 haplotype | <4 CCL3L1 copies | 4 CCL3L1 copies | >4 CCL3L1 copies | M-H Test | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | χ2 | P | |
| HHA | 0.41 | 0.01–4.34 | 5.06 | 0.34–72.7 | 1.38 | 0.35–4.87 | 2.75 | 0.2531 |
| HHC | 1.22 | 0.54–2.81 | 0.93 | 0.30–3.10 | 0.65 | 0.32–1.33 | 1.54 | 0.4627 |
| HHE | 0.86 | 0.39–1.86 | 1.24 | 0.40–3.78 | 0.98 | 0.50–1.89 | 0.35 | 0.8398 |
| HHF*1 | --- | --- | 0.00 | 0.00–6.08 | 0.48 | 0.01–4.42 | 3.08 | 0.2139 |
| HHF*2 | 0.92 | 0.42–1.98 | 0.66 | 0.21–2.02 | 0.98 | 0.50–1.91 | 0.43 | 0.8064 |
The last column shows the results of Mantel-Haenszel test for homogeneity of results across CCL3L1 copy number.
In our cohort, of the 25 subjects who were resistant to IVIG, 18 (72%) developed CAL. By contrast, of the 68 who responded to IVIG treatment, only 5 (7.3%) developed CAL. This association between IVIG resistance and CAL development was highly significant (Fisher's exact p = 1.4×10−9). Evaluation of the association for the outcome of CAL revealed that possession of the CCR2-64I-bearing CCR5 HHF*2 haplotype was associated with a reduced risk of developing CAL which trended towards statistical significance (OR = 0.44, 95% CI = 0.18–1.07, p = 0.071). However, we did not observe a significant association between CCL3L1 copy number and the risk of developing CAL.
We next determined whether CCR5 haplotypes and CCL3L1 copy number associated with IVIG responses. In the full model (Table 3), possession of CCR5 HHF*2 haplotype was associated with beneficial IVIG responses (OR = 0.21, 95% CI = 0.54–0.83, p = 0.026). We also found that possession of <4 CCL3L1 copies was significantly associated with an increased risk of IVIG resistance (OR = 10.93, 95% CI = 1.17–101.99, p = 0.036). Although possession of >4 CCL3L1 copies was also associated with an increased risk of IVIG resistance (OR = 5.12, 95% CI = 0.57–46.34, p = 0.146) (Table 3, Full Model), this did not achieve statistical significance. In the final model (Table 3), possession of <4 CCL3L1 copies remained associated with an increased risk of IVIG resistance (OR = 2.56, 95% CI = 0.96–6.87, p = 0.061) while possession of CCR5 HHF*2 haplotype was associated with a salutary IVIG response (OR = 0.34, 95% CI = 0.12–0.95, p = 0.040). Departure from the population average of 4 copies (i. e, < or >4 copies) was associated with a higher risk of IVIG resistance (OR = 6.26, 95% CI 0.76–51.9, p = 0.089).
Table 3. Association of CCR5 haplotypes and CCL3L1 copy number with IVIG response.
| CCR5 haplotype/CCL3L1 copy number | OR | 95% CI | P value |
| Full Model | |||
| CCR5 HHA | 0.83 | 0.12–5.79 | 0.851 |
| CCR5 HHC | 0.62 | 0.15–2.52 | 0.499 |
| CCR5 HHE | 0.45 | 0.13–1.51 | 0.194 |
| CCR5 HHF*2 | 0.21 | 0.54–0.83 | 0.026 |
| CCL3L1 <4copies | 10.93 | 1.17–101.99 | 0.036 |
| CCL3L1 >4 copies | 5.12 | 0.57–46.34 | 0.146 |
| Final Model (Probability Criterion of 0.1) | |||
| CCL3L1 <4copies | 2.56 | 0.96–6.87 | 0.061 |
| CCR5 HHF*2 | 0.34 | 0.12–0.95 | 0.040 |
Full model shows results from a logistic regression model including all the indicated predictors while final model indicates the results from the stepwise regression using a retention criterion of 0.1; OR – Odds Ratio; CI – Confidence Interval.
