Abstract
The purposes of the present study were to determine the autonomic origins of a bradycardiac response to a moderate intensity nonsignal auditory stimulus and the changes in autonomic cardiac control of this response as a function of habituation. Pure tone stimuli were repeatedly presented to participants while phasic changes in heart period (HP), preejection period (PEP), and respiratory sinus arrhythmia (RSA) were observed. Tone stimuli initially elicited an increase in HP, an increase in RSA, and a decrease in PEP, suggesting a coactivation of the parasympathetic and sympathetic inputs mediating changes in the bradycardiac HP response. As expected, HP responses habituated with repeated presentations of the tones. PEP and RSA responses, however, demonstrated different habituation rates than HP. These data demonstrate that cardiodeceleratory responses to nonsignal stimuli can arise from changes in activity of both autonomic divisions and document the importance of considering the autonomic origins of habituating cardiac responses in order to fully understand the process of response habituation.
Keywords: Sympathetic, Parasympathetic, Respiratory sinus arrhythmia, Preejection period, Heart period
Cardiac responses to nonsignal stimuli have received considerable attention in the developmental, attention, and emotion literatures (Campbell, Hayne, & Richardson, 1992; Lang, Simons, & Balaban, 1997; Siddle, 1983). These responses are often used to infer the occurrence of central nervous system processes such as orientation to a novel stimulus or defense, although consistently differentiating among these central states solely on the basis of target organ responses such as heart rate or skin conductance has been difficult (Turpin, 1986; Turpin, Schaefer, & Boucsein, 1999). One reason for these difficulties is that the context in which orienting or defensive responses are elicited may alter the pattern of cardiac response (Berntson, Boysen, & Cacioppo, 1992; Cook & Turpin, 1997). Thus, although the prototypic cardiac orienting response is characterized by heart rate deceleration and rapid response decrement with repeated elicitation (Graham, 1979, 1992, 1997; Turpin, 1986; Turpin & Siddle, 1983), there are exceptions to this general pattern that depend on the context in which the stimulus is embedded (e.g., Richardson, Siegel, & Campbell, 1988; Saiers, Richardson, & Campbell, 1990). Indeed, the organism's motivational or affective state, the basal state of the autonomic nervous system, ongoing motor activity, features of the stimulus, and the context in which the stimulus is embedded may alter the patterning of the orienting response. Specific features of the eliciting stimulus such as intensity, rise time, and the interstimulus interval, as well as the context also influence the response (Sokolov & Cacioppo, 1997). For example, orienting to a novel stimulus in a context previously associated with threat may manifest in a different autonomic response than orienting in an innocuous context (Cook & Turpin, 1997).
New conceptualizations of autonomic control of target organ function provide potential explanatory power for these contextually mediated variations in nonsignal stimulus-elicited cardiac responses. Advances in our understanding of the functional control of target organs by the autonomic nervous system have emphasized that a particular cardiac response can arise from different patterns of underlying autonomic activity (Berntson, Cacioppo & Quigley, 1991; 1993). For example, a cardiodeceleratory response can occur as a result of solely parasympathetic activation (uncoupled mode of control), parasympathetic activation coupled with sympathetic withdrawal (reciprocal mode), or even coactivation of the two autonomic divisions where parasympathetic activation masks the cardiac manifestation of concurrent sympathetic activity (coactivational mode). These considerations require that we move away from a sole reliance on overall target organ responses, such as heart period, which are ambiguous with regard to their underlying autonomic origins (Berntson et al., 1991; Berntson, Cacioppo, & Quigley, 1993). If changes in parasympathetic and sympathetic activity are preferentially produced by particular demands, then measures of independent autonomic influences on cardiac function will be invaluable for examining the role of context on an organism's responses to nonsignal stimuli.
To our knowledge, the autonomic origins of cardiac orienting responses to date have been assessed only in animals where pharmacological autonomic blockades and surgical manipulations have been used to derive estimates of the parasympathetic and sympathetic origins of the cardiac response. The earliest of these studies suggested that the cardiac orienting response in preweanling rats (ages 16–20 days) was mediated predominantly by parasympathetic activation (Haroutunian & Campbell, 1982; Saiers, Richardson, & Campbell, 1989). However, these studies relied exclusively on either vagotomy or parasympathetic autonomic blockade, making conclusions about the potential influence of the sympathetic division difficult. Indeed, in both studies, a small cardiodecelera-tory response remained following vagotomy or vagal blockade, suggesting that the unblocked sympathetic innervation also may have influenced the overall cardiac response. A more recent study using pharmacological blockades of both autonomic divisions revealed that the cardiac orienting response to an olfactory stimulus can be mediated by potent parasympathetic activation accompanied by a small, short-lived (4 s) sympathetic withdrawal (Hunt, Hess, & Campbell, 1994). These findings suggest that heart rate deceleration in response to novel stimuli in the preweanling rat arises primarily from an increase in parasympathetic input to the heart in combination with modest sympathetic withdrawal. The prominent parasympathetic activation elicited by an orienting stimulus in the preweanling rat appears to be a function of the animal's ability to phasically activate the parasympathetic branch to change cardiac rate (Hofer & Reiser, 1969; Hunt et al., 1994; Quigley, Shair, & Myers, 1996) before it can phasically activate the sympathetic branch (although circulating catecholamines are present and provide adrenergic tone on the heart; Tucker, 1985).
