Abstract
Capillary electrophoresis (CE) is a high resolution separation technique broadly used in the biotechnology industry for carbohydrate analysis. The standard sample preparation protocol for CE analysis of glycans released from glycoproteins generally requires derivatization times of overnight at 37°C, using ≥100 fold excess of fluorophore reagent, 8-aminopyrene-1,3,6-trisulfonic-acid (APTS), if the sample is unknown, or it is a regulated biotherapeutic product, possibly containing terminal sialic acid(s). In this paper, we report on significant improvements for the standard CE sample preparation method of glycan analysis. By replacing the conventionally used acetic acid catalyst with citric acid, as low as 1 : 10 glycan to fluorophore molar ratio (vs the typical 1 : ≥100 ratio) maintained the >95% derivatization yield at 55°C with only 50 minutes reaction time. Terminal sialic acid loss was negligible at 55°C during the derivatization process, and thus the kinetics of labeling at 55°C was faster than the loss of sialic acid from the glycan. The reduced relative level of APTS simplified the removal of excess reagent, important in both CE-LIF (electrokinetic injection bias) and CE-MS (ion suppression). Coupling capillary electrophoresis to electrospray ionization mass spectrometry confirmed that the individual peaks separated by CE corresponded to single glycans and increased the confidence of structural assignment based on glucose unit values.
Keywords: glycan analysis, fluorophore labeling, capillary electrophoresis
1 Introduction
There has been rapid progress in the characterization and understanding of the biological roles of the carbohydrate moieties on glycoproteins. This is particularly true in the efforts to elucidate the role of oligosaccharide microheterogeneity and site occupancy in biological recognition, in receptor-ligand or cell-cell interactions, in the modulation of immunogenicity and protein folding as well as in the regulation of protein bioactivity [1]. Glycan characterization is also of high importance in the biopharmaceutical industry as it provides indispensable information on immunogenicity [2] and efficacy [3]. Analysis of the glycosylation profile of production batches is needed to ensure consistent bioactivity [4], and rapid, robust and high resolution bioanalytical techniques must thus be available.
Challenges in carbohydrate analysis arise from the structural diversity of glycans with respect to sequence and linkage/positional isomers, as well as the lack of chromophoric / fluorophoric, and, in many instances, easily ionizable groups. Structural investigation of the glycan composition of glycoproteins begins with regular [5], or accelerated release [6] of the sugar moiety. Pressure cycling technology is an efficient, recently introduced method by our laboratory for enzymatic N-glycan release [7]. In most instances, fluorophore labeling of the isolated glycans is the next step prior to their downstream analysis [8, 9]. The most frequently used carbohydrate analysis techniques are electrophoresis, liquid chromatography, mass spectrometry and NMR[10]. NMR requires much more sample than the other methods. Hydrophilic interaction chromatography (HILIC) [11] and graphitized carbon liquid chromatography [12] of oligosaccharides with fluorescence or MS detection are LC methods of glycan analysis; however, they are typically time-consuming and do not always provide the resolution necessary to identify all linkage and positional isomers. As an alternative approach for linkage and positional glycan isomer analysis, various exoglycosidase cleavage steps can be employed resulting in characteristic shifts in LC elution or electrophoresis migration position [13]. Recently introduced microchip LC [14] and ultrahigh pressure liquid chromatography [15] have increased the separation speed and thus reduced analysis time. Mass spectrometric analysis of glycans after permethylation is also in current use [16].
Capillary electrophoresis (CE) is one of the most powerful separation tools for carbohydrate analysis, providing excellent and rapid separation of both positional and linkage isomers [17]. The technique has become a validated standard bioindustrial method [18] that can be used in a high throughput mode employing a multicapillary format, as designed for DNA sequencing [19]. Electric field mediated separation of glycans requires pre-separation labeling, usually with a negatively charged chromophore / fluorophore, since the majority of sugars are neither charged, nor possess spectrally detectable groups. Fluorophore labeling makes highly sensitive laser induced fluorescence detection (LIF) possible. Further, the presence of negative charges on the fluorophoric derivatization reagent accelerates the electrophoretic separation. In addition, for such negatively charged derivatives, electrospray - MS in the negative mode is typically used [4]. The standard derivatization protocol for CE analysis of glycans released from glycoproteins requires reaction times of several hours (at 55°C) for non-sialylated and overnight (at 37°C) for sialylated structures; however, as in most instances the glycan moiety of glycoproteins may potentially contain sialylated structures, the time consuming latter condition is routinely used..