Because we observed that the CCR5 HHF*2 haplotype was associated with a reduced risk of IVIG resistance as well as development of CAL, we next examined whether these associations were due to homozygosity and/or heterozygosity of the HHF*2 haplotype. We observed that heterozygosity but not homozygosity for HHF*2 was associated with a reduced risk of both CAL (OR = 0.37, 95% CI 0.14–0.97, p = 0.042) and IVIG resistance (OR = 0.39, 95% CI 0.14–1.11, p = 0.078).
Discussion
Our results suggest that in Japanese children, copy number variation of the segmental duplication bearing CCL3L1 associates with susceptibility to KD and IVIG response whereas the CCR2-64I-containing CCR5-HHF*2 haplotype is associated with a reduced risk of both CAL development and IVIG resistance. Our finding that deviation from the average CCL3L1 copy number (i.e., < or >4 copies) found in the Japanese population is associated with increased risk of KD is noteworthy because we have previously found that deviation from median copy number of CCL3L1 is also associated with an increased risk of systemic lupus erythematosus (SLE) [37] – a disease with broad immunological underpinnings – in three separate cohorts (TX, USA; Ohio, USA; and Medellin, Colombia). The notion that haploinsufficiency and higher gene dosages of immune response genes may influence susceptibility to immune-mediated diseases is also highlighted by our recent observation that both low and high copy numbers of the gene encoding FCGR3B was associated with increased susceptibility to SLE and primary Sjogren's syndrome [38]. Together these observations underscore the concept that departure of the gene copy number from a homeostatic copy number, i.e., higher or lower than the average found in the population, may be an important determinant of susceptibility to diseases with a strong immunologic component.
The precise mechanistic basis by which deviation from the average copy number of the CCL3L1-containing segmental duplication in our study population was associated with increased KD susceptibility as well as an increased risk of IVIG failure is unknown. As noted, CCL3L1 is the most potent CCR5 ligand and CCR5 ligands are associated with pro-inflammatory effects [39]. Additionally, a copy number of the CCL3L1-containing segmental duplication that is higher than the population average is associated with increased leukocyte chemoattraction [29], circulating levels of CCL3 [27] and CCL3L1 transcript (data not shown). In this light, it is conceivable that subjects bearing higher CCL3L1-containing segmental duplications may express higher levels of chemokines following antigenic stimulus that in turn may increase the risk of developing KD and possibly, IVIG resistance. In addition to causing an immunologic blockade of Fc receptor and inducing further antibody production, IVIG therapy is also known to play an important role in down-regulation of the cytokine/chemokine levels [40]. Conceptually then, in persons possessing high CCL3L1 gene copy numbers the currently used dose of IVIG may be insufficient to induce the desired degree of down-regulation of chemokines leading to IVIG resistance. The latter along with increased CCL3L1 associated inflammation may provide a partial explanation as to why we observed a trend for possession of a high CCL3L1 copy number and reduced clinical responsiveness to IVIG.
On the other hand, a low CCL3L1 copy number is associated with reduced CCL3-CCL3L1 chemokine expression levels [29] resulting in reduced inflammatory responses. It has been shown that there is a surge in levels of several cytokines/chemokines during the acute phase of KD [40], [41], [42], [43], [44] and we have observed that the CCL3 surge is a key feature of the acute phase of KD (data not shown). Thus, it is possible that an impaired CCL3-CCL3L1-dependent inflammatory response may partly explain increased risk of KD and reduced clearance of antigen. Consequently, the increased and decreased inflammation associated with a high and low CCL3L1-containing segmental duplication, respectively, may explain why all subjects do not respond to a single dose of IVIG and require additional treatments. This hypothesis is supported by the fact that greater than half of IVIG-resistant patients who receive an additional dose of IVIG become afebrile [10]. While appealing, laboratory or clinical data that directly evaluates these hypotheses regarding mechanisms by which a high or low CCL3L1 gene copy associates with KD and IVIG non-responsiveness are currently lacking and require validation.
The role of CCR5 polymorphisms in KD susceptibility has been investigated previously. Significant attention has focused on the widely recognized 32-bp deletion (Δ32) mutation present in the coding region of CCR5 that is found mainly in populations of European ancestry. We reported previously that there was an inverse relationship between the global distribution of Δ32 allele and the incidence of KD [15]. Also, in our large family-based study in US-trios we had observed an asymmetric transmission of the CCR5-Δ32 allele across generations [15]. Further, we had found that the KD-influencing effects of the CCR5-Δ32-bearing HHG*2 haploype were modified by CCL3L1 copy number [15]. Breunis et al [26] replicated our observations in a Northern European population and observed that the frequency of the CCR5-Δ32 allele was lower in cases (6.5%) compared to controls (10.7%).
