Abstract
A new method of nanoparticle formulation for low water-soluble materials was demonstrated on the example of curcumin. The drug was dissolved in organic solvent that is miscible with water (ethanol) and drug nucleation was initiated by gradual worsening the solution with addition of aqueous polyelectrolyte assisted by ultrasonication. Curcumin crystals of 60-100 nm size were obtained depending on the component concentrations, sonication power, and an initial solvent. A layer-by-layer shell assembly with biocompatible polyelectrolytes was used to provide particle coating with high surface potential and stabilization of drug nanocolloids. Polyelectrolyte layer-by-layer encapsulation allowed sustained drug release from nanoparticles over the range of 10-20 hours.
Many anticancer drugs are poorly soluble in water (curcumin, paclitaxel, tamoxifen, etc). Currently there is no established nanoscale delivery system for the continuous slow release of such drugs other than micelles, although micelles contain a very small percentage of the desired drugs1-3. Other approaches for drug nanoformulation include ultrasonicated decomposition of drug powder4 and drug loading to polymeric (often gelatin or polysaccharide) coacervates5-8. For such formulations, a usual size of drug carriers is 200-300 nm, which is not small enough for the most efficient medical applications.
Natural polyphenolic compounds are interesting as substances with variety of biological activity. High antioxidant, antiviral, and anticancer activities have been proven for plant extracts and polymeric tannins including their isolated individual compounds (such as curcumin) 9-12. In this paper we describe a new approach for the preparation of drug nanocolloids with diameters less than 100 nm which is based on sonication assisted nucleation of drug particles from their solutions in organic solvents. An aggregation of the formed nanoparticles was avoided by polycation adsorption onto particles followed by the polyelectrolyte layer-by-layer (LbL) nanoshell formation 13-18. The following procedure was used for formation stable curcumin nanocolloids through controlled crystallization initiated by worsening saturated curcumin alcohol solutions (Scheme 1). Curcumin powder was dissolved in 60 % ethanol / water solution (curcumin was obtained from Sabinsa and all other chemicals were purchased from Sigma-Aldrich). After curcumin been completely dissolved, we added aqueous polycations, poly(allylamine hydrochloride), PAH, or biodegradable protomine sulfate, (PS) and started ultrasonication with UIP1000, Hielscher instrument, at 100 Wt per mL of solution. During the sonication, water was slowly added into the solution. Because of the adding water, solvent becomes more polar, causing decrease of curcumin solubility, and eventually, its equilibrium concentration exceeds the solubility threshold resulting in the curcumin supersaturated conditions. Then, crystal nucleation starts. Under high power ultrasonication, the drug particle growth is ceased at initial stages.
Scheme 1.
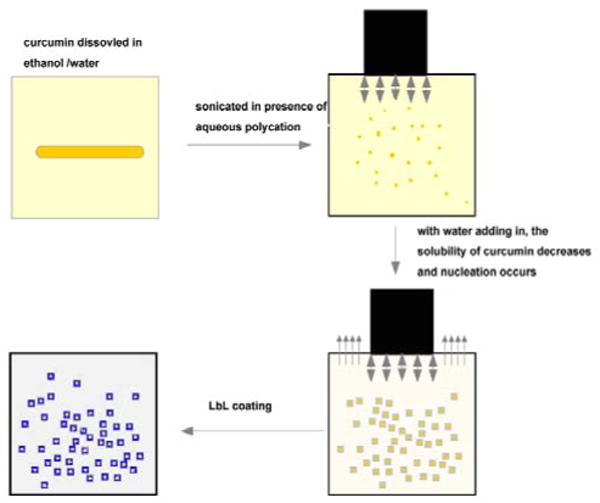
Conversion of curcumin powder into stable nanocolloids.
