Abstract
Background
We measured 34 metabolites of current-use pesticides and other precursor compounds in urine samples collected twice during pregnancy from 538 women living in the Salinas Valley of California, a highly agricultural area (1999–2001). Precursors of these metabolites included fungicides, carbamate, organochlorine, organophosphorus (OP), and pyrethroid insecticides, and triazine and chloroacetanilide herbicides. We also measured ethylenethiourea, a metabolite of the ethylene-bisdithiocarbamate fungicides. Repeat measurements of the compounds presented here have not been reported in pregnant women previously. To understand the impact of the women’s regional environment on these findings, we compared metabolite concentrations from the CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) cohort with U.S. national reference data for 342 pregnant women sampled by the National Health and Nutrition Examination Survey (1999–2002).
Results
The eight metabolites detected in > 50% of samples [2,4-dichlorophenol (2,4-DCP); 2,5-dichlorophenol (2,5-DCP); 1- and 2-naphthol; ortho-phenylphenol (ORTH); para-nitrophenol (PNP); 2,4,6-trichlorophenol (2,4,6-TCP); and 3,4,6-trichloro-2-pyridinol (TCPy)] may be related to home or agricultural pesticide use in the Salinas Valley, household products, and other sources of chlorinated phenols. More than 78% of women in this study had detectable levels of at least one of the OP pesticide-specific metabolites that we measured, and > 30% had two or more. The 95th percentile values of six of the most commonly detected (> 50%) compounds were significantly higher among the CHAMACOS women after controlling for age, race, socioeconomic status, and smoking [(2,4-DCP; 2,5-DCP; ORTH; PNP; 2,4,6-TCP; and TCPy); quantile regression p < 0.05].
Conclusions
Findings suggest that the CHAMACOS cohort has an additional burden of precursor pesticide exposure compared with the national sample, possibly from living and/or working in an agricultural area.
Keywords: ETU, exposure, NHANES, organophosphate, pesticides, pregnancy, prenatal, urinary metabolites, women
Prenatal exposure to pesticides, including organophosphorus (OP) and organochlorine (OC) compounds, has been shown to increase the risk of adverse developmental outcomes in children (Eskenazi et al. 2008; Jurewicz and Hanke 2008; Perera et al. 2005). However, few studies have comprehensively investigated maternal exposures to currently used pesticides during pregnancy. Biomonitoring results from the National Health and Nutrition Examination Survey (NHANES) (1999–2002) [Centers for Disease Control and Prevention (CDC) 2005] and other studies suggest that many pregnant women are exposed to a mixture of insecticidal and fungicidal compounds (Berkowitz et al. 2003; Bradman et al. 2003, 2005; Takser et al. 2004; Ye et al. 2008).
There are multiple pesticide exposure pathways, but recent research (Lu et al. 2006; McKone et al. 2007) suggests that diet is the dominant route of exposure for the general population. Para-occupational or take-home exposure from farmworkers and ambient exposure from living in proximity to agricultural activity can add to total pesticide exposure in agricultural communities (Goldman et al. 2004; McKone et al. 2007). Biomonitoring can help to quantify the additional exposure for pregnant women in agricultural communities by allowing for the direct comparison of detection frequencies and metabolite levels between populations.
The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) is a longitudinal birth cohort study investigating in utero and postnatal environmental exposures and their effects on the health of children residing in the largely agricultural Salinas Valley of Monterey County, California (Eskenazi et al. 2003). The growing season in this productive region is nearly year-round. Row crops are grown with a predominance of strawberries, lettuce, and broccoli in the north and warmer-weather crops such as grapes in the south. Previously we presented CHAMACOS prenatal measurements of three specific metabolites of OP pesticides (Eskenazi et al. 2004) and ethylenethiourea (ETU), a toxic metabolite of the ethylene bisdithiocarbamate (EBDC) fungicides (Montesano et al. 2007). In this study, we present exposure data on an additional 30 pesticide metabolites in repeat samples collected from pregnant women late in the first and second trimesters. To better understand the impact of the regional agricultural environment on these findings, we also compare levels in the CHAMACOS cohort with those measured in a representative national sample of pregnant women participating in the NHANES (1999–2002) (CDC 2005).
Methods
Study location characteristics
The Salinas Valley includes approximately 91,054 irrigated hectares devoted to row, vineyard, and orchard crops. In 2000, approximately 570,000 kg herbicide, EBDC fungicide, and carbamate, OP, OC, and pyrethroid pesticide active ingredients were applied for agricultural and landscaping purposes in this region, a level typical of recent years (10% herbicides; 27% EBDC fungicides; 44% OP and 2% OC pesticides; and 4% pyrethroid pesticides) [California Department of Pesticide Regulation (CDPR) 2000a, 2006]. Of the total kilograms of nonfumigant pesticides applied in the Salinas Valley (excluding sulfur and petroleum products), 65% percent devolve into one of the 34 urinary metabolites we measured. Applications of maneb, diazinon, malathion, acephate, and chlorpyrifos account for 78% of the kilograms of pesticides applied whose urinary metabolites were detectable using the analytic methods available in this study (Table 1).
Table 1.
Description of 34 analytes in urine and amount of precursor compound applied in the Salinas Valley in 2000.