The CCR5-Δ32-bearing HHG*2 haplotype is rarely found in Asian populations. The results of two prior studies in subjects with KD of European ancestry [15], [26] and a separate study of KD patients from Korea [19] suggested that other polymorphisms at the CCR5 locus also associate with susceptibility to KD. However, in the present study of Japanese subjects, we did not find an association between CCR5 haplotypes and KD susceptibility. By contrast, we did find an association of CCR5 haplotypes with KD outcomes and IVIG-resistance.
Early coronary lesions demonstrate marked infiltration of neutrophils [45] whereas at later time points show infiltration predominantly of T-cells and monocytes/macrophages [43]. Members of the chemokine system, including CCR5 and CCL3L1 play an important role in leukocyte trafficking and activation as well as the pathogenesis of coronary artery diseases such as arteriosclerosis, hypertension and myocardial infarction [46]. In our previous study of European-descent KD patients, we found that the Δ32-bearing CCR5-HHG*2 haplotype was associated with not only reduced KD susceptibility, but also a lower risk of CAL [15]. In the present study, we observed that the CCR5 HHF*2 haplotype which bears the CCR2-64I polyrmorphism is associated with a reduced the risk of IVIG-resistance and CAL formation. Whether this association suggests an involvement of CCR2, a receptor critically involved in monocyte trafficking and activation, in KD pathogenesis and therapy responses is unclear because the CCR2-64I polymorphism is in linkage disequilibrium with promoter polymorphisms in CCR5 [32]. Notwithstanding this quandary, it is conceivable that the beneficial associations observed for the CCR2-64I-bearing CCR5 HHF*2 haplotype with KD-related outcomes may relate either directly or indirectly to inflammation.
Many demographic and laboratory factors such as patient age, white blood cell count, and plasma levels of aspartate amino transferase and C-reactive protein have been identified as risk factors for IVIG resistance [3], [6], [8], [47], [48], [49]. Onouchi et al [22] observed that a functional polymorphism in the ITPKC gene was associated with response to IVIG in US KD children. The results of the present study extend the notion that host genetic factors may influence IVIG resistance. IVIG has been shown to be effective across a range of autoimmune, inflammatory and infectious conditions, as well as for post-infectious complications [25]. This suggests that IVIG may have a broad immunomodulatory mechanism of action, beyond merely inhibiting antibody-triggered inflammation. Park-Min et al showed recently that IVIG blocks cellular activation by interferon-γ (IFNγ) [50], a proinflammatory cytokine that plays a key role in cellular immune responses and Th1-type-driven inflammatory/infectious diseases [51], [52]. In this respect it is notable that CCR5 is expressed on Th1 cells [53], and thus it is conceivable that polymorphisms in this gene and its ligands by influencing Th1 pathways may influence IVIG responses. Because IVIG is far from an optimized therapeutic, and responses to IVIG vary considerably among patients, future studies are warranted to identify the broader range of host genetic factors that underlie the observed inter-subject differences in IVIG responses.
Although a limitation of our study is the small sample size, our results are concordant with previous data suggesting a role for variations in CCL3L1 and CCR5 in KD [15], [19], [26]. Recent data suggest that the Japanese population may not be as homogeneous [54] as once thought and the possibility of population stratification exists since our controls and cases came from different regions of Japan. However, despite this limitation it is noteworthy that similar to the CCR5-Δ32 polymorphism which has been intensively scrutinized in persons of European descent, the CCR2-64I polymorphism has also been associated with variable susceptibility to multiple diseases as well. Interestingly, while the CCR5-Δ32 polymorphism is rare among persons of Asian descent, the CCR2-64I-containing HHF*2 haplotype is very common, and has been associated with salutary effects (reduced risk) among persons of Japanese descent for several diseases with immunologic underpinnings including multiple sclerosis [55], sarcoidosis [56], and HIV [57]. Also, it is well-known that CAL is associated with IVIG resistance [10], [47] and concordantly, we found that IVIG-resistant subjects had a higher proportion of CAL and that the CCR2-64I-containing HHF*2 haplotype was associated with beneficial effects for both of these outcomes. Another limitation of our study is that we had to consider CAL outcomes as a dichotomous variable as the clinical centers in Japan did not uniformly use Z scores to characterize the dimension of the lumen of the coronary arteries [58]. Analyzing these data with arterial internal diameter normalized for body surface area as a continuous variable may have yielded more robust results.
