Abstract
Cleft lip/palate comprises a large fraction of all human birth defects, and is notable for its significant lifelong morbidity and complex etiology. Several studies have shown that genetic factors appear to play a significant role in the etiology of cleft lip/palate. Human chromosomal region 9q21 has been suggested in previous reports to contain putative cleft loci. Moreover, a specific region (9q22.3-34.1) was suggested to present a ∼45% probability of harboring a cleft susceptibility gene. Fine mapping of fifty SNPs across the 9q22.3-34.11 region was performed to test for association with cleft lip/palate in families from United States, Spain, Turkey, Guatemala, and China. We performed family-based analysis and found evidence of association of cleft lip/palate with STOM (rs306796) in Guatemalan families (P=0.004) and in all multiplex families pooled together (P=0.002). This same SNP also showed borderline association in the US families (P=0.04). Under a nominal value of 0.05, other SNPs also showed association with cleft lip/palate and cleft subgroups. SNPs in STOM and PTCH genes and nearby FOXE1 were further associated with cleft phenotypes in Guatemalan and Chinese families. Gene prioritization analysis revealed PTCH and STOM ranking among the top fourteen candidates for cleft lip/palate among 339 genes present in the region. Our results support the hypothesis that the 9q22.32-34.1 region harbors cleft susceptibility genes. Additional studies with other populations should focus on these loci to further investigate the participation of these genes in human clefting.
Keywords: cleft lip and palate, chromosome 9q, fine mapping, craniofacial defect
Introduction
Oral-facial clefts arise as a failure of facial embryonic processes to completely merge and/or fuse. They comprise a large fraction of all human birth defects, and may occur as part of single-gene Mendelian syndromes, as part of chromosomal abnormalities, or due to teratogen exposure [Murray, 2002]. Clefts of the lip and clefts of the palate are notable for their significant lifelong morbidity and complex etiology, and are considered different entities: cleft lip can occur with or without cleft palate (cleft lip/palate) while cleft palate may occur as an isolated defect [Fogh-Andersen, 1942]. Cleft lip/palate affects about 1/700 births with wide variability related to geographic origin and socioeconomic status. In general, Native American and Asian populations present the highest frequencies, sometimes at 1/500 or higher, followed by Caucasian, and African-derived populations showing the lowest frequencies around 1/2500 births [Mossey and Little, 2002].
Several studies have shown that genetic factors appear to play a significant role in the etiology of cleft lip/palate. In recent years, it has become evident that cleft lip/palate is heterogeneous, and different chromosome regions such as 1q, 2p, 4q, 6p, 14q, 17q, and 19q have been claimed to contain a clefting locus [Vieira, 2008]. Association of specific genes such as MSX1 [Lidral et al., 1998; Beaty et al., 2001; Jezewski et al., 2003; Vieira et al., 2003], IRF6 [Zucchero et al., 2004; Ghassibe et al., 2005; Park et al., 2007; Vieira et al., 2007; Jia et al., 2009; Jugessur et al., 2009]; PVRL1 [Sozen et al., 2001; Avila et al., 2006], and genes of the FGF signaling pathway [Riley et al., 2007; Menezes et al., 2008] have been reported as well. Recently, genome-wide association studies with German, American and European populations have revealed a new major susceptibility locus for cleft lip/palate located in a gene desert region on chromosome 8q24 [Birnbaum et al., 2009; Grant et al., 2009; Nikopensus et al., 2009].
Human chromosomal region 9q21 has also been suggested in previous reports [Marazita et al., 2002; 2004; 2009], and in a mouse model [Juriloff et al., 2004] to contain putative cleft loci. A marker (D9S1122) located at 9q21.13 showed positive association with cleft lip/palate in Chinese multiplex families [Marazita et al., 2002]. Furthermore, a meta-analysis of a 10-cM genome scan of 388 extended multiplex families with cleft lip/palate from multiple populations revealed potential candidate genes in six chromosomal regions, including 9q21 (heterogeneity LOD score [HLOD] = 6.6). Positive association results (indicating close proximity to a clefting locus) were also found with four of the five selected candidate genes in the region [Marazita et al., 2004]. Moreover, the PPL (posterior probability of linkage) statistical test pointed towards a nearby region, from bands 9q22.3 to q34.1 (between 95Mb and 128Mb), as presenting ∼45% probability of harboring a causative gene for clefting (Govil et al., unpublished observations). The FOXE1 (forkhead box E1) gene, located in this region and implicated in syndromic cleft palate (Bamforth-Lazarus syndrome, OMIM 241850) has recently been reported to be associated with both isolated cleft lip with or without cleft palate and isolated cleft palate, and suggested as a key player in primary palatogenesis [Moreno et al., 2009].
To follow up on this data, we performed association studies with densely spaced markers spanning the 9q22.3-34.1 region to test for association with cleft lip/palate.
Materials and Methods
Subjects and Samples
Details of the population data sets are presented in Table I. Briefly, we assessed 291 multiplex cleft families (one or more members affected with an oral cleft) from the United States, Spain, Turkey, Guatemala and China. All cases had nonsyndromic cleft lip with or without cleft palate. Families were ascertained through probands, and additional relatives were recruited. Individuals presenting syndromic clefts, cleft palate only, or unknown cleft types, as well as control individuals presenting positive family history of clefting were excluded. This study was approved by local and the University of Pittsburgh institutional review boards. A signed informed consent sheet was obtained from all study participants after being explained the objectives and procedures of the study.
Table I.
Summary of families and individuals ascertained for the study.
| Group | Ancestral Origin | No. of Families | No. of Individuals | Affected Individuals | Unaffected Individuals | Affected Individuals by Phenotype | |
|---|---|---|---|---|---|---|---|
| CL1 | CL/P2 | ||||||
| Families | |||||||
| North America | US | 89 | 529 | 145 | 384 | 39 | 106 |
| Central America | Guatemala | 77 | 514 | 93 | 421 | 20 | 73 |
| Europe | Spain | 36 | 136 | 43 | 93 | 10 | 33 |
| Turkey | 29 | 288 | 38 | 250 | 17 | 21 | |
| East Asia | China | 60 | 180 | 60 | 120 | 14 | 46 |
| TOTAL | 291 | 1647 | 379 | 1268 | 100 | 279 | |
CL, cleft lip;
CL/P, cleft lip with or without cleft palate.
Blood or saliva samples were collected to obtain genomic DNA. DNA samples from the populations from China, Turkey, and United States recruited until 2005 were obtained from blood. DNA samples from the population from Guatemala, Spain, and United States recruited after 2005 were obtained from saliva. DNA extraction was performed according to previously published protocols [Trevillato and Line, 2000]. There was no difference in the performance of the samples obtained from blood or saliva in regards to our genotyping approach described below.
SNP Selection and Genotyping
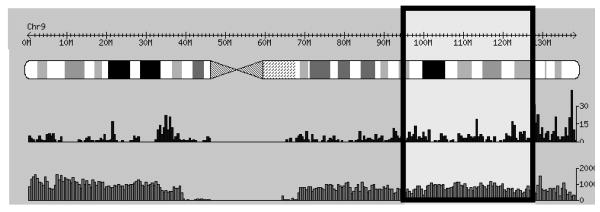
We used the International HapMap Project Database to guide our selection of single nucleotide polymorphisms (SNPs) to be genotyped. We based our selection using the approach devised by Carlson et al. [Carlson et al., 2004], selecting from a set of SNPs which maximally represented the linkage disequilibrium structure of the 9q22.3-34.1 region. Fifty SNPs spanning the 9q22.3-34.1 region were selected (Figure I). Preference was given to tag SNPs with a minor allele frequency of at least 10%, taking into consideration the various ethnicities represented in this study.