Although absolute heart period is considerably shorter in rats than humans, in many ways, autonomic control of cardiac rate is similar in the two species. For example, fetal humans and early postnatal rats both show large bradycardias to environmentally significant events that appear to be mediated mostly by parasym-pathetic influences (although the evidence concerning autonomic mediation in humans is scarce; Hon, Bradfield, & Hess, 1961; Renou, Newman, & Wood, 1969). In addition, in adult humans and rats, we see the same relatively broad range of control over heart period by the parasympathetic branch compared to a much narrower range of sympathetic control over heart period (approximately 6:1 in rats and 7:1 in humans; Berntson et al., 1993), and the same relatively linear relationship between autonomic inputs to the heart and heart period (Berntson, Cacioppo, & Quigley, 1995). Thus, the similarities in autonomic control of heart period between rats and humans appear to be sufficient to permit some comparison of autonomic control of the cardiac orienting response across these species.1
In the only study we know of that assessed the autonomic origins of the orienting response in adult animals, Quigley & Berntson (1990) demonstrated that a low intensity (55 dB; instantaneous rise time) nonsignal tone elicited a cardiodeceleratory response that reverted to cardioacceleration following the administration of a parasympathetic blockade agent. These data suggested that the cardiodeceleration elicited by the stimulus was the result of concurrent parasympathetic and sympathetic activation. Consistent with this interpretation, administration of the sympathetic antagonist atenolol enhanced the cardiodeceleratory response (see also Figure 4 in Berntson, Cacioppo, Quigley, & Fabro, 1994). Based on these initial studies in rats, it has been suggested that cardiac deceleration to moderate intensity novel stimuli is mediated primarily by increased parasympathetic stimulation of the heart, with the occasional appearance of concurrent sympathetic activation (Campbell, Wood, & McBride, 1997).2 Because there are relatively few studies examining the autonomic origins of the orienting response and because of developmental differences across these studies, the potential similarities of the autonomic mediation of the orienting response across species remains an open question. As has been suggested (Sokolov & Cacioppo, 1997), noninvasive estimates of parasympathetic and sympathetic control of the heart may provide the necessary tools for examining the autonomic origins of orienting responses in humans. Indeed, the potential problems associated with pharmacological blockades (e.g., possible reflexive adjustments in the unblocked autonomic division) highlight the importance of documenting the autonomic origins of the responses to nonsignal stimuli using noninvasive methods.
The availability of multiple modes of autonomic control also has important implications for the process of habituation of phasic cardiac responses. Because each autonomic division may independently alter heart period, the habituation function of heart period responses alone will reveal little about potential habituation or sensitization in each autonomic division. If sensitization and habituation processes act at the level of the autonomic inputs to the heart, rather than at the level of heart period per se, then responses on later trials could arise from a different mode of autonomic control than those elicited on the initial trial. In one of the few studies to assess changes in autonomic activity as a function of habituation, Richardson, Wang, and Campbell (1996), demonstrated that the initial bradycardiac startle response in adult rats was primarily mediated by parasympathetic activation, and some modest amount of sympathetic activation. However, in later trials where the startle stimulus elicited tachycardia, the mode of control shifted to uncoupled sympathetic activation. Although in this case, the directionality of the cardiac response changed over trials, the lesson of the multiple modes of autonomic control is that even when response direction does not change with stimulus repetition, the autonomic mode underlying a response can still be changing. Thus, measuring sympathetic and parasympathetic origins of the cardiac response over time could reveal important features of habituation that are not apparent from the cardiac response alone.
The primary objectives of the present study were (a) to examine the contributions of the sympathetic and parasympathetic nervous systems to the initial cardiac response to a moderate intensity nonsignal stimulus and (b) to examine the subsequent changes in the autonomic origins of this cardiac response with repeated stimulus presentations. Auditory stimuli known to elicit relatively longlasting (i.e., 30 s or more) phasic cardiac decelerations were presented repeatedly to human participants while heart period and noninvasive estimates of sympathetic cardiac activity (preejection period; PEP) and parasympathetic cardiac control (respiratory sinus arrhythmia: RSA) were obtained. The stimulus used in the current study is slightly more intense than the typical orienting stimulus, but was chosen because it produced a longer response and, therefore, enhanced our ability to detect autonomic changes using noninvasive methods. If a cardiac orienting response is associated with an uncoupled increase in parasympathetic activation as has been demonstrated in young rats (Hunt et al., 1994), then cardio-deceleration to a low-intensity stimulus should be accompanied by increased RSA and no change in PEP. Conversely, if the cardiac orienting response is mediated by autonomic coactivation as has been demonstrated in the adult rat, then we would expect simultaneous increases in RSA and decreases in PEP (i.e., corresponding to an increase in sympathetic activity). Further, the sympathetic and parasympathetic branches may contribute differentially to the overall rate of cardiac orienting response habituation. Thus, we assessed whether each of the two divisions have their own, independent habituation functions.
Method
Participants
Participants were 20 undergraduate students (14 female) recruited from an introductory psychology course and given course credit for their participation. Participants were screened to exclude (a) those with acute or chronic cardiovascular or respiratory illness, (b) those with a body mass above or below 35% of normal body weight, (c) individuals engaged in more than 20 hr per week of exercise, (d) those who used illicit drugs, and (e) those with high blood pressure (exceeding 140 mmHg systolic or 90 mmHg diastolic). From the original sample of 20 participants, 3 subjects were excluded from analyses because (a) data from more than three trials were unavailable due to equipment problems during signal acquisition (n = 1), (b) an arrhythmia detected during scoring (n = 1), and (c) the B-point in the impedance cardiographic signal could not be detected (n = 1). Analyses were conducted on the data from the remaining 17 participants (14 females).
