Abstract
Hepatitis C virus (HCV) is neurovirulent and has been shown to be associated with neuropsychological (NP) deficits in a subset of infected individuals. Despite these previous findings, little work has been done to examine neurobehavioral symptoms associated with HCV infection. We examined 34 HCV seropositive (HCV+) individuals and 35 healthy comparison participants (HCV−) with the self-rating form of the Frontal Systems Behavior Scale (FrSBe). Results showed that at the group level, only the FrSBe apathy subscale mean was clinically elevated (T-score >65) among HCV+ persons; executive dysfunction, disinhibition, and total subscale means were not clinically elevated. At the individual level, a significantly higher proportion of HCV+ individuals reported clinically elevated FrSBe T-scores as compared to HCV− individuals. Moreover, HCV+ individuals were nearly 3 times as likely to report clinically elevated FrSBe T-scores of apathy, executive dysfunction, and disinhibition as compared to HCV− participants. A multiple regression that included substance use disorders, neuropsychological impairment and age indicated that HCV status was an independent predictor of self-reported FrSBe total T-scores. Across all participants, small, yet significant, correlations were found between elevated self-reported FrsBe T-scores and dependence in activities of daily living. These results show that a subset of HCV infected individuals report clinically elevated behavioral symptoms. Clinical implications for the assessment and management of elevated behavioral symptoms in HCV are discussed.
Keywords: Hepatitis, Executive functions, Prefrontal cortex, Apathy, Neuropsychology, Substance abuse
The Hepatitis C Virus (HCV) is a major cause of acute hepatitis and chronic liver disease, including cirrhosis and liver cancer. Globally, an estimated 170 million persons are chronically infected with HCV and 3 to 4 million persons are newly infected each year. In the United States, HCV is a growing health problem that affects approximately 4 million adults (Alter et al., 1999; Shepard, Finelli, & Alter, 2005).
Increasingly, evidence supports the hypothesis that HCV affects the central nervous system (CNS) in a subset of infected individuals. The exact mechanism by which HCV enters the CNS is not known; however, it has been postulated that HCV may cross the blood-brain-barrier via infected monocytes using a “Trojan horse” mechanism (Forton, Taylor-Robinson, & Thomas, 2006). Further evidence of HCV entering the CNS comes from a recent study of co-infected HCV/HIV individuals, which revealed the presence of HCV antigens in the white matter, basal ganglia and frontal cortex (Letendre et al., 2007). Also, imaging studies have found elevations in metabolic markers of neuronal injury and neuroinflammation in the basal ganglia and frontal white matter of persons with HCV (Forton et al., 2002).
Several studies have demonstrated evidence of mild neurocognitive impairment across a variety of ability areas among a subset of HCV-infected individuals (see Forton et al. 2006 for a review). The severity and precise neurocognitive domains affected vary across the literature (Fontana et al., 2005); for example, McAndrews et al., (2005) found only mild overall cognitive impairment in a cohort of non-confounded HCV+ individuals with specific impairments in the learning domain as compared to seronegative participants. Other data suggest that some HCV-infected individuals may have more diffuse neurocognitive deficits in neuropsychological domains associated with prefrontal systems, including deficits in complex information processing (Hilsabeck, Hassanein, Carlson, Ziegler, & Perry, 2003; Hilsabeck, Perry, & Hassanein, 2002), motor skills (Ryan, Morgello, Isaacs, Naseer, & Gerits, 2004), and executive functions (Córdoba et al.,2003; Bieliauskas et al., 2006). More specifically, Bieliauskas and colleagues (2006) reported that approximately 30% of HCV infected individuals were impaired on a test that required mental flexibility, abstract reasoning, and concept formation. Similarly, Córdoba and colleagues (2003) found that HCV infection was associated with mild deficits on tasks that assessed verbal response inhibition and verbal fluency. In summary, mild neurocognitive impairments are observed among a subgroup of HCV+ persons even in the absence of frank liver disease.
HCV-infected individuals can experience substantial declines in health-related quality of life (Bonkovsky et al., 2007) and performance of activities of daily living (ADLs) (Vigil et al., 2008). Several studies have examined the link between HCV-associated neuropsychological impairment and everyday functioning (Kramer et al., 2002). This literature shows that HCV+ persons with neuropsychological impairment are at greater risk of poorer functional outcomes, including physical and instrumental ADLs (Vigil et al., 2008), and poorer health-related quality of life (Bieliauskas et al., 2006). However, no study has examined whether HCV infection is specifically associated with everyday behavioral problems that are commonly associated with frontal systems (i.e., apathy, disinhibition, executive dysfunction). Also, no study has examined whether these day-to-day behavioral difficulties are related to neuropsychological impairment, and/or substance dependence in HCV, nor whether they are associated with an increased risk of ADL dependence.
