Abstract
Object
This study was designed to investigate delayed erythropoietin (EPO) treatment for traumatic brain injury (TBI) in rats comparing efficacy of a single dose with triple doses.
Methods
Young adult male Wistar rats were randomly divided into the following groups: 1) Sham group (n = 6); 2) TBI + Saline group (n = 6); 3) TBI + EPOx1 group (n = 6); and 4) TBI + EPOx3 group (n = 7). TBI was induced by controlled cortical impact over the left parietal cortex. EPO (5,000 U/kg) or saline was administered intraperitoneally at 1 day (EPOx1 group) or at days 1, 2, and 3 (EPOx3 group) post injury. Neurological function was assessed using a modified neurological severity score (mNSS), footfault and Morris water maze tests. Animals were sacrificed 35 days after injury and brain sections stained for immunohistochemistry.
Results
Compared to the saline treatment, EPO treatment in both the EPOx1 and EPOx3 groups significantly reduced hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, and significantly improved neurological functional outcome. The EPOx3 group exhibited significantly improved functional and histological outcomes compared with the EPOx1 group.
Conclusions
These data demonstrate that delayed posttraumatic administration of EPO significantly improves histological and long-term functional outcomes in rats after TBI. The triple doses of delayed EPO treatment exhibit better histological and functional outcomes in rats although a single dose of EPO provides substantial benefits compared to saline treatment.
Keywords: angiogenesis, cell proliferation, erythropoietin, neurogenesis, rat, sensorimotor, spatial learning, traumatic brain injury
Traumatic brain injury (TBI) is a common cause of mortality and morbidity in the United States, particularly among the young.39 The most prevalent and debilitating features in survivors of TBI are cognitive deficits and motor dysfunctions.10 To date, there is no effective treatment identified to promote functional recovery after TBI except for routine medical intervention and care.29,31
Erythropoietin (EPO) and the EPO receptor (EPOR), essential for erythropoiesis, are also expressed in the neurons, astrocytes, and cerebral endothelial cells. EPO shows neuroprotection in animal models of stroke,14,40 spinal cord injury,4,15,17 concussive brain injury,3 kainate-induced seizure activity,3 and autoimmune encephalomyelitis.5,32 Most studies for TBI investigated either a single dose or multiple doses of EPO pretreatment (prior to injury) or treatment within 6 hours post injury.3,8,18,30 Several lines of evidence demonstrate that a single dose of EPO administered within 6 hours post TBI reduces lesion volume.8,16,18 Our recent work further demonstrates that delayed treatment (24 hours post injury) with EPO provides long-term benefits in rats after TBI 21,26 and after stroke.40
Although delayed EPO treatment enhances angiogenesis in rats after stroke 40, it is unclear whether delayed therapy induces angiogenesis after TBI. Delayed EPO therapy improves long-term sensorimotor functional recovery after stroke 40 and spatial learning performance after TBI.26 However, it is not known whether delayed EPO administration improves long-term sensorimotor functional recovery after TBI. In addition, there is no study comparing the efficacy of multiple doses of EPO with one single dose for treatment of TBI. Accordingly, using a controlled cortical impact (CCI) TBI rat model, we tested the hypotheses and demonstrated that: 1) delayed administration of EPO reduces brain injury, increases cell proliferation, neurogenesis and angiogenesis, and improves long-term sensorimotor function and spatial learning recovery; and 2) the efficacy of three doses of EPO is better than that of a single dose for treatment of TBI in rats.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System.
TBI Model
A CCI model of TBI in the rat was utilized for the present study.12,25 Young adult male Wistar rats (300–400 g) were anesthetized intraperitoneally (ip) with chloral hydrate (350 mg/kg body weight). Rectal temperature was maintained at 37°C using a feedback-regulated water-heating pad. A CCI device was used to induce injury. Rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy allowed for lateral movement of cortical tissue. The dura mater was kept intact over the cortex. Injury was delivered by impacting the left cortex (ipsilateral cortex) with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/second and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Experimental Groups and Treatment
Young adult male Wistar rats were randomly divided into 4 groups: 1) Sham group (n = 6); 2) TBI + Saline group (n = 6); 3) TBI + EPOx1 group (n = 6); and 4) TBI + EPOx3 group (n = 7). TBI was induced by CCI over the left parietal cortex. Sham rats underwent surgery without injury. EPO at a dose of 5000 U/kg body weight (Epoetin alpha, AMGEN, Thousand Oaks, CA) was administered intraperitoneally at 1 day (for the EPOx1 group) or at Days 1, 2 and 3 (for the EPOx3 group) after TBI. The doseof EPO was selected based on previous studies.21,46 Animals in the saline-treated group received an equal volume of saline at Days 1, 2 and 3 after surgery. For labeling proliferating cells, 5-bromo-2′-deoxyuridine(BrdU, 100 mg/kg; Sigma, St. Louis, MO) was injected intraperitoneally into rats daily for 10 days, starting 1 day after TBI. Allrats were sacrificed at 35 days after TBI or surgery.