A Beltone audiometer was used to test participants’ hearing. During testing, each individual was fitted with earphones and was presented with 20 dB pure tones in each ear. The individual was asked to raise his or her hand when a tone was heard. Tones ranged from 250–6000 Hz in octave and one-half octave intervals with every tone presented in each ear. Hearing tests were completed 45–60 min prior to testing. Although we felt it important to test auditory acuity in our college-aged sample, no potential participants were excluded based on the acuity criterion.
Auditory Stimuli
Each pure tone (1000 Hz) stimulus was presented at 75 dB SPL (Quest Technologies sound meter, Model 1800) for 1.5 s (24-ms rise and fall times) and was repeated 12 times using a random 90–120-s intertrial interval (ITI).3 All stimuli were presented monaurally over a set of Telephonics headphones and controlled by an IBM-compatible computer.
Physiological Recording
Electrocardiogram (ECG) and impedance cardiogram signals were obtained using a Minnesota Impedance Cardiograph (Model 304 B). Aluminum-coated Mylar band electrodes were used to record basal thoracic impedance (Z0), the first derivative of the pulsatile change in thoracic impedance (dZ0dt), and the ECG. Voltage (recording) electrodes were placed circumferentially around the base of the neck and around the thorax over the xiphisternal junction. Current electrodes, which passed a 100 kHz AC current (4mA), were attached at least 3 cm above (neck) and below (chest) the voltage electrodes. A respiration transducer was placed around the participant's torso to measure respiration frequency and amplitude. Physiological signals were acquired using an A0D board (12 bit) and saved for off-line processing. The ECG and dZ0dt were sampled at 500 Hz, and Z0 and respiration were sampled at 250 Hz.
Procedure
Each participant was given a verbal description of the study, then gave informed consent and completed a health questionnaire. Next, a female experimenter attached all electrodes and the respiration transducer and seated the participant in a comfortable arm chair inside a sound attenuated room (45 dB ambient; approximately 475 lux). Participants were instructed to remain as still as possible throughout the session and told that they would hear a series of sounds through headphones. Headphones were then placed on the participant and physiological recordings began.
A 10-min electrode stabilization period was followed by a 4-min baseline period during which baseline ECG, ZCG, and respiration were recorded. Participants wore headphones during these epochs, but no stimuli were presented. Following the 4-min baseline period, stimulus presentation commenced. After the session finished (approximately 1 hr, 15 min), electrodes were removed, the participant was given a brief postexperimental questionnaire, and she or he was debriefed.
Signal Reduction
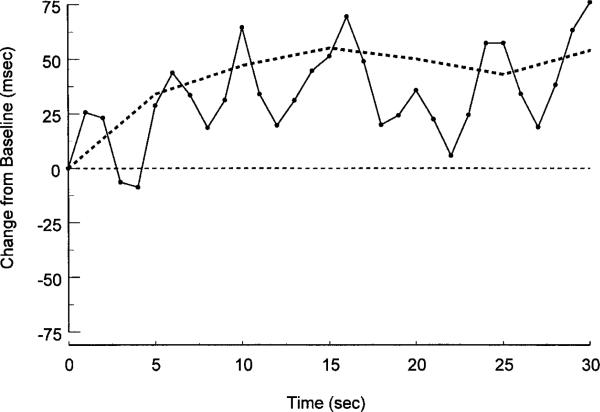
Impedance- and electrocardiogram-derived waveforms were processed off-line using a program for visualizing, editing, and ensemble averaging waveforms (Kelsey & Guethlein, 1990). Heart period (HP) was derived from the ECG as the interval in milliseconds between successive R spikes. PEP was calculated as the interval between the Q wave of the electrocardiogram and the B-point of the dZ0dt waveform. After editing the data for artifacts (<1% of the data), the program was used to calculate 5-s ensemble averages for the 10 s preceding and 30 s following stimulus onset. The resulting ensemble averages were visually inspected and event markers (e.g., the B-point) were moved manually if identified incorrectly by the program. Ensemble averages over five consecutive heart beats have been shown to provide reliable estimates of beat-to-beat impedance cardiograph-derived measures (Muzi et al., 1985). Here, ensemble-average-based estimates of baseline PEP from 5 s and successively longer epochs (10– 60 s in 5-s increments) correlated highly (range: rs = .97–.99). Likewise, ensemble-average-based estimates of baseline HP for 5 s and consecutively longer time periods (10–60 s in 5-s increments) ranged from .89–.97. Moreover, although 5-s ensemble averages smooth out short-term changes in HP, the 5-s ensemble-based mean reflects the underlying second-by-second HP changes (see Figure 2). The cardiac responses in the current study were modest and there were no shifts in posture such that changes in preload and afterload are most likely minimal and thus not problematic for our interpretation of PEP as reflecting sympathetic change (Lewis, Leighton, Forester, & Weissler, 1974).
Figure 2.
Second-by-second weighted heart period response to the tone stimulus in Trial 1 is shown overlaying the 5-s ensemble-averaged response shown in the upper panel of Figure 1.