| Analyte | Possible precursor compound(s) (kilograms active ingredient applied in 2000)a | Chemical class | Available in NHANES |
|---|---|---|---|
| Acephate (AP) | AP (33,602) | OP insecticide | No |
| Acetochlor mercapturate (ACET) | Acetochlor (0) | Herbicide (chloroacetanilide) | Yes |
| Atrazine mercapturate (ATZ) | Atrazine (4) | Herbicide (triazine) | Yes |
| Carbofuranphenol (2,3-dihydro-2,2-dimethy-7-hydroxybenzofuran) (CFP) | Carbofuran (7,288); benfuracarb (0); carbosulfan (0) | Carbamate pesticide (n-methyl) | Yes |
| 5-Chloro-1,2-dihydro-1-isopropyl-[3H]-1,2,4-triazol-3-one (CIT) | Isazaphos (0); isazaphos methyl (0) | OP insecticide | No |
| 3-Chloro-4-methyl-7-hydroxycoumarin (CMHC) | Coumaphos (0) | OP insecticide | Yes |
| cis-2,2-(Dibromo)-2-dimethylvinyl cyclopropane carboxylic acid (DBCA) | Deltamethrin (0) | Pyrethroid pesticide | No |
| cis-2,2-(Dichloro)-2-dimethylvinyl cyclopropane carboxylic acid) (cis-DCCA) | Permethrin (11,439); cypermethrin (953); cyfluthrin (6) | Pyrethroid pesticide | Yes |
| trans-2,2-(Dichloro)-2-dimethylvinyl cyclopropane carboxylic acid) (trans-DCCA) | Permethrin (11,439); cypermethrin (953); cyfluthrin (6) | Pyrethroid pesticide | Yes |
| 2,4-Dichlorophenol (2,4-DCP) | Bifenox (0); chlomethoxyfen (0); 2,4-dichlorophenoxyacetic acid (2,4-D) (0); 2,4-DB (0); 1,3-dichlorobenzene (0); dichlofenthion (0); dichlorprop (0); diclofop (0); nitrofen (0) | Herbicide (chlorinated phenoxy compound) | Yes |
| 2,5-Dichlorophenol (2,5-DCP) | Lindane (γ-HCH) (355); 1,4-dichlorobenzene (0) | Other pesticide (OC) | Yes |
| 2,4-Dichlorophenoxyacetic acid (2,4-D) | 2,4-D (and its esters) (0) | Herbicide (chlorinated phenoxy compound) | Yes |
| 2-Diethylamino-6-methylpyrimidin-4-ol (DEAMPY) | Pirimiphos methyl (0) | OP insecticide | Yes |
| Diethyl-m-toluamide (DEET) | DEET (0) | Other pesticide (repellent) | Yes |
| Dimethoate (DME) | DME (16,152) | OP insecticide | No |
| Ethylenethiourea (ETU) | Mancozeb (6,259); maneb (146,592); metiram (0); zineb (0) | EBDC fungicide | No |
| 4-Fluoro-3-phenoxybenzoic acid (4F3PBA) | Cyfluthrin (6) | Pyrethroid pesticide | Yes |
| 2-Isopropoxyphenol (IPP) | Propoxur (0) | Carbamate insecticide | Yes |
| 2-Isopropyl-4-methyl-6-hydroxypyrimidinol (IMPY) | Diazinon (56,051) | OP insecticide | Yes |
| Malathion dicarboxylic acid (MDA) | Malathion (45,710) | OP insecticide | Yes |
| Methamidaphos (MMP) | MMP (620); AP (33,602) | OP insecticide | No |
| o-Methoate (OMT) | OMT (0) | OP insecticide | No |
| Metolachlor mercapturate (MET) | Metolachlor (1,070) | Herbicide (chloroacetanilide) | Yes |
| 1-Naphthol | Carbaryl (9,190); naphthalene (0) | Carbamate insecticide; PAH | Yes |
| 2-Naphthol | Naphthalene (0) | PAH | Yes |
| para-Nitrophenol (PNP) | Parathion (3); methyl parathion (0); EPN (0); nitrobenzene (0) | OP insecticide; organic compound | Yes |
| Pentachlorophenol (PCP) | γ-HCH (355); PCP (0) | OC pesticide (fungicide/ wood preservative) | Yes |
| 3-Phenoxybenzoic acid (3PBA2) | General pyrethroid metabolite (14,938) | Pyrethroid pesticide | Yes |
| ortho-Phenylphenol (ORTH) | ORTH (0) | Other pesticide (fungicide) | Yes |
| Propylenethiourea (PTU) | Propineb (0) | EBDC fungicide | No |
| 2,4,5-Trichlorophenol (2,4,5-TCP) | γ-HCH (355); chlorinated benzenes (—); chlorinated phenols (—) | OC pesticide | Yes |
| 2,4,6-Trichlorophenol (2,4,6-TCP) | γ-HCH (355); chlorinated benzenes (—); chlorinated phenols (—) | OC pesticide | Yes |
| 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T) | 2,4,5-T (0) | Herbicide (chlorinated phenoxy acid) | Yes |
| 3,5,6-Trichloro-2-pyridinol (TCPy) | Chlorpyrifos (26,691); chlorpyrifos methyl (0) | OP insecticide | Yes |
Abbreviations: —, not available in the PUR data set. EPN, ethyl p-nitrophenol thiobenzenephosphonate; PAH, polycyclic aromatic hydrocarbon.
Kilograms active ingredient applied includes agricultural, landscape maintenance, structural pest control, and roadside pesticide usage (CDPR 2000a).
Study population
CHAMACOS
Between September 1999 and November 2000, 601 pregnant women were enrolled in the CHAMACOS birth cohort study from six local prenatal clinics. Women were eligible to participate in this study if they were ≤ 20 weeks gestation at the time of enrollment, were ≥ 18 years of age, qualified to receive poverty-based government health insurance, and planned to continue receiving prenatal care at a participating health center (Eskenazi et al. 2003, 2004). Written informed consent was obtained from all participants in accordance with procedures approved by the University of California Berkeley Committee for the Protection of Human Subjects.
NHANES 1999–2002 data set
As part of the ongoing NHANES, the CDC reported levels of current-use pesticide metabolites measured in spot urine collected from representative samples of the U.S. population stratified by age, sex, and racial/ethnic group (Barr et al. 2005; CDC 2004). Metabolite concentrations were measured in 3,048 urine samples collected from U.S. residents 6–59 years of age during 1999 and 2002 and included 342 pregnant women 15–50 years of age (CDC 2004). The public release versions of the NHANES data sets, including demographic information and metabolite data, were used for these analyses (CDC 2007a, 2007b). We applied no sample weights to the NHANES data.
Pesticide use reporting data
In California, all agricultural, right-of-way, and structural pesticides applied by farmers or licensed applicators must be reported to the state (CDPR 2000b). Agricultural pesticide applications, representing 90% of all reported use, are geocoded to 1-square-mile units based on the Public Land Survey System (PLSS). To summarize agricultural pesticide use in the Salinas Valley, we first defined the region as the area bounded by the Pacific Ocean and an isopleth at 200 feet in elevation above the Salinas River. We then identified the PLSS sections within the Salinas Valley and abstracted pesticide use from the California pesticide use reporting (PUR) data set.
Study interview
Information on demographics, health, household characteristics, and occupation of CHAMACOS participants was collected through personal interviews. Interviews were conducted in English or Spanish by bilingual, bicultural study staff. The first prenatal interview occurred shortly after enrollment in the study (mean ± SD = 13 ± 5.2 weeks gestation) and a follow-up interview occurred late in the second trimester (mean = 26 ± 2.6 weeks gestation).
Urine collection and analysis
Two spot urine samples were collected from CHAMACOS participants at each prenatal interview and analyzed by the Division of Laboratory Sciences, CDC. Women voided into a sterile urine cup in bathroom facilities at our field office or in a mobile clinic. Specimens were placed into precleaned glass containers with Teflon-lined caps, bar coded, and stored at −80°C until shipment. Samples were shipped on dry ice to the CDC and stored at −70°C until analysis.