Hematocrit
To determine the effects of EPO on hematocrit (HCT), a blood sample (50 μl) was collected via tail vein before injury, at Day 4 and weekly after TBI or sham up to 5 weeks. HCT was measured in micro-HCT capillary tubes (Fisher Scientific, Pittsburgh, PA) using standard procedures (Readacrit Centrifuge, Clay Adams, Parsippany, NJ).42
Evaluation of neurological outcome
All functional tests were performed by investigators blinded to the treatment status.
Morris Water Maze Test
To detect spatial learning impairments, a recent version of the Morris water maze (MWM) test was used.9 The procedure was modified from previous versions11,27,28,37 and has been found to be useful for chronic spatial memory assessment in rats and mice with brain injury.9,21 All animals were tested during the last five days (i.e., from 31–35 days after TBI or surgery) before sacrifice. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA.). For data collection, a blue pool (1.8 m in diameter) was subdivided into four equal quadrants formed by imaging lines. At the start of a trial, the rat was placed at one of four fixed starting points, randomly facing toward a wall (designated North, South, East and West) and allowed to swim for 90 seconds or until it found the platform. If the animal found the platform, it was allowed to remain on it for 10 seconds. If the animal failed to find the platform within 90 seconds, it was placed on the platform for 10 seconds. Throughout the test period the platform was located in the NE quadrant 2 cm below water in a randomly changing position, including locations against the wall, toward the middle of the pool or off-center but always within the target quadrant. If the animal was unable to find the platform within 90 seconds, the trial was terminated and a maximum score of 90 seconds was assigned. If the animal reached the platform within 90 seconds, the percentage of time traveled within the NE (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform and employed for statistical analysis. The advantage of this version of the water maze is that each trial takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant.33
Footfault Test
To evaluate sensorimotor function, the footfault test was carried out before TBI and at 1, 4, 7, 14, 21, 28 and 35 days after TBI or surgery. The rats were allowed to walk on a grid.43 With each weight-bearing step, a paw might fall or slip between the wires and, if this occurred, it was recorded as a footfault.1,2 A total of 50 steps were recorded for each right forelimb and hindlimb.
Modified neurological severity score (mNSS) test
Neurological functional measurement was performed using the mNSS score test.6 The test was carried out on all rats preinjury and on days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of the motor (muscle status, abnormal movement), sensory (visual, tactile and proprioceptive) and reflex tests and has been employed in previous studies.22 In this TBI model, injury in the left hemispheric cortex of rats causes sensory and motor functional deficiency with elevated scores on motor, sensory, and Beam Balance Tests in the early phase after injury (Day 1 after injury). Absent reflexes and abnormal movements can be measured on rats with severe injury. Slow recovery in asymmetry deficiency as reflected by Beam Balance Test results has been reported in unilateral brain injuries including TBI22 and ischemia.6 This test is suitable for evaluating long-term neurological function after unilateral brain injury.
Tissue Preparation and Measurement of Lesion Volume
At Day 35 after TBI, rats were anesthetized intraperitoneally with chloral hydrate and were perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Their brains were removed and post-fixed in 4% paraformaldehyde for 2 days at room temperature. The braintissue was cut into 7 equally spaced 1-mm coronal blocks and processed for paraffin sectioning. A series of adjacent 6-μm-thick sections were cut fromeach block in the coronal plane and stained with H & E. For lesion volume measurement, the 7 brain sections were traced by a microcomputerimaging device (MCID) (ImagingResearch, St. Catharine’s, Ontario, Canada), as previously described.7 The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere is subtractedfrom the area of the contralateral hemisphere),38 and the lesion volume presented as a volume percentage of thelesion compared with the contralateral hemisphere. H & E sections from Blocks E and F containing hippocampus were used to acquire images of the dentate gyrus (DG) and CA3 regions at 20 × magnification. To evaluate the cell loss after TBI, we counted the cell number per millimeter in the DG and CA3 regions.