An adsorption of polyelectrolytes onto drug nanocrystals establishes a barrier for their further growth and aggregation. Obtained crystal particles were stable and not aggregated after sonication was stopped. We assume that it was due to the increase of surface charge provided by adsorbed polyelectrolyte layer. After 45 min of sonication, curcumin nanocrystals were separated from solution by centrifugation and re-suspended in DI water. Additional polyelectrolyte multilayers were built on curcumin nanoparticles by alternate adsorption of polyanions and polycations (LbL shell assembly). For biocompatible capsules, an alternate adsorption of cationic protamine sulfate (PS) and anionic bovine serum albumin (BSA) were used. Fig. 1 shows the original curcumin crystals of approximately 2 ×10 μm size. Curcumin was dissolved in 60 % ethanol at concentration of 2 mg/mL. Ultrasonication was applied for 45 minutes with slow water adding at the rate of 0.2 mL/min. Fig. 1b shows resulted curcumin particles of cubic or rectangular shape.
Fig. 1.
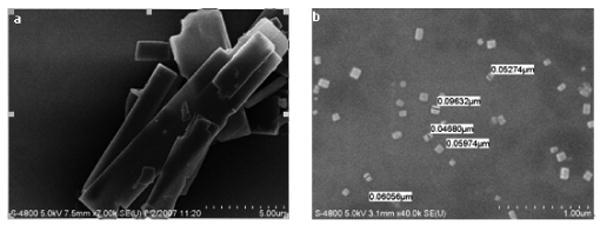
SEM (Hitachi) images of initial curcumin powder (a) and resulted curcumin nanocolloids (b)
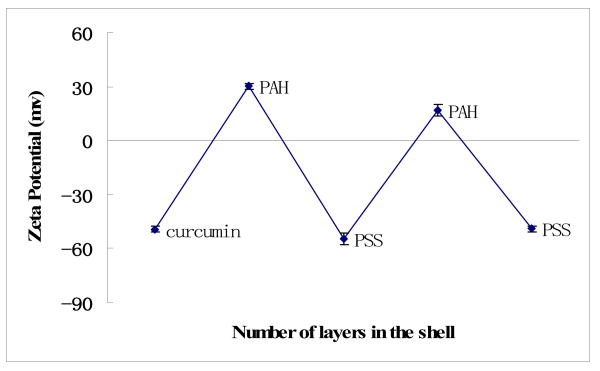
Curcumin nanoparticles of rectangular shape were obtained with edges from 60 nm to 90 nm, and overage size of 80 ± 20 nm were estimated with SEM imaging (Hitachi) and light scattering experiments (Brookhaven Inc., ZetaPlus). Due to the adsorption of cationic PAH, the surface ξ-potential of these nano-particles was positive of ca +30 mV (Fig. 2). High surface charge increases colloidal stability for this formulation. For example, curcumin nanocolloid sample of 0.5 mg/mL drug concentration was preserved for two months as a stable dispersion. The rectangular shape of the nanoparticles is associated with crystalline nature of the obtained curcumin particles.
Fig. 2.
ξ-potential during curcumin nanoparticle LbL coating via alternate adsorption of polyelectrolytes, pH 6.5, PSS-sodium poly(styrene sulfonate), Brookhaven Inc., ZetaPlus instrument
To examine the crystal structure of the curcumin nanoparticles, X-ray powder diffraction analysis was carried out with Bruker-D8 XRD instrument. Bragg pick positions in the X-ray pattern obtained from dried nanoparticle powder coincided with the pick positions for bulk curcumin powder, but the picks were wider due to smaller crystallite sizes (look supplement materials). These X-ray data indicate that the crystal structure of curcumin was preserved, and polycations added during drug crystal formation did not form a complex with curcumin. We assume that polycations were layered predominantly on the crystal surface, as it follows from electrical ξ-potential data given below.
To minimize the curcumin particle size, we prepared series of samples processed under various conditions (different alcohol / water ratios, drug concentrations, ultrasonication power, time, and speed of the solvent worsening for crystallization initiation). Two major factors that affect the crystal size were the rate of water addition (speed of the solvent worsening) and an initial curcumin concentration. Fig. 3a gives the dependence of particle size on the rate of water addition. Water addition rate was varied from 0.05 mL/min up to 0.4 mL/min for the sample volume of 50 mL, and higher rate resulted in formation of larger particles. We observed particles of ca 320 nm overage size at 0.4 mL/min rate versus ca 120 nm particles for 0.05 mL/min rate. An increase of curcumin concentration at the initial solution also resulted in larger nanoparticles as shown in Fig 3b. This may be associated with fast formation of curcumin crystals related to its quicker supersaturation. Slightly larger sizes of curcumin nanoparticles obtained with light scattering technique (ca 120 nm) as compared with mean 80 nm size overage over thirty particles in SEM image may be explained by surface hydrated water and the fact that light scattering is more sensitive to admixture of larger particles in the sample.