The peak–valley method (Grossman, van Beek, & Wientjes, 1990; Katona & Jih, 1975) was used to derive RSA estimates from three consecutive 10-s poststimulus epochs and one 10-s prestimulus epoch. This method was chosen because it provides the best temporal resolution of the methods used to estimate RSA. A customized software package (Uijtdehaage, 1994) was used to determine the difference in milliseconds between the minimum heart period value during inspiration and the maximum heart period value during the subsequent expiration for each respiratory cycle. Phase differences between respiration and interbeat intervals were accommodated by extending inspiratory and expiratory windows by either 1,000 ms or by the average interbeat interval for the trial, whichever was longer (Uijtdehaage, 1994). An RSA estimate for each 10-s epoch was calculated from the average of peak–valley differences observed during that time period. This method is similar to the approach used by Reyes del Paso, Godoy, and Vila (1993), however, we used mean RSA values for fixed 10-s epochs, whereas Reyes del Paso et al. used median RSA values for variable-length epochs. Although it has been suggested that the peak–valley estimation of RSA may be altered by slow periodicities in the heart period data, the measurement conditions here would be expected to result in minimal nonstationarities. In particular, the data epochs are brief, the subjects are resting and do not perform tasks or make overt movements, and the stimuli do not result in large changes in cardiac state (Berntson et al., 1997; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). Indeed, even using a more evocative and motorically demanding reaction time task, the peak–valley method correlated highly with the Porges-Bohrer method that removes slow periodic trends from the heart period data (Gross-man et al., 1990; Porges & Bohrer, 1990). In addition, in adherence with suggestions from a recent Committee Report for the analysis of heart period variability (Berntson et al., 1997), we spaced stimulus presentations irregularly and outside of the frequency range of the heart period variation of interest. In addition, they note that stimulus-evoked changes in heart period variability are most valid when the cardiac response to the stimuli is small to moderate, as was true here.
Data Quantification
Average prestimulus HP and PEP estimates were derived by averaging the two 5-s ensemble averages from the 10-s period immediately preceding stimulus onset. When data from one or two trials were missing for a participant due to equipment problems, the group means were assigned to the missing trial (n = 1); otherwise, the data were not analyzed (see Participants section for details). Trial 1 HP, PEP, and RSA responses were analyzed using three repeated measures analyses of variance (ANOVAs) with 5-s ensemble averages for HP and PEP and 10-s averages for RSA as factors. Both pre- and poststimulus epochs were included in the ANOVAs and trend tests were performed to determine the response profile of stimulus-elicited changes in HP, PEP, and RSA on the first trial.
Integral areas under the deceleratory portion of the poststimulus response curves were used to estimate poststimulus response amplitude for all trials.4 Square root transformations were performed on integral data prior to statistical analyses to correct distributional violations. The transformed integrals were then collapsed into blocks of two trials except for Trials 1 and 12, which represent the initial and final level of responding in the habituation series. These data were analyzed using repeated measures ANOVAs with Blocks as the repeated measure. The Huynh-Feldt epsilon (ε) was used to adjust for inflated degrees of freedom in all repeated measures analyses and a significance level of .05 was adopted. As subsidiary analyses, we also examined habituation using a more traditional approach in which we examined the Trials × Poststimulus Epoch interaction for each dependent variable. Such an interaction indicates significant change in poststimulus response across trials.
Results
Trial 1 Responses
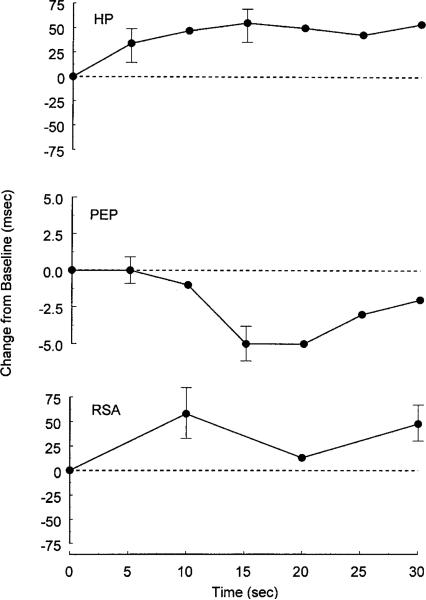
The upper panel of Figure 1 shows the average change in heart period in response to the tone stimulus on Trial 1. Pronounced cardiac deceleration occurred immediately following stimulus onset and was relatively sustained. These results were revealed by a main effect for Epoch, F(6,96) = 2.54, ε = .89, p = .03, and a significant linear trend in the HP response, F(1,16) = 7.96, p = .01. As shown in the middle panel of Figure 1, PEP decreased significantly in response to the pure tone stimulus on Trial 1, F(6,96) = 5.96, ε = .78, p = .001. Significant linear, F(1,16) = 4.34, p = .05, quadratic, F(1,16) = 6.64, p = .02, and cubic, F(1,16) = 7.78, p = .01, trends were found in the Trial 1 PEP response. The peak decrease in PEP occurred 15–20 s poststimulus and neared the prestimulus level by 30 s poststimulus. In addition to the HP lengthening and PEP shortening (upper and middle panels of Figure 1), a statistically significant increase in RSA was observed following stimulus onset on Trial 1, F(3,48) = 3.85, ε = .84, p = .02 (bottom panel of Figure 1). Further, a cubic trend in the RSA response was observed on Trial 1, F(1,16) = 11.33, p = .004.