Figure I.
Overview of chromosome 9. The lighter box in the detail indicates the investigated region (9q22.32-34.11), spanning from 95Mb to 128Mb (Obtained from HapMap International Project, available at http://www.hapmap.org). Solid black bars below chromosome structure represent genes in region. Outlined gray bars below black bars indicate number of single nucleotide polymorphisms in the region.
Genotypes were generated using Taqman chemistry [Ranade et al., 2001] on an automatic sequence-detection instrument (ABI Prism 7900HT, Applied Biosystems, Foster City, CA). Assays and reagents were supplied by Applied Biosystems (Applied Biosystems, Foster City, CA). Details of the studied polymorphisms are presented in Table II.
Table II.
Details of the SNPs investigated in the study.
| SNP No. | SNP name | Chromosome Region | Base Position1 | Mapping Gene (SNP Location) | Mapping Gene | Function | Base Change |
|---|---|---|---|---|---|---|---|
| 1 | rs357564 | 9q22.32 | 97249415 | PTCH | PTCH | coding-nonsynonymous | AG |
| 2 | rs2236407 | 9q22.32 | 97277617 | PTCH | PTCH | intron/next to exon | AG |
| 3 | rs2297088 | 9q22.32 | 97282805 | PTCH | PTCH | intron/next to exon | AG |
| 4 | rs10512248 | 9q22.32 | 97299524 | PTCH | PTCH | intron | AC |
| 5 | rs894673 | 9q22.33 | 99652091 | FOXE1 | FOXE1 | near gene 5′ UTR | AT |
| 6 | rs3758249 | 9q22.33 | 99653961 | FOXE1 | FOXE1 | near gene 5′ UTR | AG |
| 7 | rs10984103 | 9q22.33 | 99679096 | intergenic | C9orf156 | unknown | AC |
| 8 | rs2900463 | 9q22.33 | 100017278 | TBC1D2 | TBC1D2 | intron | CT |
| 9 | rs2808557 | 9q22.33 | 100283185 | GPR51 | GPR51 | intron/next to exon | GT |
| 10 | rs1031111 | 9q22.33 | 101201619 | intergenic | intergenic | unknown | AG |
| 11 | rs1526267 | 9q31.1 | 101629566 | NR4A3 | NR4A3 | 5′ UTR | AG |
| 12 | rs1226588 | 9q31.1 | 102380835 | TMEFF1 | TMEFF1 | intron | CT |
| 13 | rs1448576 | 9q31.1 | 105023498 | 340511 | 340511 | unknown | AG |
| 14 | rs1992917 | 9q31.1 | 105029464 | 340511 | 340511 | unknown | AG |
| 15 | rs1993434 | 9q31.1 | 105074129 | intergenic | intergenic | unknown | AG |
| 16 | rs2014343 | 9q31.3 | 112899132 | intergenic | intergenic | unknown | CG |
| 17 | rs4979032 | 9q31.3 | 113466796 | intergenic | intergenic | intron | AG |
| 18 | rs2762475 | 9q32 | 113928337 | SUSD1 | SUSD1 | intron | CT |
| 19 | rs1999263 | 9q32 | 114249494 | HSDL2 | HSDL2 | intron | AC |
| 20 | rs10739367 | 9q32 | 114406899 | intergenic | intergenic | intron | CT |
| 21 | rs1539339 | 9q32 | 114633550 | SNX30 | SNX30 | intron/next to exon | AC |
| 22 | rs818714 | 9q32 | 115223122 | C9orf43 | C9orf43 | intron/next to exon | CT |
| 23 | rs1008138 | 9q32 | 115849291 | ZNF618 | ZNF618 | intron/next to exon | GT |
| 24 | rs942519 | 9q32 | 116208854 | DFNB31 | DFNB31 | coding-nonsynonymous | AG |
| 25 | rs2992147 | 9q33.1 | 116889135 | TNC | TNC | coding-synonymous | CT |
| 26 | rs971037 | 9q33.1 | 117706363 | C9orf27 | C9orf27 | intron/next to exon | AT |
| 27 | rs1888636 | 9q33.1 | 117979155 | PAPPA | PAPPA | intron | AT |
| 28 | rs1418495 | 9q33.1 | 120130612 | intergenic | intergenic | unknown | AG |
| 29 | rs1335256 | 9q33.1 | 120366850 | intergenic | intergenic | unknown | CT |
| 30 | rs7859743 | 9q33.2 | 122196412 | intergenic | intergenic | intron/next to exon | AG |
| 31 | rs2300934 | 9q33.2 | 122848784 | intergenic | intergenic | intron/next to exon | AC |
| 32 | rs306796 | 9q33.2 | 123167156 | STOM | STOM | intron | CT |
| 33 | rs10739600 | 9q33.2 | 123663694 | TTLL11 | TTLL11 | unknown | AG |
| 34 | rs7853089 | 9q33.2 | 123972807 | C9orf18 | C9orf18 | intron | GT |
| 35 | rs1928623 | 9q33.2 | 124348830 | OR1N2 | OR1N2 | unknown | AC |
| 36 | rs2251495 | 9q33.2 | 124682508 | MNAB | MNAB | intron/next to exon | CT |
| 37 | rs2808416 | 9q33.2 | 125159980 | CRB2 | CRB2 | intron | AG |
| 38 | rs1928482 | 9q33.2 | 125472269 | DENND1A | DENND1A | intron | CT |
| 39 | rs2274782 | 9q33.3 | 126123895 | intergenic | intergenic | intron/next to exon | CT |
| 40 | rs10739650 | 9q33.3 | 126363597 | NR6A1 | NR6A1 | intron | CT |
| 41 | rs646527 | 9q33.3 | 126717053 | GOLGA1 | GOLGA1 | intron | CT |
| 42 | rs7853181 | 9q33.3 | 127262845 | MAPKAP1 | MAPKAP1 | intron | AG |
| 43 | rs10760403 | 9q33.3 | 127648316 | PBX3 | PBX3 | intron | GT |
| 44 | rs10987185 | 9q33.3 | 127981942 | intergenic | intergenic | unknown | CG |
| 45 | rs887659 | 9q33.3 | 128224130 | C9orf28 | C9orf28 | intron/next to exon | AG |
| 46 | rs3850585 | 9q33.3 | 128476093 | LMX1B | LMX1B | intron | CT |
| 47 | rs1890546 | 9q33.3 | 128991962 | intergenic | intergenic | intron | CT |
| 48 | rs7869023 | 9q33.3 | 129241499 | RPL12 | RPL12 | intron | AG |
| 49 | rs913989 | 9q34.1 | 129572809 | SH2D3C | SH2D3C | intron/next to exon | AC |
| 50 | rs10819354 | 9q34.1 | 129893311 | SLC25 | SLC25 | intron, near 5′ UTR | AG |
According to the UCSC Genome Browser March 2006 Assembly.