Accordingly, the present study examines the association between HCV infection and elevated self-reported behavioral symptoms (i.e., apathy, disinhibition, and executive dysfunction). In addition, we sought to determine the predictors, including neuropsychological impairment, of these behavioral symptoms. Finally, we examined whether elevated behavioral symptoms were associated with dependence on physical and/or instrumental ADLs.
Methods
Participants
Participants were recruited from the HIV Neurobehavioral Research Center (HNRC) and were enrolled in a National Institute of Drug Abuse (NIDA)-sponsored program project examining the combined central nervous system effects of methamphetamine, HIV and HCV. We identified 34 HCV seropositive (HCV+) individuals and 35 HCV seronegative (HCV−) individuals. HCV serostatus was confirmed by standard clinical antibody detection. HCV RNA was measured in serum using RT-PCR (NGI SuperQuant; National Genetics Institute, Los Angeles, CA, USA; nominal detection limit of 100 IU/mL). All HCV+ individuals were HCV treatment naïve. All participants in the present study were HIV seronegative, as confirmed by enzyme linked immunosorobent assays (ELISA). Given the known influence of methamphetamine abuse/dependence on frontal systems (see Scott et al., 2007 for a review), we chose to exclude individuals with past or current methamphetamine abuse and/or dependence. Because the parent study from which these data were drawn is specifically designed to examine the CNS effects of chronic methamphetamine use, history of methamphetamine use disorder was more chronic, severe, and recent than abuse/dependence on other substances and therefore represented a significant confound requiring exclusion. Given the potentially confounding influence of current major depressive disorder (MDD) on self-report questionnaires, we chose to exclude individuals with this diagnosis from the present study as well. Finally, parent project exclusion criteria included history of severe psychiatric illness (e.g., schizophrenia), head trauma with loss of consciousness greater than 30 minutes, neurological disease, or current substance abuse or dependence (i.e., alcohol, cocaine, opioid, marijuana, hallucinogens).
The demographic and clinical characteristics of the study participants are presented in Table 1. The study cohort was predominantly Caucasian, male, and on average completed one year of college education. The study groups were demographically comparable, with the exception that the HCV+ group was significantly older than the comparison sample. There was a relatively high prevalence of lifetime DSM-IV diagnoses of substance abuse and dependence within the HCV+ group. Specifically, a significantly greater proportion HCV+ participants met criteria for past alcohol, cocaine and opioid disorders than HCV− individuals. In terms of HCV disease makers, HCV RNA levels (which indicate HCV replication but do not correlate well with liver disease progression) are considered normal in our cohort. The AST-to-Platelet Ratio Index (APRI) is a measure that provides indirect evidence of liver fibrosis. APRI values above 1.5 are associated with greater risk for substantial liver fibrosis (stage F3 or worse) compared with values of 1.5 or below. Among our study participants, the median APRI was well below 1.5, and only two individuals had an APRI above 1.5. FrSBe scores for these two individuals with elevated APRI scores were mixed (i.e., one had clinically elevated FrSBe scores and the other did not).
Table 1.
Demographic and Clinical Characteristics of Study Cohort and Comparison Group
| HCV+ (n = 34) | HCV− (n = 35) | |
|---|---|---|
| Age, years M (SD)** | 49.5 (9.3) | 38.7 (11.2) |
| Education M (SD) | 13.7 (2.1) | 13.3 (2.3) |
| Male (%) | 56 | 69 |
| Caucasian (%) | 71 | 66 |
| Major Depression Lifetime (%) | 37 | 34 |
| NP Impaired by GDS (%)** | 32 | 9 |
| Lifetime Substance Disorder % (# individuals) | ||
| Alcohol Disorder ** | 50 (17) | 17 (6) |
| Cocaine Disorder ** | 23 (8) | 0 (0) |
| Opioid Disorder ** | 18 (6) | 0 (0) |
| Marijuana Disorder | 24 (8) | 14 (5) |
| Hallucinogens Disorder | 6 (2) | 0 (0) |
| All Combined Any Disorder ** | 53 (18) | 26 (9) |
| HCV Characteristics (Median, IQR) | ||
| HCV RNA serum (IU/ml) | 6.1 (3.2, 6.6) | |
| Total bilirubin (mg/dL) | 0.8 (0.6, 0.9) | |
| Albumin (g/dL) | 3.9 (3.8, 4.1) | |
| AST-to-Platelet Ratio Index (APRI) | 0.5 (0.3, 0.8) | |
Note. Substance use disorder was defined as meeting criteria for alcohol and/or substance abuse/dependence in the past according to the Composite International Diagnostic Interview (CIDI). Categories are not mutually exclusive. The “All Combined Any Disorder” category represents the percentage of individuals that met criteria for dependence and/or abuse on one or more substances. HCV = Hepatitis C virus; AST = aspartate aminotransferase; IQR = interquartile range; NP = neuropsychological.