Fig. 3.
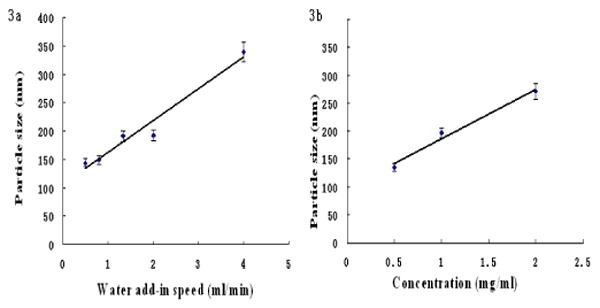
Dependence of water addition rate (a) and curcumin concentration (b) on resulted curcumin particle size.
The ξ-potential alternation demonstrates a step-wise LbL-shell formation of cationic PAH and anionic PSS on curcumin nanoparticles (Fig. 2). After the initial -50 ± 2 mV for bare curcumin microparticles, PAH adsorption during nanoparticles synthesis converts potential to +30± 2 mV. Next, anionic PSS adsorption changes the potential to −53 ± 2 mV, followed by +19 ± 2 mV with PAH, and again −50 ± 2 mV with PSS. Therefore, a multilayer of polycations and polyanions with composition of (PAH/PSS)2 was coated onto the curcumin nanoparticles. Separate QCM analysis of these polyelectrolytes assembly on silver plated 9-MHz QCM resonators (USI-System Instr., Japan) demonstrated a thickness increment of 2.0 ± 0.3 nm for the PAH/PSS bilayer, and total thickness of the two-bilayer shell on curcumin nanoparticles may be estimated as ca 4 nm. Any shell composition may be achieved with further alternate adsorption of polycations and polyanion.
In another experiment, curcumin nano-encapsulation with two bilayer shells of biocompatible protein materials was produced. A positive layer in the shell was generated with protomine sulfate, and albumin formed negative layer (PS/BSA)2 For these LbL nanocapsules, ξ-potential also regularly alternated between +30 mV and -50 mV (depending on the outermost layer) providing high colloidal stability of the samples.
For both shell compositions, curcumin nanoparticles had very thin coating resulting in high drug content of 80-90 % which is an advantage as compared with micelle or liposome formulation having typically 3-5 % drug loading 1-2.
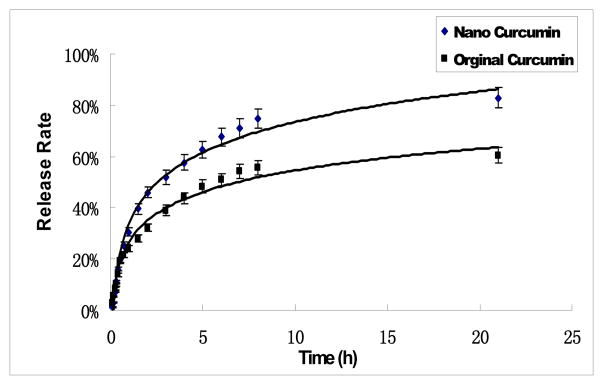
The drug release profile of the curcumin LbL-nanocolloids with the (BSA/PS)2 coating was analyzed in a diffusion chamber (sink conditions). 50 % drug release was reached in 3 hours. The release profile fits the Peppas model (Mt/M0= Kexp(tn), where Mt is the amount of drug released at time t, M0 is the amount of drug released at infinite time, n is the exponent characteristic of the release mechanism, and K is a constant). The obtained n value of 0.5 indicates that the release mechanism is Fickian diffusion 19.