Figure 1.
Heart period (upper panel), preejection period (middle panel), and respiratory sinus arrhythmia responses (bottom panel) to 75-dB pure tone stimuli in Trial 1 presented as average change (standard error of the mean) from a 10-s prestimulus baseline.
To assess whether respiration changes might account for the initial stimulus-elicited changes in RSA, respiration rate and depth were assessed for the 10-s epochs immediately preceding and following stimulus onset for Trial 1 where respiratory changes would be expected to be maximal. These data demonstrate that respiratory rate and depth in the first 10 s following the stimulus were not significantly altered by the stimulus (see Table 1; period and depth: ts < 1; ps ≥ .3). These data suggest that the changes in RSA occurring as a function of the stimulus were not a result of significant alterations in respiratory period or depth (Grossman, Karemaker, & Wieling, 1991). Indeed, Reyes del Paso and Vila (1993), using an intense stimulus (100 dB, nearly instantaneous rise time), demonstrated an approximately 50% average increase in respiratory amplitude (and no change in respiratory period) in the first 10 s following the stimulus. By comparison, we observed a 3% increase in respiratory amplitude and a 6% decrease in respiratory period.
Table 1.
Means and Standard Errors of Respiratory Period and Amplitude for 10-s Epochs Pre- and Poststimulus Onset
| Epoch |
||
|---|---|---|
| Respiratory Variable | Pre-stimulus | Post-stimulus |
| Period (ms) | 3274.51 (89.18) | 3073.53 (212.45) |
| Amplitude (arb. units) | 122.14 (12.78) | 125.27 (12.48) |
To aid comparison of these data with previous investigations of cardiac responses to nonsignal stimuli, we also show the weighted second-by-second HP response overlaying the 5-s ensemble-averaged response from Figure 1 (see Figure 2; Berntson et al., 1995). The second-by-second depiction shows an early (first two poststimulus seconds) cardiac deceleration of about 25 ms (about –2 bpm at this basal heart period) that is reminiscent of the initial component of the orienting response described by Graham (1979), and Turpin and colleagues (Turpin et al., 1999; Turpin & Siddle, 1983). In addition, a secondary deceleratory component occurring after about 5 s poststimulus has also been observed in response to moderate intensity nonsignal stimuli across species (Graham, 1979; Quigley & Berntson, 1990; Turpin, 1986). The considerable variability in the poststimulus response is not due to a respiratory artifact initiated by the stimulus (examination of individual respiratory traces shows no evidence of a inspiratory gasp or other unusual respiratory activity after the stimulus). Instead, there is simply quite large variation around the mean response and too few subjects for the variability to be smoothed in a second-by-second depiction. We do not know whether this second-by-second variation is typical of other studies, because most studies of the orienting response only report second-by-second responses for the first 10 s poststimulus.
Habituation
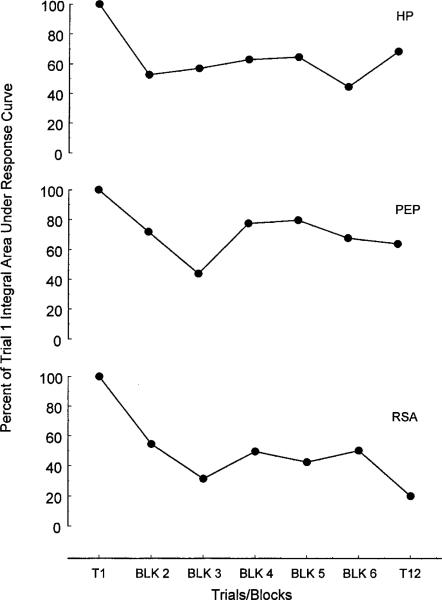
No significant effect for Block was found for the HP integrals, F(6,96) = 1.61, ε = .68, p = .18. This finding is consistent with the absence of a Trials × Poststimulus Epoch interaction on the raw HP data, F(66,1056) = 1.059, ε = .33, n.s.). However, a marginal linear trend was observed in the HP integrals across stimulus repetitions, suggesting an overall decline in response strength over trials, F(1,16) = 3.66, p = .07 (upper panel, Figure 3). No main effect for Block was found for the PEP integrals and there were no significant trends with stimulus repetitions. However, a Trials × Poststimulus Epoch interaction on the raw PEP data suggested that the PEP responses changed over stimulus repetitions, F(66,1056) = 1.92, ε = .59, p < .05. Visual inspection suggested that this interaction is due to an initial decline and then slight recovery in response strength, particularly on Blocks 4 and 5 (middle panel of Figure 3). A main effect for Block was found in the RSA integral data, F(6,96) = 4.77, ε = .85, p < .01, which suggested an overall decline in RSA response strength over trials (lower panel, Figure 3). Further, both linear and cubic trends were observed in the RSA integral data over trials, F(1,16) = 14.35, p = .002 and F(1,16) = 8.88, p = .009, respectively. Finally, a marginal Trials × Poststimulus Epoch interaction on the raw RSA data corroborated the findings with the integrals in suggesting a change in the RSA response across trials, F(44,748) = 1.60, ε = .37, p = .06.
Figure 3.
Change in the amplitude of heart period (upper panel), preejection period (middle panel), and respiratory sinus arrhythmia (bottom panel) responses to pure tone stimuli. Response amplitude represents the percent of Trial 1 integral area under the poststimulus response curve for each measure.