p <.01.
Procedure
Participants completed the self-report version of the Frontal Systems Behavior Scale (Grace & Malloy, 2001). The FrSBe is a 46-item standardized rating form that generates a total frontal dysfunction score, as well as three subscale scores: apathy, disinhibition and executive dysfunction. Ratings are on a Likert-type scale that ranges from 1 (“almost never”) to 5 (“almost always”) for each question. Higher ratings indicate more abnormal behavior. The raw scores are converted into demographically (i.e., by age, education, and gender) adjusted T-scores. A T-score cut point of 65 or higher is recommended to capture greater degrees of symptomatology and clinical significance. The scale allows for retrospective ratings from prior to the injury or illness (before), and for ratings following the injury or illness (after), creating a baseline measure with which to compare subsequent ratings (Grace & Malloy, 2001). However, for the present study only the “after” scores were analyzed because the onset of HCV symptoms can be insidious and it may have been difficult for participants to identify a particular symptom onset. The FrSBe T-scores (after) for the total score and the three subscales scores were considered the dependent variables. Although the FrSBe was designed to assess behavioral change in neurological conditions with a clear onset, (Stout, Ready, Grace, Malloy, & Paulsen, 2003) it has been widely used in other conditions in which there is no such clear onset: multiple sclerosis (Basso et al., 2008), schizophrenia (Velligan, Ritch, Sui, DiCocco, & Huntzinger, 2002) and Obsessive Compulsive Disorder (Batistuzzo et al., 2009).
Participants also completed a comprehensive neuropsychological assessment covering seven cognitive domains: Verbal Fluency, Working Memory, Speed of Information Processing, Learning, Recall, Executive Functions, and Fine-motor Coordination. Raw scores for each test were converted into demographically-adjusted T-scores. Scores were then converted to deficit scores to determine the prevalence of neurocognitive impairment in the study samples (T-score ≥40=0, 39-35=1, 34-30=2, 29-25=3, 24-29=4, ≤ 19=5). These individual deficit scores were averaged to create a Global Deficit Score (GDS). A GDS ≥ 0.5 was indicative of global neuropsychological impairment. (See Carey, et al, 2004, for details on the methodology and predictive validity of this index.)
Trained interviewers administered the Composite International Diagnostic Interview (CIDI) to the participants. The CIDI is a computer-assisted interview that provides a cross-cultural assessment of alcohol, drug, and mental disorders using DSM-IV criteria (Wittchen et al., 1991). The CIDI was used to obtain diagnoses of current (an exclusion criterion) and lifetime major depressive disorder, and psychoactive substance abuse and dependence disorders.
We also administered a modified version of the Activities of Daily Living (ADL) Scale (Lawton & Brody, 1969) to examine activities of daily living among the HCV+ individuals. The ADL scale consists of items that detail the degree to which individuals independently function in 12 different areas related to daily tasks: 5 physical ADL items (housekeeping, home repairs, bathing, dressing, and laundry) and 7 instrumental ADL items (managing finances, buying groceries, cooking, transportation, shopping, medication management, and employment). Participants separately rated their current and highest (previous) level of functioning for each activity related to an area of functioning. Items in the ADL scale are rated on either a 4-point scale (0–3) or a 3-point scale (0–2), with higher scores indicating poorer functioning. Dependence on ADLs is indicated when an individual has a decline greater than 2 points on any of the areas of functioning (a decline is indicated when an individual rated their current level of functioning lower than their past best level).