Pesticide and creatinine measurement
Thirty-four urinary metabolites were measured for the CHAMACOS cohort, including OP, OC, and pyrethroid pesticides, herbicides, and EBDC fungicides. Acephate (AP), methamidophos (MMP), omethoate (OMT), dimethoate (DME), and the metabolites ETU and propylenethiourea (PTU) are water soluble and after lyophilization were extracted with dichloromethane; they were then analyzed using isotope dilution high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry (HPLC/APCI-MS/MS) (Montesano et al. 2007). Sample preparation for the phenolic metabolites [2,4-dichlorophenol (2,4-DCP); 2,5-dichlorophenol (2,5-DCP); carbofuranphenol (CFP); 1-naphthol; 2-naphthol; para-nitrophenol (PNP); pentachlorophenol (PCP); ortho-phenylphenol (ORTH); 2,4,5-trichlorophenol (2,4,5-TCP); 2,4,6-trichlorophenol (2,4,6-TCP); and 3,5,6-trichloro-2-pyridinol (TCPy)] involved enzyme hydrolysis to liberate them from their glucuronide or sulfate conjugates, isolation of the target chemicals using solid phase extraction cartridges, a phase-transfer catalyzed derivatization, cleanup using sorbent-immobilized liquid/liquid extraction cartridges, and concentration of the sample. Derivatized samples were analyzed by capillary gas chromatography- tandem mass spectrometry using isotope dilution calibration for quantification (Bravo et al. 2005). The remaining specific metabolites of OP pesticides [5-chloro-1,2-dihydro-1- isopropyl-[3H]-1,2,4-triazol-3-one (CIT); 3-chloro-4-methyl-7-hydroxycoumarin (CMHC); 2-diethylamino-6-methylpyrimidin-4-ol (DEAMPY); 2-isopropyl-4-methyl-6-hydroxypyrimidinol (IMPY); malathion dicarboxylic acid (MDA)]; synthetic pyrethroids; and diethyl-m-toluamide (DEET) were measured as the intact metabolite after enzyme hydrolysis and solid phase extraction using HPLC/APCI-MS/MS (modification of Olsson et al. 2004). Creatinine concentrations in urine were determined using a commercially available diagnostic enzyme method (Vitros CREA slides; Ortho Clinical Diagnostics, Raritan, NJ). The same analytical laboratory at CDC performed the urinary metabolite measurements for both the NHANES and CHAMACOS studies (CDC 2004) (Table 1).
Laboratory quality control (QC) included repeat analysis of three in-house urine pools enriched with known amounts of pesticide residues whose target values and confidence limits were previously determined (Westgard 2003). Detection limits ranged from 0.03 to 1.5 μg/L. We assigned an imputed value of the limit of detection (LOD)/✓2– to levels below the detection limit (Barr et al. 1999; Hornung and Reed 1990). A total of 153 (blind) field QC samples were analyzed, representing 15% of total samples. Mean relative recoveries for the metabolites in field QC samples ranged from 78 to 120%. The mean levels of metabolites in 51 blank field samples were ≤ 1 μg/L.
The creatinine concentration in each urine sample was reported in milligrams of creatinine per deciliter of urine. Of the 601 women who enrolled in the study, adequate urine samples with valid creatinine levels were collected from 538 (90%) women at the first prenatal sampling point and 481 (80%) women at the second.
Ratio of 1-naphthol to 2-naphthol
Both naphthalene and carbaryl can devolve to urinary 1-naphthol. Because naphthalene, but not carbaryl, also metabolizes to 2-naphthol, the ratio of urinary 1-naphthol to 2-naphthol within an individual can provide information about the source of 1-naphthol. Thus, we calculated the ratio of 1-naphthol to 2-naphthol to identify subgroups within the CHAMACOS cohort and pregnant women in NHANES in which carbaryl was expected to be the primary source of 1-naphthol. We assumed that a ratio > 2 indicates carbaryl as the source of 1-naphthol (Meeker et al. 2007).
Data analysis
We calculated descriptive statistics for metabolite levels from the two CHAMACOS prenatal urine samples. For the eight CHAMACOS metabolites with detection frequencies > 50% in both samples, we computed the Spearman’s rank correlation between the first and second prenatal samples. In addition, we computed metabolite correlations within each of the two CHAMACOS samples and within samples collected from pregnant women in NHANES. To broadly compare the NHANES and CHAMACOS metabolite levels, we analyzed the 11 compounds with detection frequencies > 40% in either study. We used the Wilcoxon rank-sum test and quantile regression (95th percentile) to compare differences in metabolite concentrations between the CHAMACOS cohort and NHANES. We compared differences in detection frequencies using analysis of variance (ANOVA). For compounds with LODs that differed between the two populations (e.g., 1- and 2-naphthol), the higher LODs were applied to the metabolite concentrations of both populations for analyses of differences in detection frequencies. When comparing NHANES and CHAMACOS women’s metabolite levels using quantile regression, we used demographic variables to adjust our models for age (continuous), current smoking (yes/no), ethnicity (Mexican or Mexican American; other Hispanic; non-Hispanic white; non-Hispanic black; or other race, including multiracial), and socioeconomic status (at or below poverty threshold; within 200% of poverty threshold; above 200% of poverty threshold) (Table 2). Finally, we calculated the total number of OP pesticide metabolites detected in the first and second prenatal urine samples of each CHAMACOS woman. We did not count PNP, because compounds other than OP pesticides (e.g., nitrobenzene) are additional sources of urinary PNP.
Table 2.
Demographic characteristics of CHAMACOS cohort and pregnant women participating in NHANES 1999–2002.a,b
|
n (%) |
||
|---|---|---|
| Characteristic | CHAMACOS (n = 538) | NHANESc (n = 342) |
| Age (mean ± SD) | 26.1 ± 5.2 | 27.0 ± 5.9 |
| Education | ||
| < 12th grade | 429 (79.7) | 66 (23.2) |
| Completed high school/GED | 60 (11.2) | 66 (23.2) |
| Some college/technical school | 45 (8.4) | 79 (27.8) |
| College grad | 4 (0.7) | 73 (25.7) |
| Marital status | ||
| Married/living as married | 436 (81.0) | 233 (74.7) |
| Single | 102 (19.0) | 79 (25.3) |
| Total no. of people in household | ||
| 1–2 | 21 (4.4) | 75 (23.2) |
| 3–4 | 102 (21.6) | 154 (47.7) |
| > 4 | 350 (74.0) | 94 (29.1) |
| Parity (no. of live births) | ||
| 0 | 193 (35.9) | 20 (6.2) |
| ≥ 1 | 345 (64.1) | 303 (93.8) |
| Country of birth | ||
| Mexico | 458 (85.1) | 52 (16.1) |
| United States | 68 (12.6) | 239 (74.0) |
| Other | 12 (2.2) | 32 (9.9) |
| Ethnicity | ||
| Mexican or Mexican American | 512 (95.2) | 84 (26.0) |
| Other Hispanic | 9 (1.7) | 20 (6.2) |
| Non-Hispanic white | 8 (1.5) | 148 (45.8) |
| Non-Hispanic black | 0 (0) | 50 (15.5) |
| Other race including multiracial | 9 (1.7) | 21 (6.5) |
| Poverty | ||
| ≤ Poverty threshold | 332 (61.8) | 75 (25.5) |
| 200% poverty threshold | 184 (34.3) | 64 (21.8) |
| > 200% poverty threshold | 21 (3.9) | 155 (52.7) |
| Prepregnancy BMId | ||
| Underweight (< 18.5) | 3 (0.6) | 26 (6.2) |
| Normal (18.5–24.9) | 196 (37.5) | 231 (55.1) |
| Overweight (25–29.9) | 205 (39.2) | 98 (23.4) |
| Obese (≥ 30) | 119 (22.7) | 64 (15.3) |
| Woman currently smokes | ||
| Yes | 8 (1.5) | 21 (7.4) |
| No | 529 (98.5) | 263 (92.6) |
| Other household members who smoke | ||
| Yes | 38 (7.1) | 55 (17.1) |
| No | 496 (92.9) | 265 (82.6) |
| Don’t know | 0 (0) | 1 (0.3) |
At time of the first CHAMACOS prenatal visit (~ 13 weeks gestation).