Statistical Analyses
The transmission disequilibrium test was used to assess association of alleles at each marker with cleft lip with or without cleft palate with the use of the Family Based Association Test (FBAT) software [Horvath et al., 2001]. We also performed haplotype analyses using the “hbat” function of the FBAT software to identify if sets of markers were in linkage disequilibrium with cleft lip/palate. We also analyzed the entire dataset according to the following cleft subgroups: CLO + CLCLP (families where all affecteds have cleft lip only plus families where at least one affected has cleft lip only and one affected has cleft lip and palate, excluding any family where an affected has cleft palate only); and CLO +CLP +CLCLP (families where all affecteds have cleft lip only plus families where all affecteds have cleft lip and palate plus families where at least one affected has cleft lip only and one affected has cleft lip and palate, excluding any family where an affected has cleft palate only). Populations were analyzed individually and as a pooled dataset. P-values below 0.001 (0.05 divided by 50 markers) were considered statistically significant.
Gene Prioritization
We attempted to identify candidate genes present in the 9q22.3-34.1 region using the gene prioritization software Endeavour [Aerts et al., 2006; Tranchevent et al., 2008]. Endeavour is a web server that allows users to prioritize candidate genes with respect to their biological processes or diseases of interest. It relies on the similarity between candidate genes and models built with a set of manually inputted reference genes. We used 10 genes to compose the reference set: IRF6, MSX1, PVRL1, TGFB3, CLPTM1, TGFA, FGFR1, RARA, BCL3, and TBX22. We did not use PTCH or FOXE1 as reference genes in the target region as they would be automatically removed by the program due to redundancy between reference and test sets of genes.
Results
Fine Mapping Association Studies
We genotyped fifty SNPs spanning 339 genes present in chromosomal region 9q22.3-q34.1. Detailed information on allele frequencies per population and additional replication analyses of specific markers are available as Supplemental Material. We performed family-based analysis to assess genotype and allelic associations between the investigated SNPs and cleft lip/palate. After correcting for multiple testing, we found borderline association of cleft lip/palate with a SNP in STOM (rs306796, 9q33.2) in Guatemalan families (P=0.004) and all families pooled together (P=0.002). This same SNP (rs306796) also showed a trend for association in the US population (P=0.04) (Table III). Of note, SNPs in or nearby STOM also showed modest association with cleft lip/palate in four of the five populations studied (P=0.04). Under a nominal value of 0.05, other SNPs also showed association with cleft lip/palate and cleft subgroups (Tables III and IV).
Table III.
Summary of results for association tests with markers in the chromosome 9q region and cleft lip/palate in the studied populations.
| SNP Name | UCSC base position1 | Mapping Gene (SNP Location) | Family-Based Analyses (P-values2) | |||||
|---|---|---|---|---|---|---|---|---|
| Individual Populations | Pooled Populations | |||||||
| USA | Guatemala | Spain | Turkey | China | ||||
| rs357564 | 95,289,149 | PTCH | 0.40 | 0.19 | 0.49 | 0.74 | 0.50 | 0.09 |
| rs2236407 | 95,317,351 | PTCH | 0.49 | 0.16 | 0.40 | 0.64 | 1.00 | 0.52 |
| rs2297088 | 95,322,539 | PTCH | 0.66 | 0.64 | 0.71 | 0.64 | 0.89 | 0.87 |
| rs10512248 | 95,339,258 | PTCH | 0.75 | 0.16 | 1.00 | 0.64 | 0.58 | 0.90 |
| rs894673 | 97,691,825 | FOXE1 | 0.85 | 0.93 | 0.48 | 0.82 | 0.19 | 0.58 |
| rs3758249 | 97,693,695 | FOXE1 | 0.60 | 0.93 | 0.43 | 0.65 | 0.26 | 0.71 |
| rs10984103 | 97,718,830 | C9orf156 | 0.89 | 0.86 | 0.27 | 0.67 | 0.24 | 0.52 |
| rs2900463 | 98,057,012 | TBC1D2 | 0.63 | 0.52 | 0.87 | 0.06 | 0.18 | 0.11 |
| rs2808557 | 98,322,919 | GPR51 | 0.93 | 0.47 | 0.78 | 0.53 | 0.18 | 0.62 |
| rs1031111 | 99,241,353 | intergenic | 0.66 | 0.85 | 0.55 | 1.00 | 0.71 | 0.99 |
| rs1526267 | 99,669,300 | NR4A3 | 0.49 | 0.65 | 0.05 | 0.12 | 0.48 | 0.79 |
| rs1226588 | 100,420,569 | TMEFF1 | 0.06 | 0.88 | 0.74 | 0.37 | 0.66 | 0.25 |
| rs1448576 | 103,063,232 | 340511 | 0.56 | 0.65 | 1.00 | 0.68 | 0.04 | 0.13 |
| rs1992917 | 103,069,198 | 340511 | 0.68 | 0.89 | 0.86 | 0.70 | 0.28 | 0.84 |
| rs1993434 | 103,113,863 | intergenic | 0.92 | 0.66 | 0.42 | 0.84 | 0.76 | 0.59 |
| rs2014343 | 110,938,866 | intergenic | 0.31 | 0.01 | 0.49 | 0.03 | 0.28 | 0.01 |
| rs4979032 | 111,506,520 | intergenic | 0.89 | 0.39 | 0.74 | 0.39 | 0.58 | 0.50 |
| rs2762475 | 111,968,071 | SUSD1 | 0.47 | 0.84 | 0.49 | 1.00 | 0.79 | 0.89 |
| rs1999263 | 112,289,228 | HSDL2 | 0.71 | 0.20 | 1.00 | 0.25 | 0.15 | 0.97 |
| rs10739367 | 112,446,633 | intergenic | 0.97 | 0.26 | 0.59 | 0.67 | 0.65 | 0.87 |
| rs1539339 | 112,673,284 | SNX30 | 0.94 | 0.83 | 0.44 | 1.00 | 0.09 | 0.25 |
| rs818714 | 113,262,855 | C9orf43 | 0.12 | 0.53 | 0.39 | 0.64 | 0.86 | 0.16 |
| rs1008138 | 113,889,024 | ZNF618 | 0.46 | 0.14 | 0.12 | 0.80 | 0.63 | 0.25 |