Statistical Analyses
In order to accommodate departures from normality in individual variable distributions, we used nonparametric statistical tests as indicated, including the Wilcoxon Rank Sum test for between-group comparisons and Spearman’s ρ for correlations. Cohen’s d statistic and odds ratio calculations were used to measure the effect sizes of the group comparisons. Those variables that proved to be significantly different between the groups were included in a least squares regression analysis to determine the relative independence of HCV as a predictor of FrSBe T-scores.
Results
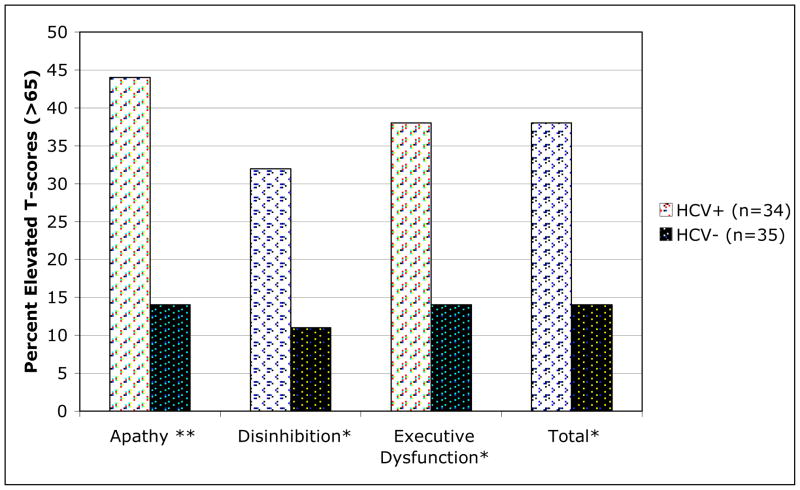
Compared to HCV− participants, HCV+ individuals had significantly higher T-scores on the FrSBe total, as well as on the apathy, disinhibition and executive dysfunction subscales (all ps< .05). These significant group differences were accompanied by medium effect sizes (Table 2). However, at the group level, only the FrSBe apathy subscale mean was clinically elevated (T-score >65) among HCV+ persons. Not all HCV+ individuals reported clinically elevated behavioral symptoms; however, the proportion of HCV+ participants reporting clinical elevations was significantly higher as compared to HCV-seronegative comparisons. The proportion of HCV+ individuals reporting clinically elevated behavioral symptoms ranged from 32.4% on the disinhibition subscale to 44.1% on the apathy subscale (see Figure 1). The odds ratio, representing the odds of being classified as having elevated behavioral symptoms rather than non-elevated among the HCV+ persons relative to those same odds among the HCV− individuals, were apathy subscale: 3.09 (95% CI = 1.00, 7.56), disinhibition subscale: 2.83 (95% CI = 1.00, 8.03), executive dysfunction subscale: 2.68 (95% CI = 1.07, 6.70) and total scale: 2.68 (95% CI = 1.07, 6.70). Importantly, we ran these same analyses excluding all the individuals with a lifetime diagnosis of cocaine abuse and/or dependence and found that these effects remained significant (ps < .05).
Table 2.
FrSBe Subscales and Total T-scores and Effect Sizes
| FrSBe Scale M (SD) | HCV+ (n = 34) | HCV− (n = 35) | p value | d | 95% Confidence Interval |
|---|---|---|---|---|---|
| Apathy | 64.7 (18.6) | 50.8 (18.9) | 0.003 | 0.73 | 0.25, 1.22 |
| Disinhibition | 56.4 (18.4) | 47.0 (13.4) | 0.017 | 0.58 | 0.10, 1.06 |
| Executive Dysfunction | 58.5 (14.2) | 47.7 (16.5) | 0.004 | 0.69 | 0.21, 1.18 |
| Total | 61.8 (18.2) | 48.1 (18.9) | 0.003 | 0.73 | 0.24, 1.22 |
Note. Data represent means and standard deviations. HCV = Hepatitis C virus; d = Cohen’s d.
Figure 1.
A significantly higher proportion of HCV+ individuals had clinically elevated FrSBe T-scores compared to HCV− comparison participants.
Note. *p < 0.05 ** p < 0.01.
In terms of neuropsychological functioning, HCV+ individuals had significantly greater overall neuropsychological deficits, albeit mild, than HCV− persons. Based on their GDS score (≥ 0.5 = impaired), 32% of HCV+ individuals were considered cognitively impaired whereas only 9% of the HCV− persons were impaired (p = 0.007)
In terms of the association between behavioral symptoms and neuropsychological functioning in the HCV+ positive group, both FrSBe executive dysfunction and FrSBe total T-scores were significantly correlated with GDS (all ps <0.05). When examining the HCV− group, none of the FrSBe subscales were significantly correlated with GDS (Table 3).