Sulfonated polyaromatic fluorophores are favored labeling reagents, due to their high fluorescent yield and strong negative charge over a broad pH range. One of the most frequently used tagging agents is 8-aminopyrene-1,3,6-trisulfonic-acid (APTS) [17], which is considered the industry standard in capillary electrophoresis-based glycan analysis today. However, the APTS derivatization reaction typically requires a large molar excess (≥100 fold) for sugar labeling via reductive amination in the presence of an acid catalyst. In spite of the favorable wavelength shift between the conjugated sugar and the free APTS, the latter producing 10 times lower signal than the derivatized glycan with the 488 nm laser system [20], the large reagent excess can still represent a problem in CE-LIF (electrokinetic injection bias) and CE-MS (ion suppression with possible co-migrating species ) analysis. Moreover, one should consider that removal of the large excess of reagent might also lead to loss of derivatized glycans of interest. The APTS – sugar reaction rate strongly depends on the pK of the acid catalyst in the reaction mixture, e.g., stronger acids, such as citric or succinyl acid have been shown to generate an increased reaction rate, relative to acetic acid [21].
In this paper we modified the fluorophore labeling conditions of glycans at 55°C by using the low pK citric acid catalyst and found that 10 times less APTS was sufficient to obtain the high derivatization yield of the standard method in less than 1 hour. In addition, using these modified reaction conditions, negligible terminal sialic acid loss was observed. On-line CE-MS coupling is shown to lead to straightforward confirmation of relative migration based structural assignments [22] and peak purity.
2 Materials and methods
2.1 Chemicals
Citric acid, acetic acid, lithium hydroxide, ammonium hydroxide, maltose, maltopentaose, maltoheptaose, 8-aminopyrene-1,3,6-trisulfonic-acid trisodium salt (APTS), sodium cyanoborohydrate (1 M solution in tetrahydrofuran), human transferrin, bovine fetuin, sodium dodecylsulfate (SDS) and Triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO). PNGase F, N-acetylglucosamine (GlcNAc) and the disialo-galactosylated biantennary oligosaccharide (A2 glycan) were from Prozyme (San Leandro, CA).
2.2 Enzymatic release of N-glycans from transferrin and fetuin
PNGase F enzyme (50 U, Sigma-Aldrich) was dissolved in 100 μL HPLC grade water, and 2 mg of glycoprotein was dissolved in 500 μL of 50 mM phosphate buffer (pH 7.5). 10 μL of 2 % SDS in 100 mM 2-mercapthethanol solution was added to 50 μL of glycoprotein solution, and the mixture was incubated at 100°C for 10 minutes. After this denaturing step, the solution was cooled to room temperature, and 50 μL of 50 mM phosphate buffer (pH 7.5) and 10 μL of 10% Triton X-100 was added. Finally, 1 U of PNGase F enzyme was added to the solution of denatured protein, and the deglycosylation was carried out under standard conditions (overnight at 37°C). After PNGase F digestion, the deglycosylated proteins were precipitated by the addition of 300 μL of ice cold ethanol and centrifuged at 11,000 g for 20 minutes. The glycan containing supernatant was used in the subsequent glycan labeling and CE experiments.
2.3 APTS labeling of glycans
Various concentrations of APTS solutions (5, 10, 20, 50 and 100 mM) were prepared in 1.2 M citric acid. For comparative purposes, APTS solution at a concentration of 0.2 M in 15% acetic acid was also examined. Solutions of sugar standards (maltose, maltopentaose, maltoheptaose, GlcNAc and A2 glycan) as well as the released N-glycans from transferrin and fetuin were evaporated to dryness in a centrifugal vacuum evaporator (Centrivap, Labconco Co., Kansas City, MO). 1 μL of each APTS solution and 1 μL of 1 M NaBH3CN (in THF) were added to the dry samples and incubated in a water bath at 55°C for the reaction rate experiments and overnight at 37°C as control. The reactions were stopped by the addition of 200 μL of HPLC water, and a 40 μL aliquot was diluted with 360 μL of acetonitrile prior to the purification process of the labeled glycans. Normal phase polyamide resin filled pipette tips (5 μL bed volume, PhyNexus, San Jose, CA) were used to remove the residual (unconjugated) APTS from the reaction mixture as described in [23].