Total number of observations varies because of missing data.
NHANES mean gestational age (5.8 ± 2.0 months).
Prepregnancy BMI (kg/m2) calculated from women’s self-reported prepregnancy height and weight.
All analyses were conducted using Stata software, version 10 (StataCorp LP, College Station, TX).
Results
Demographic characteristics
Table 2 presents the demographic characteristics of pregnant women in the CHAMACOS cohort and NHANES. Compared with pregnant women in NHANES, the CHAMACOS women were less educated (80% vs. 23% attained less than a 12th grade education), lived with more people in their households (74% vs. 29% live with ≥ 4 household members), and were poorer (62% vs. 25% living at or below the federal poverty threshold). Ninety-five percent of CHAMACOS women were Mexican or Mexican American compared with 26% in NHANES. Forty-four percent of CHAMACOS women were employed as farm workers at some point during their pregnancy, and 81% percent shared a home with at least one agricultural worker (Eskenazi et al. 2004). In addition, the CHAMACOS women tended to have higher prepregnancy body mass indexes (BMIs) compared with NHANES (23% vs. 15% with BMI > 30, respectively), but fewer CHAMACOS women smoked (1.5%) compared with pregnant women in NHANES (7.4%).
Creatinine
Median CHAMACOS creatinine levels decreased slightly from the first to the second prenatal sample, with levels of 93.7 mg/dL [interquartile range (IQR) = 51.5–139] and 90.8 mg/dL (IQR = 60.8–129), respectively. Median creatinine levels among pregnant women in CHAMACOS [combined first and second prenatal samples = 92.7 mg/dL (55.8–135)] were significantly lower than those of NHANES [108 mg/dL (IQR = 64.0–153); p < 0.05]. In both populations, women’s urinary creatinine levels decreased significantly with increasing weeks of gestation (p < 0.05). This trend is consistent with medical reference data (Becker et al. 1992; Davison and Noble 1981; Davison et al. 1980). The NHANES prenatal samples were collected at approximately 24 weeks gestation (mean = 24.4 ± 9.5 weeks).
Urinary metabolite concentration data
Table 2 describes the 34 analytes measured and the amount of precursor compound active ingredients applied in the Salinas Valley in 2000 (CDPR 2000a). Table 3 presents CHAMACOS and NHANES descriptive statistics for the most frequently detected metabolites (detection frequency > 50%) in the CHAMACOS cohort. The CHAMACOS detection frequencies ranged from 0 to 81%, with eight metabolites detected in both urine samples at frequencies over 50% (2,4- and 2,5-DCP; 1- and 2-naphthol; PNP; ORTH; 2,4,6-TCP; and TCPy). The NHANES detection frequencies for the metabolites ranged from 0 to 100%, with seven metabolites detected at frequencies over 50% [2,4- and 2,5-DCP; 1- and 2-naphthol; 3-phenoxybenzoic acid (3PBA2); 2,4,6-TCP; and TCPy].
Table 3.
Summary of eight CHAMACOS first and second prenatal and NHANES urinary metabolite concentrations (μg/L) with detection frequencies > 50%.
| Analyte | Prenatal sample | n | LOD (μg/L) | DF (%) | Percentile |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | 95th | Max | |||||
| 2,4-DCP | 1 | 523 | 0.2 | 63.7 | < LOD | 1.8 | 12.3 | 35.5 | 76.9 | 5313.6 |
| 2 | 478 | 57.5 | < LOD | 1.1 | 8.9 | 37.7 | 152.6 | 5948.6 | ||
| NHANES | 223 | 0.3 | 53.4 | < LOD | 0.29 | 2.6 | 7.8 | 19.8 | 141.8 | |
| 2,5-DCP | 1 | 523 | 0.1 | 83.8 | < LOD | 21.5 | 176.5 | 1060.3 | 1935.2 | 10442.2 |
| 2 | 479 | 77.2 | < LOD | 18.5 | 158.6 | 1035.8 | 1950.0 | 6063.2 | ||
| NHANES | 223 | 0.1 | 67.3 | < LOD | 3.7 | 37.0 | 187.7 | 419.3 | 3077.8 | |
| 1-Naphthola | 1 | 520 | 0.2 | 75.4 | 0.3 | 1.9 | 2.9 | 5.7 | 10.8 | 413.8 |
| 2 | 479 | 66.2 | < LOD | 2.4 | 3.7 | 5.8 | 8.2 | 167.0 | ||
| NHANES | 118 | 6.2 (ng/L) | 100a | 0.9 | 1.6 | 3.1 | 10.8 | 31.7 | 297.4 | |
| 2-Naphthola | 1 | 522 | 0.4 | 66.5 | < LOD | 1.7 | 5.0 | 10.9 | 16.3 | 45.9 |
| 2 | 479 | 68.7 | < LOD | 1.8 | 5.4 | 10.3 | 15.3 | 77.9 | ||
| NHANES | 118 | 2.4 (ng/L) | 100 | 1.6 | 3.0 | 6.1 | 14.1 | 22.7 | 138.7 | |
| PNP | 1 | 538 | 0.1 | 51.5 | < LOD | 0.2 | 0.9 | 2.2 | 4.4 | 44.8 |
| 2 | 481 | 58.0 | < LOD | 0.3 | 1.0 | 2.6 | 4.5 | 69.1 | ||
| NHANES | 223 | 0.1–0.8 | 30.5 | < LOD | < LOD | 0.9 | 2.2 | 2.9 | 24.0 | |
| ORTH | 1 | 523 | 0.2 | 60.6 | < LOD | 0.5 | 1.1 | 1.9 | 4.3 | 117.1 |
| 2 | 479 | 51.8 | < LOD | 0.3 | 1.5 | 3.2 | 7.5 | 67.7 | ||
| NHANES | 224 | 0.3 | 34.4 | < LOD | < LOD | 0.6 | 1.7 | 2.8 | 19.0 | |
| 2,4,6-TCP | 1 | 523 | 0.6 | 56.2 | < LOD | 1.4 | 5.5 | 11.9 | 18.2 | 142.0 |
| 2 | 478 | 74.1 | 0.4 | 4.5 | 9.0 | 17.4 | 23.4 | 61.7 | ||
| NHANES | 223 | 1–1.3 | 59.6 | < LOD | 1.8 | 4.1 | 8.6 | 12.6 | 68.0 | |
| TCPy | 1 | 538 | 0.3 | 71.2 | < LOD | 2.1 | 5.4 | 11.4 | 16.9 | 56.1 |
| 2 | 481 | 81.9 | 1.0 | 3.2 | 7.1 | 13.4 | 17.9 | 38.2 | ||
| NHANES | 224 | 0.4 | 79.0 | 0.6 | 1.6 | 3.1 | 5.5 | 7.4 | 61.0 | |
Abbreviations: DF, detection frequency; Max, maximum. CHAMACOS prenatal sample 1 was collected at approximately 13 weeks gestation, and prenatal sample 2 was collected at approximately 26 weeks gestation; the NHANES prenatal samples were collected at approximately 24 weeks gestation (mean = 24.4 ± 9.5 weeks).