Table 3.
Spearman’s correlations between FrSBe Scores and the Global Deficit Score (GDS)
| FrSBe Scale | GDS All Participants (N=69) | GDS HCV+ (n=34) | GDS HCV− (n=35) |
|---|---|---|---|
| Apathy | 0.26* | 0.20 | −0.01 |
| Disinhibition | 0.23 | 0.23 | 0.14 |
| Executive Dysfunction | 0.31** | 0.46** | −0.10 |
| Total | 0.31** | 0.34* | −0.03 |
Note.
p < 0.05
p < 0.01.
No significant correlations were found between FrSBe scores and biologic indicators of HCV severity such as HCV viral load and other disease markers (all ps > 0.10). Age was correlated with FrSBe apathy, executive dysfunction and total scales T-scores (all ps < 0.05). However, age was not significantly correlated with FrSBe T-scores within the HCV+ group (all ps > 0.5)
In terms of past substance abuse and dependence, we created a summary “substance disorder” variable that reports whether an individual met lifetime criteria for either abuse and/or dependence for each substance (alcohol, marijuana, cocaine, opioids and hallucinogens). These combined variables (one for each substance) allowed for fewer statistical analyses. We compared FrSBe T-scores on each of the subscales and found significant differences between the individuals that did and did not meet criteria for past alcohol disorder, regardless of HCV status on the FrSBe executive dysfunction and total subscales (both ps < 0.05). No other significant differences were found. When looking at the HCV+ group alone, no significant differences were found on any of the FrSBe subscales between the individuals that met criteria for a past substance disorder and those who did not (all ps > 0.05).
In an attempt to identify the relative contributions of various factors on behavioral symptoms, we conducted a regression analysis for each of the FrSBe scales using those variables found to be significantly different between the HCV+ and the HCV− groups. Regression variables included HCV status, age, neuropsychological impairment (GDS impaired vs. unimpaired), alcohol disorder, cocaine disorder and opioid disorder. For the FrsBe apathy subscale the overall model accounted for a significant amount of variance (R2 = 0.18, p < 0.01), and revealed that HCV status (β= −6.74, p = 0.01) and older age (β= 0.46 p = 0.04) were significant independent predictors of higher FrSBe apathy T-scores. The overall model for FrSBe executive dysfunction also accounted for a significant amount of variance (R2 = 0.19, p < 0.01) and revealed that a diagnosis of alcohol disorder (β= −5.40, p = 0.02) was the only significant independent predictor of higher FrSBe executive dysfunction T-scores, however, HCV status approached significance (β= −4.16, p = 0.06). For the FrSBe total subscale, where the overall model was again significant (R2 = 0.15, p < 0.01), the only significant predictor was HCV status (β= −5.94, p = 0.03). The overall model for FrSBe disinhibition subscale was not significant (p = 0.11). When examining the HCV+ group alone, none of the models accounted for a significant amount of variance (ps > .10).
We also examined dependence on ADLs by HCV status and found that 12% of the HCV+ individuals were dependent on their instrumental ADLs (IADLs). In comparison, none of the HCV− individuals were dependent on IADLs. The rates represented significant differences between HCV+ and HCV− individuals on IADL dependence (p < 0.05). In terms of physical ADLs (PADLs), 9% of the HCV+ individuals were deemed dependent and only 3% of the HCV− individuals were considered dependent on these activities. These differences in PADL dependency were not found to be statistically different between the groups (p > 0.05). When looking at FrSBe T-scores between the individuals that were dependent on IADLs as compared to those who were not, regardless of HCV status, we found significantly higher mean T-scores on the FrSBe apathy, executive dysfunction and total subscales (all ps < 0.05). Also, when looking at both groups together, significant correlations were found between IADLs and FrSBe executive dysfunction subscale (ρ = 0.25 p = 0.04), between PADLs and FrSBe apathy subscale (ρ = 0.26 p = 0.03), and between PADLs and FrSBe executive dysfunction subscale (ρ = 0.25 p = 0.04). Correlations between FrSBe total and IADLs (ρ = 0.23 p = 0.06), and PADLs (ρ = 0.21 p = 0.08) approached significance. No significant correlations were found when examining the HCV+ group alone.