2.4 CE-LIF and CE-MS analysis
Capillary electrophoretic analysis of the APTS labeled glycans were carried out by means of a laboratory-built system, consisting of a high voltage power supply (30 kV, Spellman High Voltage Electronics Corporation, Hauppauge, NY), a ZETALIF laser induced fluorescent detector (Picometrics, Toulouse, France) and a solid state 488 nm laser (Cyan, Picarro Inc., Sunnyvale, CA). The total length of the 50 μm i.d. PVA coated capillary (Agilent Technologies, Santa Clara, CA) was 60 cm. The pre-fixed ellipsoid lens was placed at 40 cm, defining in this way the effective column length. 15 mM (pH 4.75) acetate buffer was used as background electrolyte, and the separations were carried out at ambient temperature by applying 15 kV voltage. Samples were injected hydrostatically (Δ 10 cm/20 sec).
CE-MS experiments were performed on a previously described laboratory built system [24]. Briefly, the CE-MS system consisted of a 30 cm long, 50 μm i.d. PVA coated separation capillary (Agilent) attached to a pressurized liquid junction ESI interface. The separation capillary was coupled to an LTQ-MS (Thermo Scientific, San Jose, CA) system using a PicoView interface (New Objective, Woburn, MA) that allowed precise positioning of the ESI tip in front of the MS orifice. There was no pressure driven flow in the separation capillary because both the background electrolyte reservoir (injection end) and the liquid junction ESI solution reservoir were maintained at the same pressure (2 kPa). However, a flow of roughly 200 nL/min was maintained in the ESI tip to facilitate a stable electrospray. In order to permit independent control of the separation (7500 V) and ESI (1800 V) voltages, two high voltage power supplies (Spellman) were employed. The LTQ-MS was operated in the negative ESI mode. Similar to CE-LIF, the APTS-labeled glycans were separated in 15 mM acetate buffer (pH 4.75). Samples were injected electrokinetically at -5 kV for 15 sec. The ESI liquid was the same as that of the background electrolyte (15 mM acetate, pH 4.75), except it contained 40% (v/v) isopropanol.
3 Results and discussion
The analysis of N-linked oligosaccharides by capillary electrophoresis consists of several sample preparation steps, including the time consuming derivatization (most often overnight) with the large excess of charged fluorophore. Optimization of the fluorophore reagent molar ratio and type of acid catalyst in the labeling reaction were evaluated with respect to decrease in the APTS to glycan ratio in the reaction mixture and acceleration of the reaction while maintaining negligible sialic acid loss. Structural assignments of the separated highly sialylated transferrin and fetuin glycans were based on their measured glucose unit (GU) values [22]. Structures of the transferrin glycans were also verified by CE-MS based molecular mass measurements.
3.1 Optimization of the APTS to glycan molar ratio for fluorophore labeling
8-aminopyrene-1,3,6-trisulfonic-acid (APTS) was used for oligosaccharide labeling via an acid catalyzed reductive amination reaction for CE-LIF and CE-MS analysis. One of the most frequently used catalysts for such a reaction is acetic acid, in spite of the fact that it requires ≥100 fold excess of APTS to ensure >95% labeling and overnight incubation at 37°C for sensitive, e.g., sialylated, glycans [8]. This large excess of APTS leaves a significant amount of unreacted fluorophore reagent in the reaction mixture, making injections with electrokinetic focusing (injection bias) difficult. The large amount of APTS in the sample can also affect the analysis of smaller glycans as they may be lost in the tailing end of the large APTS peak, in spite of the fact that the free APTS is 10 fold less detectable with the 488 nm excitation / 520 nm emission wavelength LIF setting, compared to APTS derivatized glycans [20]. Furthermore, in CE-MS, co-migration with the tailing end of the APTS peak can represent a potential problem due to possible ion suppression. Removal of the remaining large amount of unreacted APTS from the reaction mixture thus requires an additional sample preparation step.