| rs942519 | 114,248,587 | DFNB31 | 0.80 | 0.18 | 0.45 | 0.47 | 0.53 | 0.52 |
| rs2992147 | 114,928,868 | TNC | 0.33 | 0.78 | 0.85 | 0.37 | 0.88 | 0.37 |
| rs971037 | 115,746,096 | C9orf27 | 0.81 | 0.78 | 0.52 | 0.09 | 0.56 | 0.56 |
| rs1888636 | 116,018,888 | PAPPA | 0.06 | 0.61 | 0.43 | 0.81 | 0.89 | 0.39 |
| rs1418495 | 118,170,345 | intergenic | 0.04 | 0.91 | 0.42 | 0.68 | 0.53 | 0.27 |
| rs1335256 | 118,406,583 | intergenic | 0.69 | 0.63 | 0.71 | 0.82 | 0.39 | 0.65 |
| rs7859743 | 120,236,145 | intergenic | 0.75 | 0.18 | 0.04 | 0.37 | 0.79 | 0.19 |
| rs2300934 | 120,888,517 | intergenic | 0.52 | 0.04 | 0.89 | 0.39 | 0.04 | 0.91 |
| rs306796 | 121,206,889 | STOM | 0.04 | 0.004 | 0.26 | 0.41 | 0.06 | 0.002 |
| rs10739600 | 121,703,427 | TTLL11 | 0.92 | 0.93 | 0.49 | 0.82 | 0.09 | 0.82 |
| rs7853089 | 122,012,540 | C9orf18 | 0.44 | 0.45 | 0.87 | 0.22 | 0.81 | 0.73 |
| rs1928623 | 122,388,563 | OR1N2 | 0.71 | 0.17 | 0.41 | 0.25 | 0.16 | 0.06 |
| rs2251495 | 122,722,241 | MNAB | 0.18 | 0.92 | 1.00 | 0.32 | 0.89 | 0.73 |
| rs2808416 | 123,199,713 | CRB2 | 0.51 | 0.25 | 0.21 | 0.83 | 0.74 | 0.55 |
| rs1928482 | 123,512,002 | DENND1A | 0.03 | 0.41 | 0.39 | 0.24 | 0.65 | 0.24 |
| rs2274782 | 124,163,628 | intergenic | 0.32 | 0.44 | 0.39 | 0.13 | 0.28 | 0.95 |
| rs10739650 | 124,403,330 | NR6A1 | 0.21 | 0.15 | 0.56 | 0.20 | 0.37 | 0.94 |
| rs646527 | 124,756,786 | GOLGA1 | 0.67 | 0.40 | 0.95 | 0.32 | 0.22 | 0.78 |
| rs7853181 | 125,302,578 | MAPKAP1 | 0.56 | 0.32 | 0.49 | 0.58 | 0.69 | 0.95 |
| rs10760403 | 125,688,049 | PBX3 | 0.52 | 0.90 | 0.65 | 0.41 | 0.54 | 0.90 |
| rs10987185 | 126,021,675 | intergenic | 0.42 | 0.42 | 0.71 | 0.81 | 0.34 | 0.64 |
| rs887659 | 126,263,863 | C9orf28 | 0.85 | 0.63 | 0.83 | 0.49 | 0.47 | 0.82 |
| rs3850585 | 126,515,826 | LMX1B | 0.95 | 0.73 | 0.21 | 0.62 | 0.79 | 0.64 |
| rs1890546 | 127,031,695 | intergenic | 0.47 | 0.22 | 0.20 | 0.43 | 0.22 | 0.64 |
| rs7869023 | 127,281,232 | RPL12 | 0.58 | 0.65 | 0.05 | 0.65 | 0.17 | 0.09 |
| rs913989 | 127,612,542 | SH2D3C | 0.52 | 0.22 | 0.20 | 0.67 | 0.41 | 0.68 |
| rs10819354 | 127,933,044 | SLC25 | 0.27 | 0.37 | 0.13 | 0.49 | 0.05 | 0.87 |
According to the UCSC Genome Browser March 2006 Assembly.
Family-Based Association Test.
Table IV.
Summary of results for association tests with markers in the chromosome 9q region and families with cleft lip only plus cleft lip and palate in Guatemala and China.
| CLO + CL,CLP1 | |||
|---|---|---|---|
| P-values | |||
| SNP Name | SNP Location | Guatemala | China |
| rs357564 | PTCH | 0.41 | -- |
| rs2236407 | PTCH | 0.004 | 0.11 |
| rs2297088 | PTCH | 0.01 | 0.11 |
| rs10512248 | PTCH | 0.01 | 0.07 |
| rs894673 | FOXE1 | 0.07 | 0.05 |
| rs3758249 | FOXE1 | 0.13 | 0.05 |
| rs10984103 | C9orf156 | 0.07 | 0.16 |
| rs2900463 | TBC1D2 | 0.78 | 0.03 |
| rs2808557 | GPR51 | 0.16 | 0.16 |
| rs1031111 | intergenic | 0.04 | 0.11 |
| rs1526267 | NR4A3 | 0.87 | 0.76 |
| rs1226588 | TMEFF1 | 0.05 | 0.25 |
| rs1448576 | 340511 | 0.28 | 0.32 |
| rs1992917 | 340511 | 0.94 | 0.05 |
| rs1993434 | intergenic | 0.80 | 0.76 |
| rs2014343 | intergenic | 0.05 | 0.81 |
| rs4979032 | intergenic | 0.84 | 0.32 |
| rs2762475 | SUSD1 | 0.18 | 0.76 |
| rs1999263 | HSDL2 | 0.28 | 0.64 |
| rs10739367 | intergenic | 0.32 | 0.44 |
| rs1539339 | SNX30 | 0.34 | 0.03 |
| rs818714 | C9orf43 | 0.26 | 0.56 |
| rs1008138 | ZNF618 | 0.13 | 0.29 |
| rs942519 | DFNB31 | 0.43 | 0.48 |
| rs2992147 | TNC | 1.00 | 0.71 |
| rs971037 | C9orf27 | 0.41 | 0.83 |
| rs1888636 | PAPPA | 0.49 | 0.71 |
| rs1418495 | intergenic | 0.55 | 0.11 |
| rs1335256 | intergenic | 0.83 | 0.37 |
| rs7859743 | intergenic | 0.64 | 0.76 |
| rs2300934 | intergenic | 0.03 | 0.53 |
| rs306796 | STOM | 0.01 | 0.32 |
| rs10739600 | TTLL11 | 1.00 | 1.00 |
| rs7853089 | C9orf18 | 0.49 | 0.65 |
| rs1928623 | OR1N2 | 0.49 | -- |
| rs2251495 | MNAB | 0.41 | 0.48 |
| rs2808416 | CRB2 | 0.07 | 0.74 |
| rs1928482 | DENND1A | 0.20 | 1.00 |
| rs2274782 | intergenic | 0.74 | 0.76 |
| rs10739650 | NR6A1 | 0.65 | 1.00 |
| rs646527 | GOLGA1 | 1.00 | 0.29 |
| rs7853181 | MAPKAP1 | 0.94 | 0.74 |
| rs10760403 | PBX3 | 0.18 | 0.08 |
| rs10987185 | intergenic | 0.65 | 0.48 |
| rs887659 | C9orf28 | 0.13 | 0.10 |
| rs3850585 | LMX1B | 0.20 | 0.17 |
| rs1890546 | intergenic | 0.22 | 1.00 |
| rs7869023 | RPL12 | 0.83 | 0.37 |
| rs913989 | SH2D3C | 1.00 | 0.32 |
| rs10819354 | SLC25 | 0.40 | 0.74 |
CLO + CL,CLP, families where all affecteds have cleft lip only plus families where at least one affected has cleft lip only and one affected has cleft lip and palate. Excludes any family where an affected has cleft palate only.
When analyzing families according to cleft subgroups, we found association of markers in PTCH (rs2234607, P=0.004; rs2297088, P=0.01; and rs10512248, P=0.01) and in/nearby STOM (rs306796, P=0.01; and rs2300934, P=0.03) with the CLO+CLCLP subgroup in Guatemalan families. We also found borderline association of markers nearby FOXE1 in Chinese (rs2900463; P=0.03) and Guatemalan (rs1031111; P=0.04) families (Table IV). Results for the CLO +CLP +CLCLP groups did not differ from the association results in individual populations (data not shown).