Discussion
Recent studies have shown that a proportion of HCV+ individuals have mild cognitive impairment, including impairment on tests designed to assess frontal systems (Cordoba et al., 2003). Despite these findings, no study has examined self-reported behavioral symptoms, as measured by the FrSBe among individuals with HCV. Results from the present study show that HCV+ individuals endorse greater levels of overall behavioral symptom disruptions, as well as impairments in the domains of apathy, disinhibition and executive dysfunction as compared to individuals without HCV. These medium effects were found in the absence of traditionally confounding variables such as HIV infection, current substance abuse or dependence, and current major depressive disorder.
Across all subscales, a significantly higher proportion of HCV+ individuals had clinically elevated T-scores, however, apathy was the only FrSBe subscale to be clinically elevated at a group level among HCV+ participants. Indeed, only 38.4% of HCV+ individuals reported clinically elevated behavioral symptoms on the FrSBe total, while 44.1% reported elevated apathy symptoms, 32.4% reported elevated disinhibition symptoms, and 38.2% reported elevated executive dysfunction symptoms. Although only a subset of HCV infected individuals reported clinically elevated behavioral symptoms, the proportion of HCV+ individuals classified as having elevated FrSBe T-scores on apathy was three times that of HCV− individuals and over twice as many HCV+ individuals were classified as having clinically elevated FrSBe T-scores on disinhibition and executive dysfunction as HCV− individuals. These results suggest that a subset of HCV-infected individuals may experience more difficulties initiating spontaneous behaviors, may have more irritability and emotional lability, and may have problems with the planning and execution of self-generated activities. The current study design does not allow us to determine whether the observed behavioral disruptions are HCV-induced or whether they were pre-existing among persons who became HCV-infected. For example, it has been shown that a high proportion of HCV+ individuals met criteria for substance abuse and dependence (Abou-Saleh & Foley, 2008), which in turn has been shown to affect frontal systems (Garavan & Stout, 2005). Moreover, individuals with substance abuse disorders have been shown to report clinically elevated behavioral symptoms (Verdejo-Garcia, Rivas-Perez, Lopez-Torrecillas, & Perez-Garcia, 2006). Nonetheless, clinicians should be aware that behavioral disruptions occur more frequently among HCV+ persons as compared to those without HCV infection.
In terms of neuropsychological functioning, a significantly greater proportion of HCV+ individuals were considered neuropsychologically impaired than HCV− individuals. These findings are consistent with the literature on neuropsychological functioning in HCV, which shows that neuropsychological deficits are present in about a third of the HCV+ individuals even in the absence of greater liver disease (e.g., Cherner et al., 2005; Ryan et al., 2004). Our findings are consistent with those of the McAndrews et al. (2005), who found the severity of overall NP impairment among HCV+ individuals to be generally mild.
These data also revealed that neuropsychological impairment was associated with elevated FrSBe T-scores. Within the HCV+ group, a cognitive summary score was related to self-reported difficulties with FrSBe executive dysfunction T-scores and overall FrSBe T-scores. Although correlation coefficients were relatively small (possibly due to differences in mode of ascertainment and measure content), these correlations were nonetheless significant. When accounting for all factors, however, neuropsychological impairment was not a significant predictor of FrSBe scores above and beyond other factors (e.g., HCV status, age). One possible explanation for our study findings is that HCV disrupts prefrontal systems, and leads to the self-reported behavioral problems reported herein. Alternatively, it could be that persons with HCV infection have pre-existing behavioral symptoms such as differences in personality, temperament, sensation seeking, and anxiety as compared to HCV negative individuals. For example, individuals that are highly disinhibited tend to engage in risky behaviors (e.g., sharing needles to inject drugs, having sex without protection), which could have led them to contract HCV infection. Due to the cross-sectional nature of the present study, we are unable to determine whether HCV drives behavioral disturbance or whether these factors are pre-existing among individuals who contract HCV.
Findings from this study also suggest that age is associated with elevated FrSBe T-scores, especially with apathy. This finding is consistent with the literature on apathy, which has reported that apathy is a common disorder among older individuals (Marin, 1990). However, the aforementioned findings have been reported mostly among people 50 and older. Since our cohort is relatively young (age mean: 44.1, SD: 11.6) with only 31.8% over the age of 50, we believe it is unlikely that the self-reported FrSBe apathy T-scores are due to the aging process alone. Supporting this argument is the fact that HCV remained a significant independent predictor of apathy after controlling for age.