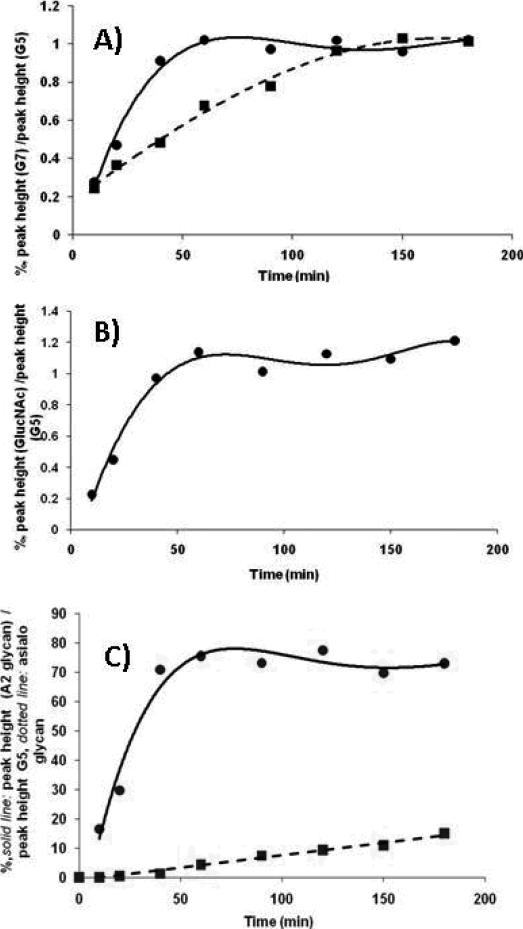
To increase the speed of the derivatization reaction, citric acid, a stronger catalyst was recommended to substitute for acetic acid [21]. In our work; however, we were interested if significant decrease in the relative amount of APTS to glycan molar ratio would still provide >95% derivatization yield. The APTS labeling efficiency was studied in the presence of the citric acid catalyst as a function of reaction time at 55°C. A model oligosaccharide, maltoheptaose (5 nmol), was mixed with 5, 10, 20, 50 and 100 nmol APTS in 1.2 M citric acid [21], respectively, incubated at 55°C in the presence of sodium cyanoborohydride and compared to the standard 500 nmol APTS in 15% acetic acid catalyst based reaction. The labeling efficiency was calculated by comparing the peak heights, after CE separation and LIF detection, of the APTS derivatized maltoheptaose substrate to the APTS derivatized maltopenatose internal standard (5 nmol added to the reaction mixture). Dissolving the derivatization agent in 1.2 M citric acid, only limited labeling was observed at a 1:1 APTS to maltoheptaose ratio. When the APTS to sugar ratio was increased to 10 : 1, >95% labeling was obtained in 50 minutes, as shown by the solid line in Figure 1A. In contrast, the use of acetic acid catalyst with 100 : 1 APTS to sugar ratio (ten fold higher ratio) required 150 min to reach the same derivatization yield (Figure 1A, dotted line). Our results show that with the use of the stronger citric acid catalyst, not only the reaction time decreased but also, significantly, less derivatization agent was required for quantitative tagging. The lower relative amount of APTS should facilitate sample clean-up, relative to the standard method.
Figure 1. Optimization of APTS labeling of glycans.
A) Time dependence of APTS derivatization of 5 nmol maltoheptaose. APTS labeled maltopentaose was used as internal quantification standard. Labeling efficiency was calculated from the peak height ratios. Solid line: 10x excess of APTS dissolved in 1.2 M citric acid as catalyst; Dotted line: 100x excess of APTS with acetic acid catalyst;
B) GlcNAc labeling efficiency as a function of reaction time. 10x excess of APTS was used with citric acid catalyst and APTS labeled maltopentaose as internal standard;
C) Labeling efficiency of disialo-galactosylated biantennary oligosaccharide glycan using 10x excess of APTS with citric acid catalyst (solid line) as a function of reaction time. Dotted line: sialic acid loss (measured by the amount of asialo-galactosylated biantennary structure) as a function of reaction time.
Another aspect of the derivatization reaction was to evaluate the APTS labeling efficiency on the reducing end of the glycans after their release from the polypeptide chain, i.e., on N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc), in case of N- and O-linked glycans, respectively. It is well know that N-linked glycans in human glycoproteins are attached to asparagine residues in the polypetide backbone through a chitobiose linker, a dimer of two β-1,4-N-acetylglucosamines [1]. On the other hand, O-linked core structures are linked to serine and threonine residues via a single N-acetylgalactosamine. Thus, the labeling efficiency for both GlcNAc and GalNAc must be carefully assessed in the derivatization procedure for the analysis of N- and O-linked glycan structures. Figure 1B shows the GlcNAc labeling efficiency using a 10 fold excess of APTS in the citric acid catalyst containing reaction mixture. As the plot in Figure 1B demonstrates, similar to the maltoheptaose labeling experiment, >95% yield was achieved in 50 min for GlcNAc. The labeling efficiency followed the same pattern for GalNAC (data not shown). Thus, we concluded that the APTS labeling efficiency using citric acid catalyst at 55°C offers high derivatization efficiency for the reducing terminal sugar units.