NHANES detection frequencies for 1- and 2-naphthol become 99% and 93%, respectively, when recalculated using the CHAMACOS LODs.
Table 4 summarizes the CHAMACOS and NHANES levels of the metabolites with average CHAMACOS detection frequencies < 50%. The CHAMACOS LODs were the same for the first and second prenatal sampling points and, except for 1- and 2-naphthol, were similar to those reported in NHANES (Tables 3 and 4) (CDC 2005). Among compounds with > 40% detection, the CHAMACOS and NHANES detection frequencies were statistically different for 2,5-DCP, 1- and 2-naphthol, ORTH, 3PBA2 and 2,4,5-TCP. The CHAMACOS first and second prenatal 2,5-DCP, ORTH, and 2,4,5-TCP detection frequencies were significantly higher than NHANES, and the NHANES 1- and 2-naphthol and 3PBA2 detection frequencies were significantly higher than CHAMACOS (ANOVA p < 0.05).
Table 4.
Summary of CHAMACOS first and second prenatal and NHANES urinary metabolites (μg/L) with detection frequencies < 50%.
| Analyte | Prenatal sample | n | LOD (μg/L) | DF (%) | Percentiles |
|||
|---|---|---|---|---|---|---|---|---|
| 75th | 90th | 95th | Max | |||||
| AP | 1 | 479 | 0.3 | 5.6 | < LOD | < LOD | < LOD | 46.9 |
| 2 | 466 | 6.4 | < LOD | < LOD | < LOD | 20.7 | ||
| NHANES | — | — | — | — | — | — | — | |
| CFP | 1 | 523 | 0.2 | 1.2 | < LOD | < LOD | < LOD | 2.5 |
| 2 | 479 | 2.5 | < LOD | < LOD | < LOD | 14.9 | ||
| NHANES | 224 | 0.4 | 8.9 | < LOD | < LOD | 0.4 | 5.1 | |
| CIT | 1 | 538 | 1.5 | 12.5 | < LOD | 2.3 | 6.2 | 70.9 |
| 2 | 481 | 9.2 | < LOD | < LOD | 2.5 | 6.8 | ||
| NHANES | — | — | — | — | — | — | — | |
| cis-DCCA | 1 | 538 | 0.2 | 5.6 | < LOD | < LOD | 0.3 | 162.8 |
| 2 | 481 | 8.7 | < LOD | < LOD | 0.5 | 55.7 | ||
| NHANES | 223 | 0.1 | 32.7 | 0.15 | 0.4 | 0.8 | 40.0 | |
| trans-DCCA | 1 | 538 | 0.4 | 12.5 | < LOD | 0.5 | 0.9 | 397.5 |
| 2 | 481 | 16.6 | < LOD | 0.9 | 1.5 | 339.0 | ||
| NHANES | 223 | 0.4 | 26.9 | 0.4 | 0.8 | 1.5 | 78.0 | |
| 2,4-D | 1 | 538 | 0.2 | 13.8 | < LOD | 0.3 | 0.5 | 8.6 |
| 2 | 481 | 21.2 | < LOD | 0.4 | 0.8 | 8.6 | ||
| NHANES | 221 | 0.2–1.0 | 31.2 | 0.1 | 0.4 | 0.5 | 2.3 | |
| DEAMPY | 1 | 538 | 0.2 | 5.6 | < LOD | < LOD | 0.3 | 29.7 |
| 2 | 481 | 4.4 | < LOD | < LOD | < LOD | 9.1 | ||
| NHANES | 124 | 0.2 | 7.3 | < LOD | < LOD | 0.6 | 2.9 | |
| DEET | 1 | 538 | 0.1 | 5.6 | < LOD | < LOD | 0.1 | 1.9 |
| 2 | 481 | 1.9 | < LOD | < LOD | < LOD | 0.3 | ||
| NHANES | 224 | 0.1–0.4 | 17.0 | < LOD | 0.1 | 0.2 | 1.4 | |
| DME | 1 | 479 | 0.03 | 0.8 | < LOD | < LOD | < LOD | 1.2 |
| 2 | 466 | 3.7 | < LOD | < LOD | < LOD | 2.0 | ||
| NHANES | — | — | — | — | — | — | — | |
| ETU | 1 | 446 | 0.1 | 23.5 | < LOD | 0.7 | 1.5 | 14.4 |
| 2 | 466 | 7.7 | < LOD | < LOD | 0.4 | 11.0 | ||
| NHANES | — | — | — | — | — | — | — | |
| 4F3PBA | 1 | 538 | 0.2 | 3.2 | < LOD | < LOD | < LOD | 75.2 |
| 2 | 481 | 1.9 | < LOD | < LOD | < LOD | 26.0 | ||
| NHANES | 222 | 0.2 | 1.4 | < LOD | < LOD | < LOD | 5.6 | |
| IMPY | 1 | 538 | 0.7 | 2.2 | < LOD | < LOD | < LOD | 7.7 |
| 2 | 481 | 2.7 | < LOD | < LOD | < LOD | 7.3 | ||
| NHANES | 222 | 0.7–7.0 | 11.7 | < LOD | 1.3 | 1.8 | 12.0 | |
| MDA | 1 | 359 | 0.3 | 32.0 | 0.5 | 1.3 | 2.3 | 57.5 |
| 2 | 265 | 26.4 | 0.4 | 1.3 | 1.9 | 45.1 | ||
| NHANES | 92 | ≤ 2.6 | 41.3 | 0.6 | 0.9 | 1.8 | 7.4 | |
| MET | 1 | 538 | 0.2 | 7.3 | < LOD | < LOD | 0.23 | 0.3 |
| 2 | 481 | 1.9 | < LOD | < LOD | < LOD | 0.5 | ||
| NHANES | 127 | 0.2 | 1.6 | < LOD | < LOD | < LOD | 0.3 | |
| PCP | 1 | 523 | 0.9 | 4.0 | < LOD | < LOD | < LOD | 8.3 |
| 2 | 479 | 2.3 | < LOD | < LOD | < LOD | 29.5 | ||
| NHANES | 224 | 0.3–0.5 | 10.3 | < LOD | 0.4 | 1.5 | 15.2 | |
| 3PBA2 | 1 | 538 | 0.1 | 21.4 | < LOD | 0.5 | 0.9 | 222.6 |
| 2 | 481 | 27.0 | 0.1 | 0.5 | 1.1 | 225.0 | ||
| NHANES | 224 | 0.1 | 67.0 | 0.5 | 1.3 | 2.2 | 58.7 | |
| 2,4,5-TCP | 1 | 517 | 1.0 | 45.1 | 7.9 | 45.6 | 203.1 | 2145.5 |
| 2 | 479 | 48.9 | 8.3 | 37.4 | 65.2 | 554.1 | ||
| NHANES | 224 | 0.9 | 24.1 | 0.6 | 2.5 | 5.0 | 95.5 | |
Abbreviations: DF, detection frequency; Max, maximum. CHAMACOS prenatal sample 1 was collected at approximately 13 weeks gestation, and prenatal sample 2 was collected at approximately 26 weeks gestation. NHANES samples were collected around 24 weeks gestation (mean = 24.4 ± 9.5 weeks). ACET, ATZ, CMHC, DBCA, IPP, MMP, OMT, PTU, and 2,4,5-T, were not included because these compounds were detected in < 3% of prenatal samples (DF ≤ 3%).