We also performed haplotype analysis of the investigated SNPs by population (Table V) and by population according to cleft subgroup (Table VI). Altered transmission of haplotypes involving markers in/nearby STOM was evident in families from the US (rs1928623-rs2251495-rs2808416; P=0.02), Turkey (rs1928623-rs2251495-rs2808416-rs1928482; P=0.02), Guatemala (rs2300934-rs306796; P=0.02 and rs306796-rs10739600; P=0.03), and China (rs2300934-rs306796; P=0.04) (Table V). Altered transmission was also observed for PTCH rs357654-rs2236407-rs2297088 haplotype (P=0.03) and for were PTCH rs357654-rs2236407-rs2297088-rs10512248 haplotype (P=0.02) in Guatemalan families (Table V). When analyzed by cleft subgroup, interesting results were observed for haplotype markers involving PTCH (rs357654-rs2236407; P=0.01) and PTCH and FOXE1 (rs10512248-rs894673) in families from Guatemala (P=0.01) and China (P=0.03) presenting CLO + CLCLP (Table VI).
Table V.
Results of haplotype transmission analysis in families with cleft lip with or without cleft palate by population.
| Marker Haplotype | Genes in Haplotype | Population | HBAT P-value2 |
|---|---|---|---|
| rs1928623-rs2251495-rs2808416 | Intergenic-MNAB (∼1Mb of STOM)- CRB2 | US | 0.02 |
| rs1888636-rs1418495-rs1335256-rs7859473 | PAPPA-intergenic-intergenic-CDK5RAP2 | US | 0.02 |
| rs7869023-rs913989 | ZNF79-SH2D3C | Spain | 0.03 |
| rs1928623-rs2251495-rs2808416-rs1928482 | Intergenic-MNAB (∼1Mb of STOM)- CRB2-KIAA1608 | Turkey | 0.02 |
| rs2014343-rs4979032 | Intergenic-GNG10 | Guatemala | 0.04 |
| rs357654-rs2236407-rs2297088 | PTCH-PTCH-PTCH | Guatemala | 0.03 |
| rs357654-rs2236407-rs2297088-rs10512248 | PTCH-PTCH-PTCH-PTCH | Guatemala | 0.02 |
| rs2300934-rs306796 | C5-STOM | Guatemala | 0.03 |
| rs306796-rs10739600 | STOM-TTLL11 (∼500kb of STOM) | Guatemala | 0.03 |
| rs306796-rs10739600-rs7853089-rs1928623 | STOM-TTLL11 (∼500kb of STOM)-C9orf18 (∼800kb of STOM) | Guatemala | 0.02 |
| rs2300934-rs306796 | C5-STOM | China | 0.04 |
| rs306796-rs10739600-rs7853089-rs1928623 | STOM-TTLL11 (∼500kb of STOM)-C9orf18 (∼800kb of STOM) | China | 0.04 |
Comprises analyses of US, Spain, and Turkey families pooled together.
Transmission distortion significant when p<0.05.
Table VI.
Results of haplotype transmission analysis according to cleft subgroup by population.
| Marker Haplotype | Genes in Haplotype | Cleft Group | Population | HBAT P-value2 |
|---|---|---|---|---|
| rs357654-rs2236407 | PTCH-PTCH | CLO + CLCLP1 | Guatemala | 0.01 |
| rs10512248-rs894673 | PTCH-FOXE1 | CLO + CLCLP | Guatemala | 0.01 |
| rs2297088-rs10512248-rs894673 | PTCH-PTCH-FOXE1 | CLO + CLCLP | Guatemala | 0.01 |
| rs2236407-rs2297088-rs10512248-rs894673 | PTCH-PTCH-PTCH-FOXE1 | CLO + CLCLP | Guatemala | 0.01 |
| rs10512248-rs894673 | PTCH-FOXE1 | CLO + CLCLP | China | 0.03 |
| rs10512248-rs894673-rs3758249 | PTCH-FOXE1-FOXE1 | CLO + CLCLP | China | 0.03 |
| rs10512248-rs894673-rs3758249-rs10984103 | PTCH-FOXE1-FOXE1-C9orf156 | CLO + CLCLP | China | 0.03 |
CLO + CLCLP, families where all affecteds have cleft lip only plus families where at least one affected has cleft lip only and one affected has cleft lip and palate. Excludes any family where an affected has cleft palate only.
Transmission distortion significant when p<0.05.
Gene Prioritization
We found 339 genes within the 9q22.3-34.1 region with Endeavour (Supplementary Table I). The algorithm prioritized all genes within the 9q22.3-34.1 region in relation to cleft lip/palate. The highest priority gene according to Endeavour was TGFBR1. The associated genes, PTCH and STOM, ranked in tenth and fourteenth places in the priority output, respectively. FOXE1 ranked in 39th place. Detailed results are available as Supplemental Material.
Discussion
We investigated a region on chromosome 9q identified in previous studies as to harbor cleft susceptibility loci. Among the genes present in the region are PTCH and FOXE1, in which missense mutations and polymorphic variants have been described as having a role in cleft lip/palate [Marazita et al., 2004; 2009; Mansilla et al., 2006; Vieira et al., 2005; Moreno et al., 2009].
We performed fine mapping association studies with fifty SNPs across a 32.65 Mb region and multiple populations. Although using 50 SNPs to investigate 339 genes in a region may not seem a dense enough strategy, we used information on linkage disequilibrium of the markers to avoid choosing redundant markers. We found association of a polymorphism in STOM (rs306796) in the Guatemalan families (P=0.004) and in all families pooled together (P=0.002). STOM encodes stomatin, a membrane protein that was first isolated from human red blood cells [Zhang et al., 1999]. In mouse, the STOM homolog presents a wide pattern of expression, with high levels of mRNA in heart, liver, skeletal muscle, and testis but low levels in lung, brain, and spleen [Gallagher et al., 1995b]. Despite being associated with a variety of diseases such as cancer, kidney failure and anemia, precise functions of this protein remain unclear. In humans, the absence of stomatin is associated with a form of hemolytic anemia known as hereditary stomatocytosis [Yokoyama et al., 2006]. Lower expression of stomatin results in an increase in the basal rate of glucose transport [Zhang et al., 2001]. Interestingly, diabetic pregnant women are at an increased risk for having offspring with neural tube defects and oral-facial clefts [Spilson et al., 2001] and excessive exposure to glucose has been postulated to play a role in the pathogenesis of nonsyndromic cleft lip/palate [Krapels et al., 2004].
Suggestive evidence of association was also observed for several other markers among the populations implicating that the chromosome 9q region might contain cleft susceptibility genes (P<0.05). When analyzing the dataset according to cleft subgroups, we observed borderline association of PTCH variants (rs2236407, P=0.004; rs2297088, P=0.01; and rs10512248, P=0.01) in Guatemalan families with CLO + CLCLP (families where all affecteds have cleft lip only plus families where at least one affected has cleft lip only and one affected has cleft lip and palate). PTCH was initially suggested as a candidate for human clefting because of its mouse homolog located in the candidate region clf2 [Juriloff et al., 2001]. Although no causal mutation in Ptch coding sequence was found in mice, alterations in the regulatory sequence are not ruled out. Given the “normal” phenotype of noncleft A/∼ strain mice, a mild regulatory defect seems likely if Ptch is clf2 [Juriloff et al., 2004]. In humans, mutations in PTCH cause Gorlin syndrome with diverse developmental anomalies, often including rib and craniofacial abnormalities (and cleft palate) and a mixture of tumor types [Cohen, 1999]. In their study with PTCH and isolated cleft lip/palate, Mansilla et al. sequenced all 23 exons of the gene and found seven new normal variants spread along the entire gene and three missense mutations in cases with cleft lip/palate, one of which was not found in 1,188 control samples [Mansilla et al., 2006]. Although the authors did not find statistically significant evidence of linkage (multipoint HLOD peak=2.36), they reported over-transmission of the PTCH rs2297088–rs2236407 haplotype with borderline statistical significance (P=0.08) in Filipino families with two or more affected members. They further concluded that missense mutations in PTCH may be rare causes of isolated cleft lip/palate and yet unidentified variants near PTCH may act as modifiers of the cleft phenotype. Corroborating with these findings, we observed significant evidence of transmission distortion for the PTCH rs357654-rs2236407-rs2297088 (P=0.03) and rs357654-rs2236407-rs2297088-rs10512248 (P=0.02) haplotypes in Guatemalan families.