3.2 APTS labeling of sialylated structures
Reductive amination based fluorophore labeling of sialylated glycan structures requires special attention due to the possible instability of the terminal sialic acid residues in the presence of strong acid catalysts at 55-65°C [personal communication, Dr. Pauline Rudd]. Potential sialic acid loss was assessed using a standard disialo-galactosylated biantennary structure. As shown in Figure 1C, labeling of the disialo-galactosylated biantennary oligosaccharide at 55°C with APTS in 1.2 M citric acid catalyst reached the maximum derivatization yield in 50 min with negligible sialic acid loss. As we have noted,the conventional approach requires overnight derivatization of such sialylated structures at 37°C to avoid loss of sialic acid residues; however, the stronger catalyst allowed derivatization at 55°C with shorter (50 min) reaction times resulting in little or no loss of sialylation. As shown in Figure 1C, with extended reaction times, an increasing loss of sialic acid residues was observed (measured by the amount of the resulting asialo-galactosylated biantennary structure); however, the desialylation was still less than 10% after 150 min. Therefore, we concluded that the use of citric acid catalyst in 0.6 M final concentration in the labeling reaction mixture with 10 to 1 APTS to glycan molar ratio at 55°C for 50 min represents appropriate labeling conditions for complex sugar structures. Presumably one could reduce the time necessary for derivatization by increasing the relative amount of APTS; however, for reasons given above we did not want to have more than a 10 to 1 molar ratio.
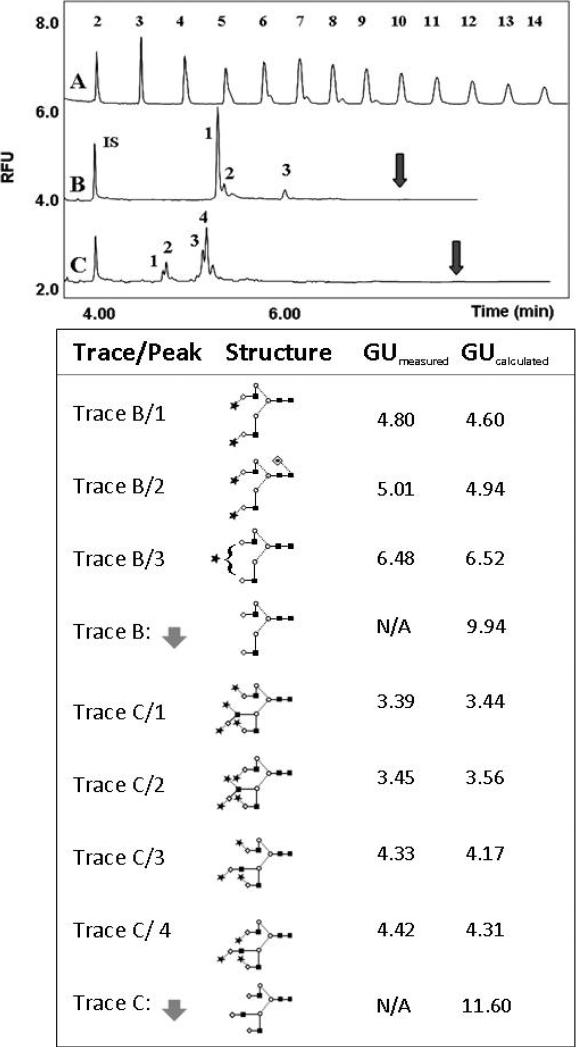
As further evidence of the stability of the terminal sialic acid(s) under the above conditions, the upper panel of Figure 2 shows the N-linked glycosylation pattern of transferrin (trace B) and fetuin (trace C), both highly sialylated glycoproteins along with the maltooligosaccharide ladder standard (trace A) using CE-LIF. The released glycans were labeled by 10:1 APTS to glycan molar ratio at 55°C in the presence of 0.6 M citric acid (final concentration in the reaction mixture). APTS labeled maltose was used as internal standard (IS) to ensure no interference with the electrophoretically faster migrating sialylated glycans. The structural assignments of the major transferrin (trace B, peaks 1-3) and fetuin (trace C, peaks 1-4) glycans were based on their glucose unit values (GU), calculated from previously published data [22, 25]. The lower panel of Figure 2 shows the structures and the corresponding GU values for the peaks marked by the numbers in the electropherograms of the APTS labeled transferrin (trace B) and fetuin (trace C) glycans, respectively. The structures depicted by the arrows in panel B and C in the lower panel of Figure 2 correspond to the asialo transferrin and asialo fetuin glycans with the calculated GU value of 9.94 and 11.6, respectively. Since there were no peaks detected at these sites in the corresponding traces (shown by the arrows), we concluded that little or no desialylation occurred during our optimized derivatization step.