Spearman correlations between the two CHAMACOS sampling points for the eight most frequently detected metabolites ranged from 0.01 to 0.31. Most correlations were weak (rho ~ 0.15). Moderate correlations were observed for 2,5-DCP (rho = 0.31) and 2-naphthol (rho = 0.26). The intraclass correlation coefficients between the two sampling points for these metabolites ranged from 0.02 to 0.30. Overall, these analyses indicate weak to moderate correlations between the two sampling points for all frequently detected compounds except 1-naphthol and ORTH [see Supplemental Material, Table 1 (doi:10.1289/ehp.0901568)].
The correlations of the eight most frequently detected metabolites within each CHAMACOS sampling time cross-section ranged from weakly negative to strongly positive (Spearman rho = –0.13 to 0.66), indicating that some participants were exposed simultaneously to several pesticides [see Supplemental Material, Table 2 (doi:10.1289/ehp.0901568)]. The strongest correlations were found between 2,4,6-TCP and TCPy within the first and second prenatal samples (rho = 0.66 and rho = 0.42, respectively; p < 0.01) and between 2,4-DCP and 2,5-DCP within the first and second prenatal samples (rho = 0.61 and rho = 0.65, respectively; p < 0.01). 1-Naphthol and 2-naphthol, which can devolve from naphthalene, were moderately correlated (rho = 0.40 and 0.55 within the first and second prenatal samples, respectively; p < 0.01). Similarly, in NHANES, 2,4,6-TCP and TCPy (rho = 0.55) and 2,4-DCP and 2,5-DCP (rho = 0.53) were strongly correlated (p < 0.01). 1-Naphthol and 2-naphthol levels were also significantly correlated (rho = 0.56; p < 0.01) in NHANES. Overall, the patterns of within-sample correlations were similar between CHAMACOS and NHANES. In all cases except PNP, when two metabolites were correlated in NHANES, the same metabolites were also significantly correlated in one or both of the CHAMACOS prenatal samples.
In the CHAMACOS cohort, the detection frequency of ETU was higher at the first (23.5%) compared with the second (7.7%) sampling time. In addition, the 95th percentile value of ETU was significantly higher in the first compared with the second prenatal sample (1.5 μg/L vs. 0.4 μg/L, respectively; p < 0.01).
Comparison of CHAMACOS and NHANES metabolite levels
Results of the Wilcoxon rank-sum test suggest that for six (2,5-DCP; MDA; 2-naphthol; 3PBA2; 2,4,5-TCP; and 2,4,6-TCP) of 11 frequently detected metabolites (i.e., detection frequencies > 40%), there were statistically significant differences between the distributions of the CHAMACOS first prenatal samples and NHANES metabolite levels (p < 0.05). Among these six compounds, the distributions of four (2,5-DCP; MDA; 2,4,5-TCP; and 2,4,6-TCP) of the first prenatal CHAMACOS metabolite levels were higher compared with those in NHANES, and the distributions of two NHANES metabolite levels (2-naphthol and 3PBA2) were higher compared with those in CHAMACOS.
In addition, there were statistically significant differences between the distributions of eight (2,5-DCP; MDA; 2-naphthol; PNP; 3PBA2; 2,4,5-TCP; 2,4,6-TCP; and TCPy) of the 11 frequently detected metabolites in the CHAMACOS second sample versus the NHANES metabolite levels (p < 0.05). Among these eight compounds, the distributions of six (2,5-DCP; MDA; PNP; 2,4,5-TCP; 2,4,6-TCP; and TCPy) of the second prenatal CHAMACOS metabolites were higher compared with those in NHANES, and the distributions of two NHANES metabolite levels (2-naphthol and 3PBA2) were higher compared with those in CHAMACOS. The NHANES prenatal samples were collected at approximately 24 weeks gestation, and the CHAMACOS first and second prenatal samples were collected at approximately 13 and 26 weeks gestation, respectively. Thus, the CHAMACOS second prenatal metabolite levels may be more comparable with those from pregnant women in NHANES.
We compared median concentrations of the six compounds with detection frequencies > 50% in both populations using quantile regression and adjusting for age, race, socioeconomic status, and smoking. The CHAMACOS first prenatal median 2,4-DCP and 2,5-DCP concentrations were higher than NHANES (p < 0.05). Further, the CHAMACOS second prenatal median 2,5-DCP, 2,4,6-TCP, and TCPy concentrations were also higher than NHANES (p < 0.05). By contrast, the NHANES 2-naphthol median levels were significantly higher than the CHAMACOS first and second prenatal levels (p < 0.05) (Table 3).
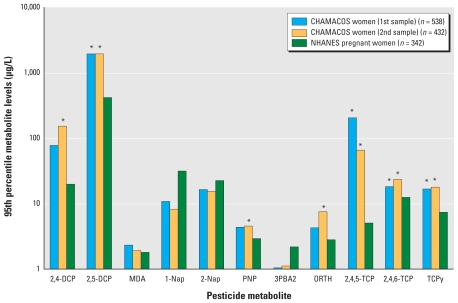
Figure 1 presents a comparison of the 95th percentile metabolite values for the CHAMACOS cohort (both first and second prenatal samples) and NHANES for the 11 metabolites detected at > 40% in either study. Using quantile regression, the CHAMACOS first and/or second prenatal 95th percentile values were statistically higher compared with NHANES for seven of the 11 metabolites. These seven included six of the most commonly detected metabolites (2,4-DCP; 2,5-DCP; ORTH; PNP; 2,4,6-TCP; and TCPy). [Probability plots examining the z-scores of CHAMACOS vs. NHANES TCPy, 2,4,5-TCP, and 2,5-DCP levels are presented in the Supplemental Material (doi:10.1289/ehp.0901568).] We found no significant differences between the CHAMACOS first and second prenatal 95th percentile metabolite levels presented in Figure 1.
Figure 1.
CHAMACOS first and second prenatal 95th percentile metabolite levels compared with pregnant women in NHANES 1999–2002. Nap, naphthol.
*95th percentile quantile regression adjusting for age, race, socioeconomic status, and smoking; p < 0.05.
A metabolite of several commonly used pyrethroids (3PBA2), including permethrin, cypermethrin, cyhalothrin, deltamethrin, and fenvalerate, was detected in urine samples of 67% of NHANES pregnant women compared with 21% and 27% in the CHAMACOS first and second prenatal samples, respectively. In addition, the CHAMACOS 3PBA2 95th-percentile metabolite levels were lower than the NHANES level, albeit not significantly (Figure 1). We could not compare the CHAMACOS and NHANES metabolite levels of several high-use precursor pesticides (dimethoate, acephate, methamidophos, and maneb) because they have not yet been reported in NHANES.