Notwithstanding these limitations, our findings underscore that genetic determinants influence not only inter-individual differences in KD susceptibility but also inter-subject variation in cardiac complications and treatment response. In conjunction with previous studies that have focused on the relationship between variations in CCR5 and its ligands with KD [15], [19], [26], the current findings showing a commonality of the genetic associations across three different KD-related phenotypes (KD susceptibility, CAL development and IVIG resistance) together suggest that CCR5 and its ligands may play an important role in the pathogenesis of different facets of KD. Of broad importance, our findings also suggest that host variations may influence responses to immune modulators such as IVIG.
Acknowledgments
We thank Tamotsu Fujimoto, MD (Pediatrics, Oita Children's Hospital) for DNA collection and Tsuyoshi Takegawa (Dept. of Pediatrics, Yamaguchi University Graduate School of Medicine, Japan) for helpful discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by grants from the National Institutes of Health, National Heart, Lung, Blood Institute, HL074864 and HL69413 awarded to JCB. This work was also supported by the Voelcker Senior Scholar Award from The Max and Minnie Tomerlin Voelcker Fund awarded to SKA. SKA is also supported by a Burroughs Wellcome Clinical Scientist Award in Translational Research, and the Doris Duke Distinguished Clinical Scientist Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 2.Harnden A, Takahashi M, Burgner D. Kawasaki disease. Bmj. 2009;338:b1514. doi: 10.1136/bmj.b1514. [DOI] [PubMed] [Google Scholar]
- 3.McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–179. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 4.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Cha S, Yoon M, Ahn Y, Han M, Yoon KL. Risk factors for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. J Korean Med Sci. 2008;23:718–722. doi: 10.3346/jkms.2008.23.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egami K, Muta H, Ishii M, Suda K, Sugahara Y, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149:237–240. doi: 10.1016/j.jpeds.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Fukunishi M, Kikkawa M, Hamana K, Onodera T, Matsuzaki K, et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr. 2000;137:172–176. doi: 10.1067/mpd.2000.104815. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 9.Sittiwangkul R, Pongprot Y, Silvilairat S, Phornphutkul C. Management and outcome of intravenous gammaglobulin-resistant Kawasaki disease. Singapore Med J. 2006;47:780–784. [PubMed] [Google Scholar]
- 10.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–121. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura M, Ohki H, Tsuchihashi T, Yamagishi H, Katada Y, et al. Coronary risk factors in Kawasaki disease treated with additional gammaglobulin. Arch Dis Child. 2004;89:776–780. doi: 10.1136/adc.2003.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzman-Cottrill JA, Shulman ST. Recent developments and controversies in Kawasaki disease. Minerva Pediatr. 2004;56:51–61. [PubMed] [Google Scholar]
- 13.Rowley AH, Shulman ST. New developments in the search for the etiologic agent of Kawasaki disease. Curr Opin Pediatr. 2007;19:71–74. doi: 10.1097/MOP.0b013e328012720f. [DOI] [PubMed] [Google Scholar]
- 14.Biezeveld M, Geissler J, Merkus M, Kuipers IM, Ottenkamp J, et al. The involvement of Fc gamma receptor gene polymorphisms in Kawasaki disease. Clin Exp Immunol. 2007;147:106–111. doi: 10.1111/j.1365-2249.2006.03266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns JC, Shimizu C, Gonzalez E, Kulkarni H, Patel S, et al. Genetic variations in the receptor-ligand pair CCR5 and CCL3L1 are important determinants of susceptibility to Kawasaki disease. J Infect Dis. 2005;192:344–349. doi: 10.1086/430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns JC, Shimizu C, Shike H, Newburger JW, Sundel RP, et al. Family-based association analysis implicates IL-4 in susceptibility to Kawasaki disease. Genes Immun. 2005;6:438–444. doi: 10.1038/sj.gene.6364225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung YF, Huang GY, Chen SB, Liu XQ, Xi L, et al. Inflammatory gene polymorphisms and susceptibility to kawasaki disease and its arterial sequelae. Pediatrics. 2008;122:e608–614. doi: 10.1542/peds.2008-0646. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh KC, Lin YJ, Chang JS, Wan L, Tsai YH, et al. Influence of interleukin 18 promoter polymorphisms in susceptibility to Kawasaki disease in Taiwan. J Rheumatol. 2008;35:1408–1413. [PubMed] [Google Scholar]