Figure 2.
Comparative CE-LIF analysis of APTS labeled released glycans from human transferrin (upper panel). The lower panel shows the structures and GU values of all the glycans corresponding to the numbered peaks in the upper panel (trace B, peaks 1-3 and trace C, peaks 1-4). The arrows in the upper panel depict the calculated migration position of the desialylated transferrin (trace B) and fetuin (trace C) glycans. IS (internal standard): APTS derivatized maltose. GUmeas and GUcalc are the measured and calculated glucose unit values, respectively. Symbols: ■ GlcNac; ○ Man; ◇ Gal; * Neu5Ac, – β linkage; ... α linkage. All separations were carried out in a PVA coated capillary (effective length: 40 cm, i.d.: 50 μm) at ambient temperature in 25 mM acetate (pH 4.75) buffer applying 250 V/cm field strength. Injection: hydrostatic, Δ 10 cm/10 sec.
3.3 CE-MS analysis of APTS-labeled transferrin glycans
As discussed above, CE-LIF is a standard tool for both the qualitative and quantitative analysis of glycans, even allowing structural assignment of the separated sugars based on their glucose unit (GU) values [22]. However, the GU-based structural elucidation can be verified by additional information on the molecular mass and purity of individual glycan peaks separated by CE. CE-MS can thus be an important complementary tool to CE-LIF in the identification and quantitation of individual glycans. Detailed linkage and/or positional information, however, will not be easily determined by MS analysis, and would require MS/MS analysis under special conditions [16]. The coupling of high resolution separation to MS is thus critical for glycan analysis.
We have coupled capillary electrophoresis with ESI-MS via a pressurized liquid junction interface [24] to enable direct assignment of glycan mass information for individual peaks, as well as to detect co-migrating species (i.e. peak purity assessment). The pressurized liquid junction interface used the same neutral PVA coated capillary and background electrolyte as used in CE-LIF. The interface preserved the high efficiency of CE and allowed independent tuning of separation and ESI conditions. The pressurized liquid junction operated at only 200 nL/min ESI flow rate, resulting in improved sensitivity of the ESI process [26].
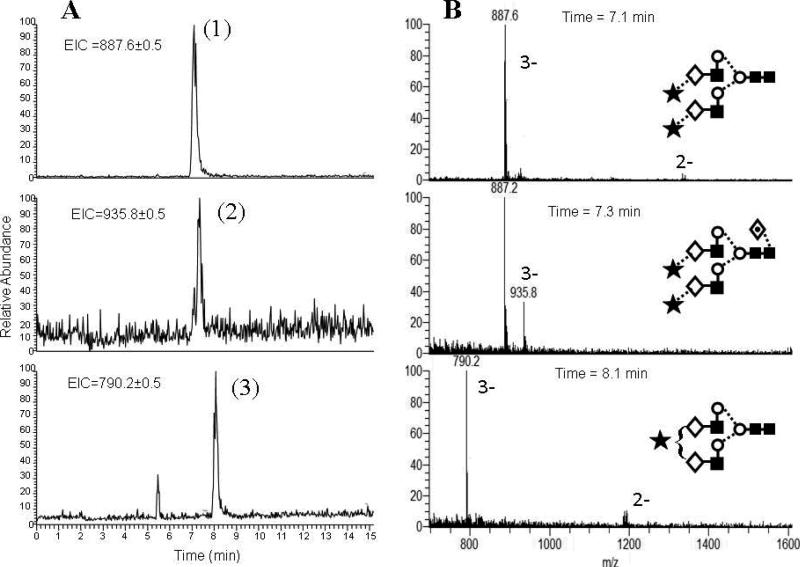
The three panels in Figure 3A depict the extracted ion electropherograms for the same three major peaks in CE-MS analysis of APTS labeled transferrin glycans. Note that these peaks were also found in CE-LIF analysis (Figure 2, trace B). Comparing the CE-LIF and CE-MS results, the peaks in the latter appeared slightly broader. However, the overlapping first and second peaks in trace B of Figure 2 were completely resolved in the mass dimension (Figure 3A, upper and middle panels). Thus, if CE separation did not result in sufficient resolution, LIF detection would be unable to determine whether the peak represented a single component; therefore a complementary method, such as MS is needed. The three panels in Figure 3B show that the triply charged ions dominated the mass spectra of the three glycans (peaks 1, 2 and 3) with m/z values of 887.6; 935.8 and 790.2, respectively, and verified the correctness of our glucose unit (GU) calculation based structural assignment by CE-LIF (upper panel in Figure 2). Consequently, coupling CE to ESI-MS as a complementary tool for CE-LIF can increase the confidence of the structural assignment based on the GU value. Recently, CE-LIF and MS have been coupled in a single experiment using an optical fiber to probe the fluorescence signal prior to the MS analysis [4].