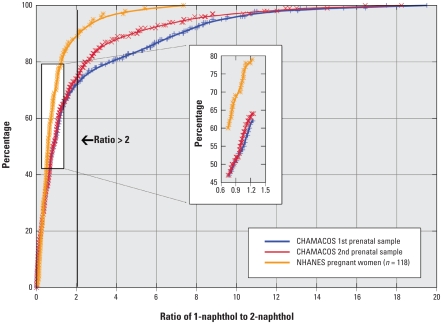
Figure 2 presents the cumulative distributions of urinary 1-naphthol to 2-naphthol ratios in the CHAMACOS cohort and NHANES. Ratios > 2 occurred in 22% and 24% of CHAMACOS first and second prenatal samples compared with 13% among pregnant women in NHANES. Conversely, the frequency of 1-naphthol to 2-naphthol ratios in the region near unity (i.e., ratios ranging from 0.75 to 1.25) were higher among women in NHANES (32%) compared with the CHAMACOS women at the first and second prenatal sampling times (19% and 20%, respectively) (Figure 2 inset).
Figure 2.
Cumulative distributions of urinary 1-naphthol to 2-naphthol ratios for the CHAMACOS cohort and pregnant women in NHANES 2000–2001.
Discussion
We measured 34 specific metabolites in two urine samples collected at approximately 13 and 26 weeks gestation from 538 pregnant women living in an agricultural area, including tests for fungicides, carbamate, OC, OP and pyrethroid insecticides, triazine and chloroacetanilide herbicides, and several chlorinated phenol compounds. To better understand the impact of the regional environment on these metabolite levels in the CHAMACOS cohort, we compared urinary metabolite concentrations from the CHAMACOS cohort with U.S. national reference data for 342 pregnant women sampled by NHANES (1999–2002).
Overall, our results suggest that this population is chronically exposed to several current-use pesticides and intermittently exposed to others. Between 78% and 86% of CHAMACOS women had urine samples with biomarkers for at least one OP pesticide, and 30–34% were exposed to two or more compounds. These findings are consistent with earlier data that we reported indicating that the nonspecific OP dialkylphosphate metabolites were present in > 90% of CHAMACOS women, with levels significantly higher than those found for women in NHANES 1999–2000 (Bradman et al. 2005).
Other studies comparing biomonitoring data with NHANES have found that population-specific factors may explain higher metabolite levels or detection frequencies (Bradman et al. 2005, 2007; Fenske et al. 2005). Of 26 possible comparisons between CHAMACOS and NHANES urinary metabolite levels, detection frequencies were > 50% for just seven metabolites measured in NHANES and eight measured in CHAMACOS. Notably, six of the frequently detected compounds were common to both studies (2,4-DCP; 2,5-DCP; 1-naphthol; 2-naphthol; 2,4,6-TCP; and TCPy). These metabolites devolve from chlorpyrifos; carbaryl; naphthalene; lindane [γ-hexachlorocyclohexane (γ-HCH)]; 1,3- and 1,4-dichlorobenzene; and PCP (Table 1), all relatively persistent in the environment. The increase in CHAMACOS concentrations at the 95th percentile for the chlorinated phenol compounds (2,4- and 2,5-DCP and 2,4,6-TCP) is likely contributed to by population-specific but nonagricultural factors such as proximity to manufacturing or waste incineration, and household product choices (e.g., mothballs, cleaning detergents, and head lice treatments). The similarity of OP pesticide metabolite detection frequencies between the CHAMACOS cohort and a U.S. national reference population support previous multimedia, multipathway modeling efforts that suggest that diet is a major route of exposure in both populations (McKone et al. 2007).
In our analysis, we have limited our metabolite level comparisons with NHANES to pregnant women. By doing so, we compare the CHAMACOS cohort with a national sample of woman also experiencing the changes in diet and metabolism that accompany pregnancy. Furthermore, through this research we have expanded the number of pesticide-specific metabolites reported in pregnant women and provided repeat measurements from samples collected at consistent prenatal time points. Many of the compounds presented here have not been measured or reported before in pregnant women.
Human exposure to naphthalene, a polycyclic aromatic hydrocarbon and component of vehicle exhaust, smoke, and mothballs, is widespread. Carbaryl (n-methylcarbamate insecticide) is commonly used in agriculture. Naphthalene devolves to 1- and 2-hydroxynaphthalene (1- and 2-naphthol) in approximately equal proportions, whereas carbaryl devolves to 1-naphthol only. Thus, a higher ratio of 1-naphthol to 2-naphthol suggests exposure to carbaryl. Median 1-naphthol concentrations in the CHAMACOS cohort were similar to median concentrations among pregnant women in NHANES 2001–2002; however, the median and underlying distribution of the 2-naphthol metabolite were statistically greater in NHANES compared with the CHAMACOS first and second prenatal samples. The higher levels in NHANES may be due to higher smoking rates in pregnant women participating in NHANES (7.4%) compared with CHAMACOS (1.5%). Our finding that 23% of CHAMACOS women had 1-naphthol to 2-naphthol ratios > 2 compared with 13% among pregnant women in NHANES suggests additional sources of carbaryl exposure in the CHAMACOS cohort (Meeker et al. 2007).
ETU is the principal metabolite of the EBDC fungicides (maneb and mancozeb). ETU is more toxic than its precursor compounds (Houeto et al. 1995). More than 150,000 kg of maneb and mancozeb are used annually in the Salinas Valley region, primarily on lettuce and wine grapes (CDPR 2000a). CHAMACOS is the first study to report repeated urinary ETU levels in a U.S. population and in pregnant women. We found that the ETU levels decreased with pregnancy, with higher detection frequencies and levels in samples collected during the first trimester compared with samples collected during the second trimester. We do not know if this change is due to differences in exposure or in metabolism over the course of pregnancy. More information on the metabolism and excretion of these compounds during pregnancy is needed. Urinary ETU levels in the CHAMACOS cohort were similar to those of nonoccupational populations studied in Italy (Aprea et al. 1996; Colosio et al. 2006; Saieva et al. 2004), but lower than those reported for pesticide applicators in other countries (Colosio et al. 2002; Panganiban et al. 2004; Steenland et al. 1997).
Our findings indicate that pregnant women in the Salinas Valley are chronically exposed to several current-use OP and OC pesticides and chlorinated phenols, with additional intermittent exposures to other pesticides. Overall, pesticide metabolite detection frequencies in our population were similar to those found in a U.S. reference population of pregnant women; however, significantly higher 95th percentiles for several metabolites (e.g., PNP; 2,4,5-TCP; TCPy) in our population suggest that a subset of CHAMACOS women experience additional exposures potentially related to regional agricultural pesticide use. The cumulative effect of these exposure levels on pregnant women or their offspring is not known.
Footnotes
This publication was made possible by research supported by grants RO1 OH007400-04 and PO1 ES009605 from National Institute of Environmental Health Sciences (NIEHS) and grant RD 83171001 from the U.S. Environmental Protection Agency (EPA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. EPA, NIEHS, or the Centers for Disease Control and Prevention.