In conclusion
A new method of nanoparticle formulation for low water-soluble materials was demonstrated on example of curcumin. The drug was dissolved in organic solvent that is miscible with water (ethanol) and drug nucleation was initiated by gradual worsening the solution with addition of aqueous polyelectrolyte assisted by ultrasonication. Polyelectrolytes coating induced higher surface charge on the formed nanoparticles and generated stable nanocolloids. This coating was extended to achieve multilayer encapsulation through alternate adsorption of oppositely charged polyelectrolytes. Unlike the commonly used emulsion methods 20, our ultrasonic assisted LbL encapsulation can achieve nanoparticles of much smaller size. For curcumin, we obtained crystalline nanoparticles with an average size of 80 nm, and ξ-potential of +30 mV or -50 mV, which ensured stability of these nanocolloids for months (kept in saturated drug solution). Formation of shells with two bilayers of biocompatible polyelectrolytes allowed slow drug release during ca 20 hours.
Fig. 4.
Curcumin release curves measured with UV adsorption at 480 nm wavelength (UV-vis Agilent-8453 instrument)
Acknowledgments
We are thankful to V. Torchilin (Northeastern University) for useful discussion. The project was supported by Award R01CA134951 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Literature
- 1.Torchilin V. Adv Drug Deliv Rev. 2006;58:1532. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Domb A, Tabata Y, Kumar MR, Farber S, editors. Nanoparticles for Pharmaceutical Applications. American Scientific Publ; Stevenson Ranch, CA: 2007. pp. 5–158. [Google Scholar]
- 3.Shabner V, Collings J, editors. Cancer Chemotherapy: Principles and Practice. Lippincott Williams & Wilkins; Philadelphia, PA: 1990. pp. 12–320. [Google Scholar]
- 4.Agarwal A, Lvov Y, Sawant R, Torchilin V. J Controlled Release. 2008;128:255. doi: 10.1016/j.jconrel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Moya S, Ma L, Gao C, Shen J. J Macromol Biosci. 2009;4:326–335. doi: 10.1002/mabi.200800188. [DOI] [PubMed] [Google Scholar]
- 6.Kommareddy S, Amiji M. Cancer Gene Therapy. 2007;14:488. doi: 10.1038/sj.cgt.7701041. [DOI] [PubMed] [Google Scholar]
- 7.Kommareddy S, Amiji M. J Pharm Sci. 2007;96:397. doi: 10.1002/jps.20813. [DOI] [PubMed] [Google Scholar]
- 8.Shutava T, Balkundi S, Vangala P, O'Neal P, Steffan J, Bigelow R, Cardelli J, Lvov Y. ACS Nano. 2009;3:2564. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- 9.Adhami V, Mukhtar H. Mol Biotech. 2007;37:52. doi: 10.1007/s12033-007-0047-8. [DOI] [PubMed] [Google Scholar]
- 10.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Cancer Research. 2006;66:1234. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 11.Surh Y. Nat Rev Cancer. 2003;3:768. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 12.Barik A, Priyadarsini K, Mohan H. Photochem Photobiol. 2003;77:597. doi: 10.1562/0031-8655(2003)077<0597:psoboc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Donath E, Sukhorukov G, Caruso F, Davis S, Möhwald H. Angew Chem, Int. 1998;37:2202. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2201::AID-ANIE2201>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Ariga K, Hill J, Ji Q. Phys Chem Chem Phys. 2007;9:2319. doi: 10.1039/b700410a. [DOI] [PubMed] [Google Scholar]
- 15.Ariga K, Hill JP, Lee MV, Vinu A, Charvet R, Acharya S. Sci Technol Adv Mater. 2008;9:014109. doi: 10.1088/1468-6996/9/1/014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Geest B, Sanders N, Sukhorukov G, Demeestera J, Smedt S. Chem Soc Rev. 2007;36:636. doi: 10.1039/b600460c. [DOI] [PubMed] [Google Scholar]
- 17.De Geest B, Sukhorukov G, Möhwald H. Expert Opinions Drug Deliv. 2009;6:613. doi: 10.1517/17425240902980162. [DOI] [PubMed] [Google Scholar]
- 18.Shutava T, Kommireddy D, Lvov Y. J Am Chem Soc. 2006;128:9926. doi: 10.1021/ja062318i. [DOI] [PubMed] [Google Scholar]
- 19.Peppas N. Pharmac Acta Helvetia. 1985;60:110. [PubMed] [Google Scholar]
- 20.Zahr A, DeVilliers M, Pishko M. Langmuir. 2005;21:403. doi: 10.1021/la0478595. [DOI] [PubMed] [Google Scholar]