It is unknown whether the associated PTCH polymorphisms (rs2236407 and rs2297088) located in introns and therefore not in coding regions or splice sites might cause a functional change in the final protein. Notwithstanding, intronic polymorphisms have been demonstrated in association with other complex diseases, including the association of IRF6 with cleft lip/palate [Zucchero et al., 2004; Ghassibe et al., 2005; Park et al., 2007; Vieira et al., 2007; Jia et al., 2009; Jugessur et al., 2009] and thus should not be disregarded as potentially damaging. Of note, in a recent association study with genes in chromosome 9q, PTCH SNP rs2297088 showed the strongest signal for Filipino families, based on the lowest p-value (P-value=6.49E-03), indicating that PTCH may have a possible role in the etiology of cleft lip with or without cleft palate in some populations.
A previous study from our group has shown that point mutations in FOXE1 may be rare causes for isolated cleft lip/palate [Vieira et al., 2005]. Following studies have shown positive association and linkage between FOXE1 and cleft lip/palate [Marazita et al., 2004; 2009], including a recent publication which identified FOXE1 in strong association with both isolated cleft lip with or without cleft palate and isolated cleft palate [Moreno et al., 2009]. We did not find significant association of FOXE1 with cleft phenotypes in the populations tested although suggestive association was observed for variants in/nearby FOXE1 in Chinese and Guatemalan families with CLO + CLCLP (P=-0.03 and P=0.04, respectively). FOXE1 belongs to a family of fork-head domain-genes involved in embryonic pattern formation, which have been identified as factors that bind to regulatory elements in mammalian genes expressed in terminally differentiated cells [Kaestner et al., 1993]. Foxe1 null mice exhibit cleft palate and either a sublingual or completely absent thyroid gland [De Felice et al., 1998]. In humans, mutations in FOXE1 result in the Bamforth syndrome, characterized by thyroid agenesis, cleft palate, spiky hair, and choanal atresia [Clifton-Bligh et al., 1998]. It is possible that variants in this gene may be in linkage disequilibrium with variants in other genes that jointly increase the susceptibility to cleft lip/palate. For example, we found overtransmission of PTCH-FOXE1 haplotypes in families Guatemalan and Chinese families presenting CLO + CLCLP (P=0.01 and P=0.03, respectively).
Using gene prioritization software, we identified 339 genes in the 9q22.3-34.11 candidate region, which were ranked based on similar roles or participation in similar biological processes with genes known to be associated with cleft lip/palate. Other investigators have used this gene prioritization approach as well, in studies of obesity and type II diabetes genes [Elbers et al., 2007; Sookoian et al., 2009], idiopathic pulmonary fibrosis [Tzoulevekis et al., 2007] and even cleft lip/palate [Osoegawa et al., 2008]. This approach however has the limitation of not allowing any control on the quality of gene annotation and quantity and quality of information across genes in different databases. It is difficult to predict how much these aspects influenced our results and the results of others but at minimal, this information adds to the bulk of the results and help interpreting the association data. In comparison with a reference set of 11 established candidate genes for human clefting, PTCH and STOM ranked in tenth and fourteenth places, respectively, among the 339 genes present in the investigated region, thus at the top five percent ranking as candidates for cleft lip/palate. These observations taken together with the association findings, suggest that those genes may be considered plausible candidates for cleft lip/palate. Meanwhile, we checked for conservation of the associated SNPs, and found that the wild-type nucleotide is conserved in the following species: rhesus monkey, dog and horse for rs2297088 in PTCH, and dog and horse for rs306796 in STOM.
In summary, we performed fine mapping analysis of chromosome 9q22.32-34.1 region previously suggested to harbor cleft susceptibility genes. Our association results support a role for PTCH as contributor for cleft lip/palate and suggest STOM as a possible new candidate gene. Haplotype and gene prioritization analyses confirmed the individual association findings with PTCH and STOM, ranking in the top five percent of the highest priority candidate genes for cleft lip/palate. We failed to replicate previous findings suggesting FOXE1 contributes to nonsyndromic cleft lip and palate. Additional studies with other populations should focus on these loci to further investigate the participation of these genes in human clefting.
Supplementary Material
Acknowledgments
We gratefully acknowledge individuals and families for their valuable collaboration. Thanks to research coordinators and staff at each collection site. Wendy Carricato helped with sample organization. Rebecca DeSensi and Kathleen Deeley provided technical assistance. Maria Adela Mansilla and Lina Maria Moreno at the University of Iowa helped with SNP marker selection. This work was supported by the National Institutes of Health grants: K99-DE018954 (to AL); K99-DE018413 (to RM); K99-DE018085 (to MG); R01-DE016148 and P50-DE016215 (to MLM); R21-DE16718 (to ARV); FAPERJ grant E-26/152.831/2006, CNPq grants 308885/2006-0 and 401467/2004-0 (to IMO); and CAPES, Brazil (to RFF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health. This paper is partly based on a thesis submitted to the graduate faculty, Federal University of Rio de Janeiro, in partial fulfillment of the requirements for the PhD degree (for RFF).
Footnotes
Web Resources: Accession numbers and URLs for data presented herein are as follows:
Applied Biosystems, http://www.appliedbiosystems.com/index.cfm
Family Based Association Test, http://www.biostat.harvard.edu/∼fbat
International HapMap Project, http://hapmap.org
Endeavour, http://www.esat.kuleuven.be/endeavour
Supplementary information is available at the American Journal of Medical Genetics website.