Discussion
In the present study, the initial presentation of a moderate intensity tone stimulus elicited a cardiodeceleratory response that was accompanied by an increase in RSA and a decrease in PEP. Cardio-deceleration is a typical response to low to moderate intensity stimuli with noninstantaneous rise times that are usually considered to reflect orienting (Graham, 1973, 1997; Turpin, 1986). Recall that the stimulus characteristics used in the current study were chosen to provide a somewhat more prolonged response (i.e., typically the HP returned to baseline in about 40 s in Trial 1) than is observed for a prototypical orienting stimulus to enhance the ability to detect autonomic changes using noninvasive estimates. Thus, the stimulus characteristics in the current study likely fell on the border between a typical orienting response and a typical defense response (Graham, 1997). Graham proposed that a 75-dB tone may be more likely to produce coactivational autonomic effects in humans than a tone of lesser intensity, which is in accord with the current findings. A look at the initial bradycardiac response in this study suggests that the initial heart period response is mediated by parasympathetic activation followed by concurrent activation of the sympathetic input to the heart (see Figure 1, middle and lower panels). The finding of an initial parasympathetically mediated cardiodeceleratory response is consistent with Graham and Clifton's (1966) speculation that short latency cardiodeceleratory responses to orienting stimuli are mediated primarily by increased parasympathetic input to the heart. Indeed, in the current study there were no reliable stimulus-related changes in respiration suggesting that the changes in RSA were predominantly a function of centrally generated changes in vagal outflow rather than a function of lung stretch receptor-mediated changes. Importantly, this finding of coactivation of autonomic outflows to the heart in response to a novel, moderate-intensity auditory stimulus parallels data in adult animals (rats; Quigley & Berntson, 1990). We further speculate, in line with Graham's conjectures, that differences in stimulus parameters would influence the exact pattern of autonomic contributions to such cardiodeceleratory responses with most low-to-moderate-intensity orienting-like stimuli producing parasympathetic activation that is sometimes accompanied by sympathetic activation (particularly with faster rise times). To assess the influence of parametric variations of stimulus variables such as intensity or rise time, we will need to further refine noninvasive estimates that can be used over shorter temporal intervals. These refinements will be crucial for understanding the autonomic origins of the relatively brief orienting response seen with low-intensity stimuli.
Several features of the autonomic origins of the Trial 1 cardiac response warrant further highlight. First, to our knowledge this is the first study to document a coactivational mode of autonomic control in humans in response to a nonsignal stimulus using noninvasive measures. The demonstration of coactivation in humans whose physiology is unaltered by autonomic blockades provides important additional documentation of the doctrine of multiple modes of autonomic control (Berntson et al., 1991). Thus, it is now clear that a coactivational mode of control is observable under normal, resting physiological conditions in humans in a benign behavioral context. Second, we will need parametric studies to test several predictions that arise from Graham's (1997) theorizing and the current findings. Two obvious predictions are that faster rise times and moderate-intensity nonsignal stimuli (e.g., 75 dB rather than 55 dB) will be expected to produce some sympathetic activation to accompany the parasympathetic activation elicited by attention-capturing stimuli. In addition, as the stimulus intensity rises beyond the moderate range, we predict that sympathetic activation will continue to rise, whereas eventually parasympathetic activation will cease.
These data demonstrate not only a coactivational mode of cardiac control on Trial 1, but also that the mode of autonomic control can change as a function of repeated stimulus presentations. As shown in Figure 2, HP, RSA, and, to some extent, even PEP response amplitudes declined after Trial 1 (see Figure 2). However, RSA declined most markedly with the mean response amplitude after Trial 1 at 69.1% of the initial response. HP declined less notably with the mean response amplitude after Trial 1 at 74.1% of initial responding. PEP declined least and nonsignificantly so with average response amplitude after Trial 1 at 94.2% of initial responding. These data suggest that the autonomic responses that underlie a habituating heart period response do not necessarily habituate at the same rate as the overall or composite cardiac response. Graham (1973) referred to this distinction as central versus response habituation where central habituation indicates habituation of the central nervous system substrates and response habituation referred to the habituation of different autonomically mediated responses. In Graham's review, she discusses the differential habituation of responses derived mostly from different organ systems such as heart rate and skin conductance. Here however, we demonstrate that the HP response habituates at a rate intermediate to that of the two contributing autonomic branches. Thus, even cardiac habituation does not appear to be a unitary phenomenon, but rather is the result of multiple inputs each with its own independent habituation function.5 These results illustrate that the measure used to represent the state of a habituating system must be chosen with care.
Several limitations to the current study should be noted. First, unlike much previous literature on habituation, we did not present a dishabituation stimulus at the conclusion of the habituation series to assess effector fatigue. Given that numerous studies have shown that such cardiac responses recover with a dishabituation stimulus, it seems unlikely that effector fatigue can explain the current findings. Moreover, even if fatigue were to play a role, we would still be left with the intriguing finding that PEP responses to this stimulus habituate to a minimal extent (at least with long ITIs), whereas RSA habituated to a significant extent. However, we cannot rule out the possibility that effector fatigue could have contributed to our findings. Another issue deserving attention in future studies is the autonomic contributions to the more fine-grained temporal features (e.g., deceleratory and acceleratory portions) of the cardiac response to nonsignal stimuli. As noted above, this will require the development of techniques that permit assessment of shorter intervals (e.g., 1 s). In the current study, we justify the use of short-term measures of RSA (i.e., 10-s epochs) on the grounds that (a) the response to the stimulus is predominantly deceleratory and relatively long-lasting (i.e., at least 30 s on initial presentation), (b) values of RSA for the 30-s poststimulus epoch corroborate the findings for 10-s epochs, and (c) the presence of increased sympathetic activity concurrent with a cardiac deceleration suggests that parasympathetic activity must be increased.