Similar to the findings in Vigil et al. (2008), HCV+ individuals were significantly more likely to be dependent on instrumental activities of daily living than HCV− individuals. FrSBe T-scores of apathy, executive dysfunction and total subscales were significantly higher among those individuals that were dependent on IADLs, regardless of HCV status (as a clarification note, this study’s cohort is different from Vigil’s 2008 cohort). Our finding is consistent with previous studies on HCV+ individuals, which reported significant declines in health related quality of life in HCV (Cordoba et al, 2003; Hussain et al., 2001). Our findings indicate that individuals with elevated FrSBe T-scores are at a higher risk for everyday functioning difficulties and points to the need to identify this possibility in clinical evaluations.
Several limitations of the present study must be acknowledged. The sample size of the present study is relatively small; however, our sample size is comparable to previously published studies examining cognitive and daily functioning deficits in HCV. Furthermore, our sample represents a carefully screened cohort of HCV infected individuals that excluded persons with several other comorbidities known to affect the frontal cortex (e.g., HIV, current and past methamphetamine dependence, current major depressive disorder). More importantly, this is the first study that examines the association between HCV and behavioral symptoms as measured by the FrSBe.
Another potential limitation of the present study is that the FrSBe was initially designed for neuropsychological conditions with distinct neurological onsets (e.g., Traumatic Brain Injury) and the lack of an acute onset in HCV may limit the usefulness of the FrSBe in this population. Despite this weakness, we still observed significant group effects by HCV status. We also recognize that the FrSBe, as well as the ADL scale, are self-report measures, and that spurious correlations are possible due to inaccurate or inflated self report. Given the relatively mild neurocognitive impairment in this group and the fact that we excluded individuals with major depressive disorder makes this possibility less likely. Also, no other collateral measure, such as the FrSBe caregiver report, was administered. This limitation could have affected our results in the sense that HCV+ individuals could have either maximized or minimized their current behavioral symptoms. Findings from Batistuzzo and colleagues (2009), suggest that symptom minimization is more likely since they reported significant differences between the FrSBe self-report form and the Family form, where individuals with a diagnosis of refractory obsessive compulsive disorder scored significantly lower on each of the self-report form subscales as compared to the Family report subscales. However, future studies using the FrSBe should strongly consider administering a collateral form, such as the caregiver form, since self-report could be affected by the very own disruption of frontal systems resulting in an individual’s poor insight into his/her condition. Also, other measures of frontal dysfunction such as the Neurospychiatric Inventory (Cummings, et al, 1994) and/or the Frontal Behavioural Inventory (Kertesz, Davidson & Fox,1997) may prove useful in HCV+ cohorts.
Additionally, we only report “current” FrSBe ratings and not “before the illness” ratings because it can be very difficult for HCV+ individuals to clearly identify the symptoms as being present “before” or “after” the insidious onset of HCV symptoms. We acknowledge that the use of “current” FrSBe ratings does not allow us to directly attribute these behavioral problems to HCV infection. As such, FrSBe elevations may reflect a variety of different pre-existing influences, including sensation seeking, anxiety, and mild mood disturbances. Finally, it is important to acknowledge that the FrSBe has been normed on a Caucasian population. Since our population is not exclusively Caucasian, the results of this study need to be interpreted with caution.
Overall, these findings highlight the presence of higher levels of self-reported behavioral symptoms among HCV+ persons as compared to HCV− persons as well as clinically significant elevations in apathy. Elevations in self-reported FrSBe T-scores could compromise an individual’s ability to function and live independently, and our results suggest that HCV+ individuals with elevated FrSBe T-scores appear to have increased difficulties in activities of daily living as compared to comparable HCV− individuals. This is important for both clinicians and family members because behavioral problems can be more distressing to caregivers than cognitive deficits (Allegri, et al, 2006). The exact etiology of behavioral symptoms among HCV+ persons is unclear. Regardless of whether these symptoms are preexisting among HCV+ persons or whether they develop as a result of the neurovirulence of HCV, behavioral symptoms appear to impact everyday functioning. The possibility that a significant proportion of HCV+ persons may have elevated behavioral symptoms regardless of the ultimate etiology seems useful for clinicians working with this population. Future studies examining behavioral symptoms in HCV would benefit from a prospective design, collateral reports of behavioral symptoms, and multimodal assessments to help tease apart the role of pre-existing conditions as compared to processes attributable to HCV.