References
- Aerts S, Lambrechts D, Maity S, Van Loo P, Coessens B, De Smet F, Tranchevent LC, De Moor B, Marynen P, Hassan B, Carmeliet P, Moreau Y. Gene prioritization through genomic data fusion. Nature Biotechnology. 2006;24:537–544. doi: 10.1038/nbt1203. [DOI] [PubMed] [Google Scholar]
- Avila JR, Jezewski PA, Vieira AR, Orioli IM, Castilla EE, Christensen K, Daack-Hirsch S, Romitti PA, Murray JC. PVRL1 variants contribute to non-syndromic cleft lip and palate in multiple populations. Am J Med Genet. 2006;140A:2562–2570. doi: 10.1002/ajmg.a.31367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Wang H, Hetmanski JB, Fan YT, Zeiger JS, Liang KY, Chiu YF, Vanderkolk CA, Seifert KC, Wulfsberg EA, Raymond G, Panny SR, McIntosh I. A case-control study of nonsyndromic oral clefts in Maryland. Ann Epidemiol. 2001;11:434–442. doi: 10.1016/s1047-2797(01)00222-8. [DOI] [PubMed] [Google Scholar]
- Birnbaum S, Ludwig KU, Reutter H, Herms S, Steffens M, Rubini M, Baluardo C, Ferrian M, Almeida de Assis N, Alblas MA, Barth S, Freudenberg J, Lauster C, Schmidt G, Scheer M, Braumann B, Bergé SJ, Reich RH, Schiefke F, Hemprich A, Pötzsch S, Steegers-Theunissen RP, Pötzsch B, Moebus S, Horsthemke B, Kramer FJ, Wienker TF, Mossey PA, Propping P, Cichon S, Hoffmann P, Knapp M, Nöthen MM, Mangold E. Key susceptibility locus for nonsyndromic cleft lip with or without cleft palate on chromosome 8q24. Nat Genet. 2009;41:473–477. doi: 10.1038/ng.333. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton-Bligh RJ, Wentworth JM, Heinz P. Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet. 1998;19:399–401. doi: 10.1038/1294. [DOI] [PubMed] [Google Scholar]
- Cohen MM., Jr Nevoid basal cell carcinoma syndrome: molecular biology and new hypotheses. Int J Oral Maxillofac Surg. 1999;28:216–223. doi: 10.1034/j.1399-0020.1999.283280314.x. [DOI] [PubMed] [Google Scholar]
- DeFelice M, Ovitt C, Biffali E. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–398. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- Elbers CC, Onland-Moret NC, Franke L, Niehoff AG, van der Schouw YT, Wijmenga C. A strategy to search for common obesity and type II diabetes. Trends Endocrinol Metab. 2007;18:19–26. doi: 10.1016/j.tem.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fogh-Andersen P. Inheritance of harelip and cleft palate. Copenhagen: Munksgaard; 1942. [Google Scholar]
- Gallagher PG, Romana M, Lieman JH, Ward DC. cDNA structure, tissue-specific expression, and chromosomal localization of the murine band 7.2b gene. Blood. 1995;86:359–365. [PubMed] [Google Scholar]
- Ghassibé M, Bayet B, Revencu N, Verellen-Dumoulin C, Gillerot Y, Vanwijck R, Vikkula M. Interferon regulatory factor-6: a gene predisposing to isolated cleft lip with or without cleft palate in the Belgian population. Eur J Hum Genet. 2005;13:1239–1242. doi: 10.1038/sj.ejhg.5201486. [DOI] [PubMed] [Google Scholar]
- Grant SF, Wang K, Zhang H, Glaberson W, Annaiah K, Kim CE, Bradfield JP, Glessner JT, Thomas KA, Garris M, Frackelton EC, Otieno FG, Chiavacci RM, Nah HD, Kirschner RE, Hakonarson H. A genome-wide association study identifies a locus for nonsyndromic cleft lip with or without cleft palate on 8q24. J Pediatr. 2009;155:909–913. doi: 10.1016/j.jpeds.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype-phenotype associations. Eur J Hum Genet. 2001;9:301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Jezewski PA, Vieira AR, Nishimura C, Ludwig B, Johnson M, O'Brien SE, Daack-Hirsch S, Schultz RE, Weber A, Nepomucena B, Romitti PA, Christensen K, Orioli IM, Castilla EE, Machida J, Natsume N, Murray JC. Complete sequencing shows a role for MSX1 in non-syndromic cleft lip and palate. J Med Genet. 2003;40:399–407. doi: 10.1136/jmg.40.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia ZL, Li Y, Li L, Wu J, Zhu LY, Yang C, Chen CH, Shi B. Association among IRF6 polymorphism, environmental factors, and nonsyndromic orofacial clefts in western china. DNA Cell Biol. 2009;28:249–257. doi: 10.1089/dna.2008.0837. [DOI] [PubMed] [Google Scholar]
- Jugessur A, Shi M, Gjessing HK, Lie RT, Wilcox AJ, Weinberg CR, Christensen K, Boyles AL, Daack-Hirsch S, Trung TN, Bille C, Lidral AC, Murray JC. Genetic determinants of facial clefting: analysis of 357 candidate genes using two national cleft studies from Scandinavia. PLoS One. 2009;4:e5385. doi: 10.1371/journal.pone.0005385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Brown CJ. Unravelling the complex genetics of cleft lip in the mouse model. Mamm Genome. 2001;12:426–435. doi: 10.1007/s003350010284. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Dewell SL. A digenic cause of cleft lip in Astrain mice and definition of candidate genes for the two loci. Birth Defects Res A Clin Mol Teratol. 2004;70:509–518. doi: 10.1002/bdra.20041. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Lee KH, Schlöndorff J, Hiemisch H, Monaghan AP, Schütz G. Six members of the mouse forkhead gene family are developmentally regulated. Proc Natl Acad Sci USA. 1993;90:7628–7631. doi: 10.1073/pnas.90.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapels IP, van Rooij IA, Ocké MC, van Cleef BA, Kuijpers-Jagtman AM, Steegers-Theunissen RP. Maternal dietary B vitamin intake, other than folate, and the association with orofacial cleft in the offspring. Eur J Nutr. 2004;43:7–14. doi: 10.1007/s00394-004-0433-y. [DOI] [PubMed] [Google Scholar]
- Lidral C, Romitti PA, Basart AM, Doetschman T, Leysens NJ, Daack-Hirsch S, Semina EV, Johnson LR, Machida J, Birds A, Parnell TJ, Rubenstein JL, Murray JC. Association of MSX1 and TGFB3 with Nonsyndromic Clefting in Humans Am J Hum Genet. 1998;63:557–568. doi: 10.1086/301956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MA, Cooper ME, Goldstein T, Castilla EE, Lopez Camelo JS, Marazita ML, Murray JC. Contributions of PTCH Gene Variants to Isolated Cleft Lip and Palate. Cleft Palate Craniof J. 2006;43:21–29. doi: 10.1597/04-169R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Field LL, Cooper ME, Tobias R, Maher BS, Peanchitlertkajorn S, Liu YE. Genome scan for loci involved in cleft lip with or without cleft palate in Chinese multiplex families. Am J Hum Genet. 2002;71:349–364. doi: 10.1086/341944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Lidral AC, Murray JC, Field LL, Maher BS, Goldstein McHenry T, Cooper ME, Govil M, Daack-Hirsch S, Riley B, Jugessur A, Felix T, Moreno L, Mansilla MA, Vieira AR, Doheny K, Pugh E, Valencia-Ramirez C, Arcos-Burgos M. Genome scan, fine-mapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype specific differences in linkage and association results. Hum Hered. 