Supplemental Material is available online (doi:10.1289/ehp.0901568 via http://dx.doi.org/).
We gratefully acknowledge the CHAMACOS staff, students, and community partners, especially the CHAMACOS participants and their families, without whom this study would not be possible.
References
- Aprea C, Betta A, Catenacci G, Lotti A, Minoia C, Passini W, et al. Reference values of urinary ethylenethiourea in four regions of Italy (multicentric study) Sci Total Environ. 1996;29;192(1):83–93. doi: 10.1016/0048-9697(96)05300-4. [DOI] [PubMed] [Google Scholar]
- Barr DB, Barr JR, Driskell WJ, Hill RH, Ashley DL, Needham LL, et al. Strategies for biological monitoring of exposure for contemporary-use pesticides. Toxicol Ind Health. 1999;15(1–2):168–179. doi: 10.1191/074823399678846556. [DOI] [PubMed] [Google Scholar]
- Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, et al. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99(3):314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Becker JG, Whitworth JA, Kincaid-Smith P. Clinical Nephrology in Medical Practice. Boston: Blackwell Scientific Publications; 1992. [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111:1782–1789. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Schwartz JM, Fenster L, Barr DB, Holland NT, Eskenazi B. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expo Sci Environ Epidemiol. 2007;17(4):388–399. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Fernandez C, Smith KD, Gallegos M, Whitehead RD, Jr, et al. Quantification of phenolic metabolites of environmental chemicals in human urine using gas chromatography-tandem mass spectrometry and isotope dilution quantification. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820(2):229–236. doi: 10.1016/j.jchromb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) 2001–2002 National Health and Nutrition Examination Survey (NHANES) 2004. [[accessed 3 June 2009]]. Available: http://www.cdc.gov/nchs/nhanes/nhanes01-02.htm.
- CDC (Centers for Disease Control and Prevention) NCEH Pub. No. 05-0570. Atlanta: National Center for Environmental Health, Centers for Disease Control and Prevention; 2005. Third National Report on Human Exposure to Environmental Chemicals. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) NHANES 1999–2000. 2007a. [[accessed 1 June 2008]]. Available: http://www.cdc.gov/nchs/nhanes/demo99_00.htm.
- CDC (Centers for Disease Control and Prevention) NHANES 2001–2002. 2007b. [[accessed 1 June 2008]]. Available: http://www.cdc.gov/nchs/nhanes/demo01_02.htm.
- CDPR (California Department of Pesticide Regulation) Pesticide Use Report 2000, Indexed by Chemical and by Crop. Sacramento: Department of Pesticide Regulation, California Environmental Protection Agency; 2000a. [Google Scholar]
- CDPR (California Department of Pesticide Regulation) Pesticide Use Reporting: An Overview of California’s Unique Full Reporting System. Sacramento: Department of Pesticide Regulation, California Environmental Protection Agency; 2000b. [Google Scholar]
- CDPR (California Department of Pesticide Regulation) Pesticide Use Report 2006, Indexed by Chemical and by Crop. Sacramento: Department of Pesticide Regulation, California Environmental Protection Agency; 2006. [Google Scholar]
- Colosio C, Fustinoni S, Birindelli S, Bonomi I, De Paschale G, Mammone T, et al. Ethylenethiourea in urine as an indicator of exposure to mancozeb in vineyard workers. Toxicol Lett. 2002;134(1–3):133–140. doi: 10.1016/s0378-4274(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Colosio C, Visentin S, Birindelli S, Campo L, Fustinoni S, Mariani F, et al. Reference values for ethylenethiourea in urine in Northern Italy: results of a pilot study. Toxicol Lett. 2006;162(2–3):153–157. doi: 10.1016/j.toxlet.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W, Ezimokhai M. 24-hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol. 1980;87(2):106–109. doi: 10.1111/j.1471-0528.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88(1):10–17. doi: 10.1111/j.1471-0528.1981.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Gladstone EA, Jaramillo S, Birch K, Holland N. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J Child Health. 2003;1(1):3–27. [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;12:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102(2):228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Curl CL, Shirai JH, Kissel JC. Biologic monitoring to characterize organophosphorus pesticide exposure among children and workers: an analysis of recent studies in Washington State. Environ Health Perspect. 2005;113:1651–1657. doi: 10.1289/ehp.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L, Eskenazi B, Bradman A, Jewell N. Risk behaviors for pesticide exposure among pregnant women living in farmworker households in Salinas, CA. Am J Ind Med. 2004;45:491–499. doi: 10.1002/ajim.20012. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- Houeto P, Bindoula G, Hoffman JR. Ethylenebisdithiocarbamates and ethylenethiourea: possible human health hazards. Environ Health Perspect. 1995;103:568–573. doi: 10.1289/ehp.95103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int J Occup Med Environ Health. 2008;21(2):21–32. doi: 10.2478/v10001-008-0014-z. [DOI] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006;114:260–263. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J Expo Sci Environ Epidemiol. 2007;17(4):314–320. doi: 10.1038/sj.jes.7500502. [DOI] [PubMed] [Google Scholar]
- McKone TE, Castorina R, Harnly ME, Kuwabara Y, Eskenazi B, Bradman A. Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California. Environ Sci Technol. 2007;41(9):3233–3240. doi: 10.1021/es0618447. [DOI] [PubMed] [Google Scholar]
- Montesano MA, Olsson AO, Kuklenyik P, Needham LL, Bradman AS, Barr DB. Method for determination of acephate, methamidophos, omethoate, dimethoate, ethylenethiourea and propylenethiourea in human urine using high-performance liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Expo Sci Environ Epidemiol. 2007;17(4):321–330. doi: 10.1038/sj.jes.7500550. [DOI] [PubMed] [Google Scholar]
- Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD, et al. A liquid chromatography–tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and DEET in human urine. Anal Chem. 2004;76(9):2453–2461. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- Panganiban L, Cortes-Maramba N, Dioquino C, Suplido ML, Ho H, Francisco-Rivera A, et al. Correlation between blood ethylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environ Health Perspect. 2004;112:42–45. doi: 10.1289/ehp.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26(4):573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Saieva C, Aprea C, Tumino R, Masala G, Salvini S, Frasca G, et al. Twenty-four-hour urinary excretion of ten pesticide metabolites in healthy adults in two different areas of Italy (Florence and Ragusa) Sci Total Environ. 2004;332(1–3):71–80. doi: 10.1016/j.scitotenv.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Steenland K, Cedillo L, Tucker J, Hines C, Sorensen K, Deddens J, et al. Thyroid hormones and cytogenetic outcomes in backpack sprayers using ethylenebis(dithiocarbamate) (EBDC) fungicides in Mexico. Environ Health Perspect. 1997;105:1126–1130. doi: 10.1289/ehp.971051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res. 2004;95(2):119–125. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Westgard JO. Westgard QC: Tools, Technology, and Training for Healthcare Laboratories. Madison, WI: Westgard QC, Inc; 2003. [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]