Acknowledgments
This research was partially supported by NIH/NIDA grant P01 DA12065-08. The HIV Neurobehavioral Research Center (HNRC) is supported by Center award MH 62512-07 from NIMH. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Melanie Sherman; Naval Hospital San Diego: Braden R. Hale, M.D., M.P.H. (P.I.); Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D.; Jennifer Marquie-Beck; Terry Alexander, R.N.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., Steven Paul Woods, Psy.D., David J. Moore, Ph.D.; Matthew Dawson; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D., Brian Schweinsburg, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Ian Everall, FRCPsych., FRCPath., Ph.D., Cristian Achim, M.D., Ph.D.; Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Ian Everall, FRCPsych.,FRCPath., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Clinical Trials Component: J. Allen McCutchan, M.D., J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., Scott Letendre, M.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman (Data Systems Manager), Daniel R. Masys, M.D. (Senior Consultant); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Christopher Ake, Ph.D.
References
- Abou-Saleh MT, Foley S. Prevalence and incidence of hepatitis C in drug users: A review. Addictive disorders and their treatment. 2008;7(4):190–198. [Google Scholar]
- Allegri RF, Sarasola D, Serrano CM, Taragona FE, Arizaga RL, Butman, et al. Neuropsychiatric Symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neurospychiatric Dis Treat. 2006;2(1):105–110. [PMC free article] [PubMed] [Google Scholar]
- Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- Basso MR, Shields IS, Lowery N, Ghormley C, Combs D, Arnett PA, et al. Self-reported executive dysfunction, neuropsychological impairment, and functional outcomes in multiple sclerosis. J Clin Exp Neuropsychol. 2008:1–11. doi: 10.1080/13803390801888733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistuzzo MC, Taub A, Nakano E, D’Alcante CC, de Mathis ME, Hoexter MQ, et al. Performance of patients with refractory obsessive-compulsive disorder in the Frontal Systems Behavior Scale. Neurocase. 2009;15(2):157–162. doi: 10.1080/13554790802709047. [DOI] [PubMed] [Google Scholar]
- Bieliauskas LA, Back-Madruga C, Lindsay KL, Snow KK, Kronfol Z, Lok AS, et al. Clinical relevance of cognitive scores in hepatitis C patients with advanced fibrosis. J Clin Exp Neuropsychol. 2006;28(8):1346–1361. doi: 10.1080/13803390500473720. [DOI] [PubMed] [Google Scholar]
- Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46(3):420–431. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Flavia M, Jacas C, Sauleda S, Esteban JI, Vargas V, et al. Quality of life and cognitive function in hepatitis C at different stages of liver disease. J Hepatol. 2003;39(2):231–238. doi: 10.1016/s0168-8278(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega MS, Gray K, Rosemberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, Bieliauskas LA, Back-Madruga C, Lindsay KL, Kronfol Z, Lok AS, et al. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. J Hepatol. 2005;43(4):614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Forton DM, Taylor-Robinson SD, Thomas HC. Central nervous system changes in hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2006;18(4):333–338. doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35(2):433–439. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Frontal Systems Behavior Scale. Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9(6):847–854. doi: 10.1017/S1355617703960048. [DOI] [PubMed] [Google Scholar]
- Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35(2):440–446. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Hussain KB, Fontana RJ, Moyer CA, Su GL, Sneed-Pee N, Lok AS. Comorbid illness is an important determinant of health-related quality of life in patients with chronic hepatitis C. Am J Gastroenterol. 2001;96(9):2737–2744. doi: 10.1111/j.1572-0241.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24(1):29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Kramer L, Bauer E, Funk G, Hofer H, Jessner W, Steindl-Munda P, et al. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002;37(3):349–354. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Letendre S, Paulino AD, Rockenstein E, Adame A, Crews L, Cherner M, et al. Pathogenesis of hepatitis C virus coinfection in the brains of patients infected with HIV. J Infect Dis. 2007;196(3):361–370. doi: 10.1086/519285. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147(1):22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, et al. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41(4):801–808. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62(6):957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor analysis of the frontal systems behavior scale (FrSBe) Assessment. 2003;10(1):79–85. doi: 10.1177/1073191102250339. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113(3):227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Lopez-Torrecillas, Perez-Garcia M. Differential impact of severity of drug use on frontal behavioral symptoms. Addictive Behaviors. 2006;31(8):1373–1382. doi: 10.1016/j.addbeh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vigil O, Posada C, Woods SP, Atkinson JH, Heaton RK, Perry W, et al. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. J Clin Exp Neuropsychol. 2008:1–11. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry. 1991;159:645–653. 658. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]