Within the context of the current study, it appears that the bradycardia elicited on Trial 1 to a moderate intensity nonsignal stimulus is a joint result of parasympathetic and sympathetic activation. Moreover, the autonomic contributions to that response change differentially as a function of repeated stimulus presentations. These data have important implications for the ongoing debate about the functional relevance of cardiac deceleration in orienting contexts. Namely, these data are consistent with Graham's (1997) suggestion that less intense stimuli will preferentially result in uncoupled parasympathetic activation, whereas more intense stimuli may evoke concurrent sympathetic activation. Thus, we may need to shift the focus of the debate on the functional significance of cardiac deceleration to the significance of the underlying autonomic modes of control of cardiac responses. Such a focus will likely provide a more complete theoretical framework for the assessment of cardiac responses to nonsignal stimuli.
Acknowledgments
This research was partially supported by a Sigma Xi Grant-in-Aid of Research to Peter J. Gianaros. The authors would like to thank David Lozano and Thomas Frank for their technical assistance. We also thank Robert Kelsey and William Guethlein for providing impedance cardiography analysis software, Gary Berntson for heart period analysis software, and Jaime Marinaccio for assistance in data collection. Finally, we thank J. Toby Mordkoff, Robert Stern, and William Ray for helpful comments on study design and analyses, and Robert Stern and Gary Berntson for comments on an earlier draft.
Footnotes
Comparisons of the autonomic cardiac responses to environmentally relevant stimuli between rats and humans are best made from studies where the state of the organism is not altered by anesthesia, restraint, or distress. Unfortunately, many earlier studies of autonomic function in animals were conducted under such nonoptimal conditions, which limits the number of studies from which appropriate comparisons with human results can be made. In addition to state factors, stimulus parameters such as intensity, duration, rise time and composition (e.g., single frequency versus mixed frequencies) differ across studies and species. Unfortunately, there has been little explicitly comparative work in this area, so we can only recommend that comparisons across species be made cautiously and with careful attention to state and stimulus variables.
Campbell et al. suggest that although cardiac orienting appears across many mammalian species (see also Berntson, Boysen, & Cacioppo, 1992), cardiac orienting responses were not observed in the few studies using amphibians, reptiles, or birds.
Prior research on the cardiac orienting response has typically used a fixed or variable ITI that ranges from approximately 10 to 55 s (e.g., Turpin, 1986; Turpin & Siddle, 1983). Fixed and short ITIs have been shown to promote more rapid habituation in a variety of response systems (Graham, 1973; Groves & Thompson, 1970). In the present study, a variable and relatively long ITI was chosen to prevent anticipatory cardiac responses, which can occur with fixed ITIs, and to ensure that there was sufficient time after the stimulus to observe the full autonomic response. In addition, this stimulus is similar enough to that used by Quigley and Berntson (1990) in rats that some comparisons are possible with data obtained under conditions of autonomic blockade.
Acceleratory response integrals under the HP response curves were also examined. However, there was little cardiac acceleration apparent, and no analyses of the acceleratory integrals were significant (all ps ≥ .1).
Although RSA and PEP both index autonomic input to the heart, they reflect different aspects of cardiac control. RSA reflects chronotropic or rate influences on the heart, whereas PEP reflects inotropic or contractility influences on the heart.
REFERENCES
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen M. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Boysen ST, Cacioppo JT. Cardiac orienting and defensive responses: Potential origins in autonomic space. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. pp. 163–200. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin. 1993;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. The metrics of cardiac chronotropism: Biometric perspectives. Psychophysiology. 1995;32:162–171. doi: 10.1111/j.1469-8986.1995.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and psychophysiological response. Psychophysiology. 1994;31:44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Campbell BA, Wood G, McBride T. Origins of orienting and defensive responses: An evolutionary perspective. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 41–67. [Google Scholar]
- Cook E, Turpin G. Differentiating orienting, startle and defense responses: The role of affect and its implications for psychopathology. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 137–164. [Google Scholar]
- Graham FK. Habituation and dishabituation of responses inner-vated by the autonomic nervous system. In: Peeke HVS, Herz MJ, editors. Habituation: Vol. 1. Behavioral Studies. Academic Press; New York: 1973. pp. 163–218. [Google Scholar]
- Graham FK. Distinguishing among orienting, defense and startle reflexes. In: Kimmel HD, Van Olst EH, Orlebeke JF, editors. The orienting reflex in humans. Erlbaum; Hillsdale, NJ: 1979. pp. 137–168. [Google Scholar]
- Graham FK. Attention: The heartbeat, the blink, and the brain. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. pp. 3–29. [Google Scholar]
- Graham FK. Afterword: Pre-attentive processing and passive and active attention. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 417–472. [Google Scholar]