- 19.Jhang WK, Kang MJ, Jin HS, Yu J, Kim BJ, et al. The CCR5 (-2135C/T) Polymorphism may be Associated with the Development of Kawasaki Disease in Korean Children. J Clin Immunol. 2008 doi: 10.1007/s10875-008-9218-z. [DOI] [PubMed] [Google Scholar]
- 20.Jibiki T, Terai M, Shima M, Ogawa A, Hamada H, et al. Monocyte chemoattractant protein 1 gene regulatory region polymorphism and serum levels of monocyte chemoattractant protein 1 in Japanese patients with Kawasaki disease. Arthritis Rheum. 2001;44:2211–2212. doi: 10.1002/1529-0131(200109)44:9<2211::aid-art375>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Kariyazono H, Ohno T, Khajoee V, Ihara K, Kusuhara K, et al. Association of vascular endothelial growth factor (VEGF) and VEGF receptor gene polymorphisms with coronary artery lesions of Kawasaki disease. Pediatr Res. 2004;56:953–959. doi: 10.1203/01.PDR.0000145280.26284.B9. [DOI] [PubMed] [Google Scholar]
- 22.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura S, Zaitsu M, Hara M, Yokota G, Watanabe M, et al. A polymorphism in the promoter of the CD14 gene (CD14/-159) is associated with the development of coronary artery lesions in patients with Kawasaki disease. J Pediatr. 2003;143:357–362. doi: 10.1067/S0022-3476(03)00330-5. [DOI] [PubMed] [Google Scholar]
- 24.Park JA, Shin KS, Kim YW. Polymorphism of matrix metalloproteinase-3 promoter gene as a risk factor for coronary artery lesions in Kawasaki disease. J Korean Med Sci. 2005;20:607–611. doi: 10.3346/jkms.2005.20.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballow M. Clinical and investigational considerations for the use of IGIV therapy. Am J Health Syst Pharm. 2005;62:S12–18; quiz S19-21. doi: 10.2146/ajhp050283. [DOI] [PubMed] [Google Scholar]
- 26.Breunis WB, Biezeveld MH, Geissler J, Kuipers IM, Lam J, et al. Polymorphisms in chemokine receptor genes and susceptibility to Kawasaki disease. Clin Exp Immunol. 2007;150:83–90. doi: 10.1111/j.1365-2249.2007.03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 28.Mummidi S, Ahuja SS, Gonzalez E, Anderson SA, Santiago EN, et al. Genealogy of the CCR5 locus and chemokine system gene variants associated with altered rates of HIV-1 disease progression. Nat Med. 1998;4:786–793. doi: 10.1038/nm0798-786. [DOI] [PubMed] [Google Scholar]
- 29.Townson JR, Barcellos LF, Nibbs RJ. Gene copy number regulates the production of the human chemokine CCL3-L1. Eur J Immunol. 2002;32:3016–3026. doi: 10.1002/1521-4141(2002010)32:10<3016::AID-IMMU3016>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, et al. Recent segmental duplications in the human genome. Science. 2002;297:1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 31.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez E, Dhanda R, Bamshad M, Mummidi S, Geevarghese R, et al. Global survey of genetic variation in CCR5, RANTES, and MIP-1alpha: impact on the epidemiology of the HIV-1 pandemic. Proc Natl Acad Sci U S A. 2001;98:5199–5204. doi: 10.1073/pnas.091056898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 34.Akagi T, Rose V, Benson LN, Newman A, Freedom RM. Outcome of coronary artery aneurysms after Kawasaki disease. J Pediatr. 1992;121:689–694. doi: 10.1016/s0022-3476(05)81894-3. [DOI] [PubMed] [Google Scholar]