2009;76:404–416. doi: 10.1159/000224636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Murray JC, Lidral AC, Arcos-Burgos M, Cooper ME, Goldstein T, Maher BS, Daack-Hirsch S, Schultz R, Mansilla MA, Field LL, Liu YE, Prescott N, Malcolm S, Winter R, Ray A, Moreno L, Valencia C, Neiswanger K, Wyszynski DF, Bailey-Wilson JE, Albacha-Hejazi H, Beaty TH, McIntosh I, Hetmanski JB, Tunçbilek G, Edwards M, Harkin L, Scott R, Roddick LG. Meta-Analysis of 13 genome scans reveals multiple cleft lip/palate genes with novel loci on 9q21 and 2q32-35. Am J Hum Genet. 2004;75:161–173. doi: 10.1086/422475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes R, Letra A, Ruff J, Granjeiro JM, Vieira AR. Studies of Genes in the FGF Signaling Pathway and Oral Clefts With or Without Dental Anomalies. Am J Med Genet. 2008;146A:1614–1617. doi: 10.1002/ajmg.a.32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno LM, Mansilla MA, Bullard SA, Cooper ME, Busch TD, Machida J, Johnson MK, Brauer D, Krahn K, Daack-Hirsch S, L'heureux J, Valencia-Ramirez C, Rivera D, López AM, Moreno MA, Hing A, Lammer EJ, Jones M, Christensen K, Lie RT, Jugessur A, Wilcox AJ, Chines P, Pugh E, Doheny K, Arcos-Burgos M, Marazita ML, Murray JC, Lidral AC. FOXE1 Association with both Isolated Cleft Lip with or without Cleft Palate; and Isolated Cleft Palate. Hum Mol Genet. 2009;18:4879–4896. doi: 10.1093/hmg/ddp444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. In: Wyszynski DF, editor. Cleft lip and Palate From origin to treatment. New York: Oxford; 2002. pp. 127–158. [Google Scholar]
- Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet. 2002;61:248–256. doi: 10.1034/j.1399-0004.2002.610402.x. [DOI] [PubMed] [Google Scholar]
- Osoegawa K, Vessere GM, Utami KH, Mansilla MA, Johnson MK, Riley BM, L'Heureux J, Pfundt R, Staaf J, van der Vliet WA, Lidral AC, Schoenmakers EF, Borg A, Schutte BC, Lammer EJ, Murray JC, de Jong PJ. Identification of novel candidate genes associated with cleft lip and palate using array comparative genomic hybridization. J Med Genet. 2008;45:81–86. doi: 10.1136/jmg.2007.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopensius T, Ambrozaityte L, Ludwig KU, Birnbaum S, Jagomägi T, Saag M, Matuleviciene A, Linkeviciene L, Herms S, Knapp M, Hoffmann P, Nöthen MM, Kucinskas V, Metspalu A, Mangold E. Replication of novel susceptibility locus for nonsyndromic cleft lip with or without cleft palate on chromosome 8q24 in Estonian and Lithuanian patients. Am J Med Genet. 2009;149A:2551–2553. doi: 10.1002/ajmg.a.33024. [DOI] [PubMed] [Google Scholar]
- Park JW, McIntosh I, Hetmanski JB, Jabs EW, Vander Kolk CA, Wu-Chou YH, Chen PK, Chong SS, Yeow V, Jee SH, Park BY, Fallin MD, Ingersoll R, Scott AF, Beaty TH. Association between IRF6 and nonsyndromic cleft lip with or without cleft palate in four populations. Genet Med. 2007;9:219–227. doi: 10.1097/GIM.0b013e3180423cca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, Pesich R, Hebert J, Chen YD, Dzau VJ, Curb D, Olshen R, Risch N, Cox DR, Botstein D. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. 2001;11:1262–1268. doi: 10.1101/gr.157801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BM, Mansilla MA, Ma J, Daack-Hirsch S, Maher BS, Raffensperger LM, Russo ET, Vieira AR, Dodé C, Mohammadi M, Marazita ML, Murray JC. Impaired FGF signaling contributes to cleft lip and palate. Proc Nat Acad Sci. 2007;104:4512–4517. doi: 10.1073/pnas.0607956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gianotti TF, Schuman M, Pirola CJ. Gene prioritization based on biological plausibility over genome wide association studies renders new loci associated with type 2 diabetes. Genet Med. 2009;11:338–343. doi: 10.1097/GIM.0b013e31819995ca. [DOI] [PubMed] [Google Scholar]
- Sözen MA, Suzuki K, Tolarova MM, Bustos T, Fernández Iglesias JE, Spritz RA. Mutation of PVRL1 is associated with sporadic, nonsyndromic cleft lip/palate in northern Venezuela. Nat Genet. 2001;29:141–142. doi: 10.1038/ng740. [DOI] [PubMed] [Google Scholar]
- Spilson SV, Kim HJ, Chung KC. Association between maternal diabetes mellitus and newborn oral cleft. Ann Plast Surg. 2001;47:477–481. doi: 10.1097/00000637-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Tranchevent LC, Barriot R, Yu S, Van Vooren S, Van Loo P, Coessens B, De Moor B, Aerts S, Moreau Y. ENDEAVOUR update: a web resource for gene prioritization in multiple species. Nucleic Acids Res. 2008;36(Web Server issue):W377–84. doi: 10.1093/nar/gkn325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1 alpha in disease pathogenesis. Am J Respir Crit Care Med. 2007;176:1108–1119. doi: 10.1164/rccm.200705-683OC. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Avila JR, Daack-Hirsch S, Dragan E, Félix TM, Rahimov F, Harrington J, Schultz RR, Watanabe Y, Johnson M, Fang J, O'Brien SE, Orioli IM, Castilla EE, Fitzpatrick DR, Jiang R, Marazita ML, Murray JC. Medical sequencing of candidate genes for nonsyndromic cleft lip and palate. PloS Genet. 2005;1:e64. doi: 10.1371/journal.pgen.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AR. Unraveling Human Cleft Lip and Palate Research. J Dent Res. 2008;87:119–125. doi: 10.1177/154405910808700202. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Modesto A, Meira R, Barbosa AR, Lidral AC, Murray JC. Interferon Regulatory Factor 6 (IRF6) and Fibroblast Growth Factor Receptor 1 (FGFR1) Contribute to Human Tooth Agenesis. Am J Med Genet. 2007;143A:538–545. doi: 10.1002/ajmg.a.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AR, Orioli IM, Castilla EE, Cooper ME, Marazita ML, Murray JC. MSX1 and TGFB3 Contribute to Clefting in South America. J Dent Res. 2003;82:289–292. doi: 10.1177/154405910308200409. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Matsui E, Akiba T, Harata K, Matsui I. Molecular structure of a novel membrane protease specific for a stomatin homolog from the hyperthermophilic archaeon Pyrococcus horikoshii. J Mol Biol. 2006;358:1152–1164. doi: 10.1016/j.jmb.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Abbud W, Prohaska R, Ismail-Beigi F. Overexpression of stomatin depresses GLUT-1 glucose transporter activity. Am J Physiol Cell Physiol. 2001;280:C1277–1283. doi: 10.1152/ajpcell.2001.280.5.C1277. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Hayashi H, Ebina Y, Prohaska R, Ismail-Beigi F. Association of stomatin (band 7.2b) with Glut1 glucose transporter. Arch Biochem Biophys. 1999;372:173–178. doi: 10.1006/abbi.1999.1489. [DOI] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, Natsume N, Yoshiura K, Vieira AR, Orioli IM, Castilla EE, Moreno L, Arcos-Burgos M, Lidral AC, Field LL, Liu YE, Ray A, Goldstein TH, Schultz RE, Shi M, Johnson MK, Kondo S, Schutte BC, Marazita ML, Murray JC. Interferon Regulatory Factor 6 (IRF6) Gene Variants and the Risk of Isolated Cleft Lip or Palate. N Engl J Med. 2004;351:769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.