Figure 3.
CE-MS extracted ion electropherograms of APTS-labeled transferrin glycans (panel A) and the MS traces of the corresponding major peaks (panel B). Symbols are the same as in Figure 2. Conditions: Separation field strength: 250 V/cm; 15 mM acetate buffer (pH 4.75), ambient temperature. Injection: -5 kV / 15 seconds. ESI voltage: -1.8 kV.
4 Conclusions
In this paper we have improved several important sample preparation steps for CE-LIF and CE-MS carbohydrate analysis with respect to reaction time as well as fluorophore reagent consumption. Replacing 15% acetic acid catalyst in the APTS reagent solution with 1.2 M citric acid allowed the use of a 10 fold lower fluorophore to glycan molar ratio (10 : 1), while maintaining >95% derivatization yield in 50 minutes reaction time at 55°C. We observed negligible loss of sialic acid residues at this higher reaction temperature within the 50 min reaction. This new procedure offered the convenience of using the same labeling conditions for both non-sialylated and sialylated sugar structures, in contrast to the current industry standard of 55°C / 2 hours for non-sialylated and 37°C / overnight for sialylated structures. Again, if the sample is unknown, or it is a regulated biotherapeutic product, information about the presence of terminal sialic acid(s) is crucial, thus the industry standard suggests overnight derivatization at 37°C. The use of the reduced amount of APTS was particularly beneficial for both CE-LIF and CE-MS based analyses with respect to electrokinetic injection efficiency and minimization of ion suppression, respectively. It is important to note that further decrease in the derivatization time is expected if a higher APTS to sugar molar ratio in the reaction mixture were used. In addition, using the recently introduced pressure cycling technology (PCT) [7] for enzymatic glycan release can further decrease the time required for glycosylation analysis. PCT also offers the possibility of simultaneous processing of 12 samples, thus high throughput glycan analysis can be envisioned by its combination with multicapillary electrophoresis systems. We envision similar advantages of the reported derivatization parameters with other labeling reagents frequently used in HPLC, such as 2-aminobenzamide (2AB) and 2-aminobenzoic acid (2AA) [10]. Finally, coupling CE to ESI-MS increased the confidence of the structural assignment based on CE-LIF data derived glucose unit values and the individual peaks corresponded to the anticipated structures. In the future, we plan to create a publically based comprehensive CE-LIF database for structural prediction of biologically relevant glycosylations and to add the option of CE-MS/MS analysis using data dependent acquisition.
Acknowledgment
This research was supported by grants NIH GM 15847 and NIH GM15847S1 (B.L.K.). The authors thank PhyNexus for their equipment gift. Contribution No. 960 from the Barnett Institute.