- 35.Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, et al. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J Biol Chem. 2000;275:18946–18961. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 37.Mamtani M, Rovin B, Brey R, Camargo JF, Kulkarni H, et al. CCL3L1 gene-containing segmental duplications and polymorphisms in CCR5 affect risk of systemic lupus erythaematosus. Ann Rheum Dis. 2008;67:1076–1083. doi: 10.1136/ard.2007.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamtani M, Anaya JM, He W, Ahuja SK. Association of copy number variation in the FCGR3B gene with risk of autoimmune diseases. Genes Immun. 2010;11:155–160. doi: 10.1038/gene.2009.71. [DOI] [PubMed] [Google Scholar]
- 39.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13:455–481. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 40.Minami T, Suzuki H, Takeuchi T, Uemura S, Sugatani J, et al. A polymorphism in plasma platelet-activating factor acetylhydrolase is involved in resistance to immunoglobulin treatment in Kawasaki disease. J Pediatr. 2005;147:78–83. doi: 10.1016/j.jpeds.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Asano T, Ogawa S. Expression of IL-8 in Kawasaki disease. Clin Exp Immunol. 2000;122:514–519. doi: 10.1046/j.1365-2249.2000.01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asano T, Ogawa S. Expression of monocyte chemoattractant protein-1 in Kawasaki disease: the anti-inflammatory effect of gamma globulin therapy. Scand J Immunol. 2000;51:98–103. doi: 10.1046/j.1365-3083.2000.00650.x. [DOI] [PubMed] [Google Scholar]
- 43.Terai M, Jibiki T, Harada A, Terashima Y, Yasukawa K, et al. Dramatic decrease of circulating levels of monocyte chemoattractant protein-1 in Kawasaki disease after gamma globulin treatment. J Leukoc Biol. 1999;65:566–572. doi: 10.1002/jlb.65.5.566. [DOI] [PubMed] [Google Scholar]
- 44.Wong M, Silverman ED, Fish EN. Evidence for RANTES, monocyte chemotactic protein-1, and macrophage inflammatory protein-1 beta expression in Kawasaki disease. J Rheumatol. 1997;24:1179–1185. [PubMed] [Google Scholar]
- 45.Takahashi K, Oharaseki T, Naoe S, Wakayama M, Yokouchi Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47:305–310. doi: 10.1111/j.1442-200x.2005.02049.x. [DOI] [PubMed] [Google Scholar]
- 46.Aukrust P, Halvorsen B, Yndestad A, Ueland T, Oie E, et al. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008;28:1909–1919. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 47.Kim T, Choi W, Woo CW, Choi B, Lee J, et al. Predictive risk factors for coronary artery abnormalities in Kawasaki disease. Eur J Pediatr. 2007;166:421–425. doi: 10.1007/s00431-006-0251-8. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi T, Inoue Y, Morikawa A. [Risk stratification and prediction of resistance to intravenous immunoglobulin in Kawasaki disease]. Nippon Rinsho. 2008;66:332–337. [PubMed] [Google Scholar]
- 49.Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–137. doi: 10.1007/s00431-006-0223-z. [DOI] [PubMed] [Google Scholar]
- 50.Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, et al. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Fietta P, Delsante G. The effector T helper cell triade. Riv Biol. 2009;102:61–74. [PubMed] [Google Scholar]
- 52.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi-Kabata Y, Nakazono K, Takahashi A, Saito S, Hosono N, et al. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet. 2008;83:445–456. doi: 10.1016/j.ajhg.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyagishi R, Niino M, Fukazawa T, Yabe I, Kikuchi S, et al. C-C chemokine receptor 2 gene polymorphism in Japanese patients with multiple sclerosis. J Neuroimmunol. 2003;145:135–138. doi: 10.1016/j.jneuroim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Hizawa N, Yamaguchi E, Furuya K, Jinushi E, Ito A, et al. The role of the C-C chemokine receptor 2 gene polymorphism V64I (CCR2-64I) in sarcoidosis in a Japanese population. Am J Respir Crit Care Med. 1999;159:2021–2023. doi: 10.1164/ajrccm.159.6.9810020. [DOI] [PubMed] [Google Scholar]
- 57.Deng XW, Terunuma H, Handema R, Hanabusa H, Taki M, et al. Polymorphisms of coreceptor-related genes associated with HIV-1 disease progression and infection in Japanese hemophiliacs; . 2002 Jul 7–12.pp. abstract no. ThPeB7222. [Google Scholar]
- 58.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, et al. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]