- Graham FK, Clifton RK. Heart rate changes as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: The need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, van Beek J, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27:702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Neural control of the heart-rate-orienting response in preweanling rats. Behavioral and Neural Biology. 1982;36:24–39. doi: 10.1016/s0163-1047(82)90212-6. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Reiser MF. The development of cardiac rate regulation in preweanling rats. Psychosomatic Medicine. 1969;31:372–388. doi: 10.1097/00006842-196909000-00003. [DOI] [PubMed] [Google Scholar]
- Hon EH, Bradfield AH, Hess OW. The electronic evaluation of the fetal heart rate. American Journal of Obstetrics and Gynecology. 1961;82:291–300. [PubMed] [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Autonomic mediation of unconditioned heart rate responses in the 16-day-old rat. Psychobiology. 1994;22:209–218. [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: Noninvasive measure of parasympathetic cardiac control. Journal of Applied Physiology. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kelsey RM, Guethlein W. An evaluation of the ensemble averaged impedance cardiogram. Psychophysiology. 1990;27:24–33. doi: 10.1111/j.1469-8986.1990.tb02173.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. [Google Scholar]
- Lewis RP, Leighton RF, Forester WF, Weissler AM. Systolic time intervals. In: Weissler AM, editor. Noninvasive cardiology. Grune & Stratton; New York: 1974. pp. 301–368. [Google Scholar]
- Muzi M, Ebert TJ, Tristani FE, Jeutter DC, Barney JA, Smith JJ. Determination of cardiac output using ensemble-averaged impedance cardiograms. Journal of Applied Physiology. 1985;58:200–205. doi: 10.1152/jappl.1985.58.1.200. [DOI] [PubMed] [Google Scholar]
- Porges SW, Bohrer RE. The analysis of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: Physical, social and inferential elements. Cambridge University Press; New York: 1990. pp. 708–753. [Google Scholar]
- Quigley KS, Berntson GG. Autonomic origins of cardiac responses to nonsignal stimuli in the rat. Behavioral Neuroscience. 1990;104:751–762. doi: 10.1037//0735-7044.104.5.751. [DOI] [PubMed] [Google Scholar]
- Quigley KS, Shair HN, Myers MM. Parasympathetic control of heart period during early postnatal development in the rat. Journal of the Autonomic Nervous System. 1996;59:75–82. doi: 10.1016/0165-1838(96)00010-0. [DOI] [PubMed] [Google Scholar]
- Renou P, Newman W, Wood C. Autonomic control of fetal heart rate. American Journal of Obstetrics and Gynecology. 1969;105:949–953. doi: 10.1016/0002-9378(69)90103-3. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Godoy J, Vila J. Respiratory sinus arrhythmia as an index of parasympathetic cardiac control during the cardiac defense response. Biological Psychology. 1993;35:17–35. doi: 10.1016/0301-0511(93)90089-q. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Vila J. Respiratory influences on the cardiac defense response. International Journal of Psychophysiology. 1993;15:15–26. doi: 10.1016/0167-8760(93)90091-3. [DOI] [PubMed] [Google Scholar]
- Richardson R, Siegel MA, Campbell BA. Effect of maternal presence on the fear response to an unfamiliar environment as measured by heart rate in rats as a function of age. Developmental Psychobiology. 1988;21:613–633. doi: 10.1002/dev.420210702. [DOI] [PubMed] [Google Scholar]
- Richardson R, Wang P, Campbell BA. Developmental and pharmacological analysis of the cardiac response to an acoustic startle stimulus. Psychophysiology. 1996;33:31–41. doi: 10.1111/j.1469-8986.1996.tb02106.x. [DOI] [PubMed] [Google Scholar]
- Saiers JA, Richardson R, Campbell BA. Pharmacological dissociation of heart rate and somatomotor components of the orienting response. Psychobiology. 1989;17:418–423. [Google Scholar]
- Saiers JA, Richardson R, Campbell BA. Disruption and recovery of the orienting response following shock or context change in preweanling rats. Psychophysiology. 1990;27:45–56. doi: 10.1111/j.1469-8986.1990.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Siddle DAT, editor. Orienting and habituation: Perspectives in human research. Wiley; New York: 1983. [Google Scholar]
- Sokolov EN, Cacioppo JT. Orienting and defense reflexes: Vector coding the cardiac response. In: Lang PJ, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. pp. 1–22. [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tucker DC. Components of functional sympathetic control of heart rate in neonatal rats. American Journal of Physiology. 1985;248:R601–R610. doi: 10.1152/ajpregu.1985.248.5.R601. [DOI] [PubMed] [Google Scholar]
- Turpin G. Effects of stimulus intensity on autonomic responding: The problem of differentiating orienting and defensive reflexes. Psychophysiology. 1986;23:1–14. doi: 10.1111/j.1469-8986.1986.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Turpin G, Schaefer F, Boucsein W. Effects of stimulus intensity, risetime, and duration on autonomic and behavioral responding: Implications for the differentiation of orienting, startle and defense responses. Psychophysiology. 1999;36:453–463. [PubMed] [Google Scholar]
- Turpin G, Siddle DAT. Effects of stimulus intensity on cardiovascular activity. Psychophysiology. 1983;20:611–624. doi: 10.1111/j.1469-8986.1983.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Uijtdehaage SH. A BASIC program for the peak-to-valley estimation of respiratory sinus arrhythmia. International Journal of Biomedical Computing. 1994;35:169–192. doi: 10.1016/0020-7101(94)90074-4. [DOI] [PubMed] [Google Scholar]