Abbreviations
- APTS
8-Aminopyrene-1,3,6-trisulfonic-acid
- CE-LIF
capillary electrophoresis – laser induced fluorescence
- CE-MS
capillary electrophoresis – mass spectrometry
REFERENCES
- 1.Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi CR, Hart GW, et al. Essentials of Glycobiology. second edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. [PubMed] [Google Scholar]
- 2.Li H, d'Anjou M. Curr Opin Biotechnol. 2009;20:678–684. doi: 10.1016/j.copbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Jefferis R. Expert Opin Biol Ther. 2007;7:1401–1413. doi: 10.1517/14712598.7.9.1401. [DOI] [PubMed] [Google Scholar]
- 4.Gennaro LA, Salas-Solano O. Anal Chem. 2008;80:3838–3845. doi: 10.1021/ac800152h. [DOI] [PubMed] [Google Scholar]
- 5. www.prozyme.com, GK80115.
- 6.Sandoval VN, Arellano F, Arnott D, Raab H, Vandlen R, Lill JR. Int. J. Mass Spectrom. 2007;259:117–123. [Google Scholar]
- 7.Szabo Z, Guttman A, Karger BL. Anal.Chem. 2010 doi: 10.1021/ac100098e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guttman A, Chen FT, Evangelista RA, Cooke N. Anal Biochem. 1996;233:234–242. doi: 10.1006/abio.1996.0034. [DOI] [PubMed] [Google Scholar]
- 9.El Rassi Z. Electrophoresis. 1997;18:2400–2407. doi: 10.1002/elps.1150181230. [DOI] [PubMed] [Google Scholar]
- 10.Townsend RR, Hotchkiss AT. Techniques in Glycobiology. Marcel Dekker; New York: 1997. [Google Scholar]
- 11.Takahashi N. J Chromatogr A. 1996;720:217–225. doi: 10.1016/0021-9673(95)00328-2. [DOI] [PubMed] [Google Scholar]
- 12.Ruhaak LR, Deelder AM, Wuhrer M. Anal Bioanal Chem. 2009;394:163–174. doi: 10.1007/s00216-009-2664-5. [DOI] [PubMed] [Google Scholar]
- 13.Rudd PM, Colominas C, Royle L, Murphy N, Hart E, Merry AH, Hebestreit HF, et al. Proteomics. 2001;1:285–294. doi: 10.1002/1615-9861(200102)1:2<285::AID-PROT285>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Bynum MA, Yin H, Felts K, Lee YM, Monell CR, Killeen K. Anal Chem. 2009;81:8818–8825. doi: 10.1021/ac901326u. [DOI] [PubMed] [Google Scholar]
- 15.Kurihara T, Min JZ, Hirata A, Toyo'oka T, Inagaki S. Biomed Chromatogr. 2009;23:516–523. doi: 10.1002/bmc.1147. [DOI] [PubMed] [Google Scholar]
- 16.Kang P, Mechref Y, Novotny MV. Rapid Commun Mass Spectrom. 2008;22:721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 17.Guttman A. Nature (London) 1996;380:461–462. doi: 10.1038/380461a0. [DOI] [PubMed] [Google Scholar]
- 18.Ma S, Nashabeh W. Anal Chem. 1999;71:5185–5192. doi: 10.1021/ac990376z. [DOI] [PubMed] [Google Scholar]
- 19.Callewaert N, Geysens S, Molemans F, Contreras R. Glycobiology. 2001;11:275–281. doi: 10.1093/glycob/11.4.275. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista RA, Liu M-S, Chen F-TA. Analytical Chemistry. 1995;67:2239–2245. [Google Scholar]
- 21.Evangelista RA, Guttman A, Chen FT. Electrophoresis. 1996;17:347–351. doi: 10.1002/elps.1150170210. [DOI] [PubMed] [Google Scholar]
- 22.Guttman A, Herrick S. Anal Biochem. 1996;235:236–239. doi: 10.1006/abio.1996.0118. [DOI] [PubMed] [Google Scholar]
- 23.Olajos M, Hajos P, Bonn GK, Guttman A. Anal Chem. 2008;80:4241–4246. doi: 10.1021/ac8002598. [DOI] [PubMed] [Google Scholar]
- 24.Thakur D, Rejtar T, Karger BL, Washburn NJ, Bosques CJ, Gunay NS, Shriver Z, et al. Anal Chem. 2009;81:8900–8907. doi: 10.1021/ac901506p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guttman A, Ulfelder KW. J Chromatogr A. 1997;781:547–554. doi: 10.1016/s0021-9673(97)00724-3. [DOI] [PubMed] [Google Scholar]
- 26.Smith RD, Shen Y, Tang K. Acc Chem Res. 2004;37:269–278. doi: 10.1021/ar0301330. [DOI] [PubMed] [Google Scholar]