Immunohistochemistry
To examine the effect of EPO on cell proliferation and angiogenesis, coronal sections were histochemically stained with mouse anti-BrdU21 and rabbit anti-human von Willebrandfactor (vWF),21 respectively. For BrdU detection, paraffin-embedded coronal sections (6 μm) were deparaffinized and rehydrated. Antigen retrieval was performed by boiling sections in 10-mM citrate buffer (pH 6.0) for 10 minutes (Chen et al., 2001). After washing with PBS, sections were incubated with 0.3 % H2O2 in PBS for 10 minutes, blocked with 1% BSA containing 0.3 % Triton-X 100 for 1 hour at room temperature, and incubated with mouse anti-BrdU (1:200; Dako, Carpinteria, CA) at 4°C overnight. After washing, sections were incubated with biotinylated anti-mouse antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 minutes. After washing, sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc., Burlingame, CA). Diaminobenzidine (Sigma, St. Louis, MO) was then used as a sensitive chromogen for light microscopy. Sections were counterstained with hematoxylin.
To identify vascular structure, brain sections were deparaffinized and then incubated with 0.4% Pepsin solution at 37°C for 1 hour. After washing, the sections were blocked with 1% BSA at room temperature for 1 hour, and then incubated with rabbit anti-human vWF (1:200; DakoCytomation, Carpinteria, CA) at 4°C overnight. After washing, sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 minutes. The subsequent procedures were the same as for BrdU staining.
BrdU-positive cells and vWF-stained vascular structures in the DG, CA3, and the cortex of ipsilateral hemispheres were examined at a magnification of 20 and counted. The cells with BrdU (brown stained) that clearlylocalized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells.
Immunofluorescent Staining
Newly generated neurons were identified by double labeling for BrdU and NeuN. After dehydration, tissue sections were boiled in 10 mM citric acid buffer (pH 6) for 10 minutes. After washing with PBS, sections were incubated in 2.4 N HCl at 37°C for 20 minutes. Sections were incubated with 1% BSA containing 0.3% Triton-X-100 in PBS. Sections were then incubated with mouse anti-NeuN antibody (1:200; Chemicon, Temecula, CA) at 4°C overnight. FITC-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch, West Grove, PA) was added to sections at room temperature for 2 hours. Sections were then incubated with rat anti-BrdU antibody (1:200; Dako, Glostrup, Denmark) at 4°C overnight. Sections were then incubated with Cy3-conjugated anti-rat antibody (1:400; Jackson ImmunoResearch, West Grove, PA) at room temperature for 2 hours. Each of the steps was followed by three 5-minute rinses in PBS. Tissue sections were mounted with Vectashield mounting medium (Vector laboratories, Burlingame, CA). Images were collected with fluorescent microscopy. NeuN/BrdU-colabeled cells in the DG and the cortex were counted at a magnification of 40.
Cell Counting and Quantitation
Cell counts were performed by observers blinded to the individual treatment status of the animals. Five sections with 50-μm intervals from the dorsal DG were analyzed with a microscope (Nikon i80) at 400× magnification via the MCID system.21 All counting was performed on a computer monitor to improve visualization and in one focal plane to avoid oversampling.44 To evaluate whether intraperitoneally administered EPO reduces neuronal damage after TBI, the number of cells was counted in the DG using the MCID system. Although H & E staining is not neuron-specific, the morphological characteristics of neuronal cells in the DG and CA3 region aid in counting them. Counts were averaged and normalized by measuring the linear distance (in mm) of the DG and CA3 for each section. Although it is just an estimate of the cell number, this method permits a meaningful comparison of differences between groups. For cell proliferation, the total number of BrdU-positive cells was counted in the lesion boundary zone and the DG,, using the MCID system. The cells with BrdU (brown stained) that clearlylocalized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells. The number of BrdU-positive cells was expressed in cells/mm2 in the lesion boundary zone or in cells/mm in the hippocampus. For analysis of neurogenesis, additional sections used in the above studies were used to evaluate neurogenesis in the DG and the cortex by calculating the density of BrdU-labeled cells and BrdU/NeuN-colabeled cells.24 We mainly focused on the ipsilateral DG and its subregions, including the subgranular zone (SGZ), granular cell layer (GCL), and the molecular layer. The number of BrdU-positive cells (red stained) and NeuN/BrdU-colabeled cells (yellow after merge) were counted in the DG and the lesion boundary zone. The percentage of NeuN/BrdU-colabeled cells over the total number of BrdU-positive cells in the corresponding regions (DG or cortex) was estimated and used as a parameter to evaluate neurogenesis.42
Statistical Analyses
All data are presented as the means ± standard deviation. Data were analyzed by analysis of variance (ANOVA) for repeated measurements of hematocrit and functional tests (spatial performance and sensorimotor function). For lesion volume, cell counting, cell proliferation and vWF-stained vascular density, a one-way ANOVA followed by post hoc Student-Newman-Keuls tests were used to compare the difference between the EPO-treated, saline-treated and sham groups. Statistical significance was set at p < 0.05.
Results
Hematocrit
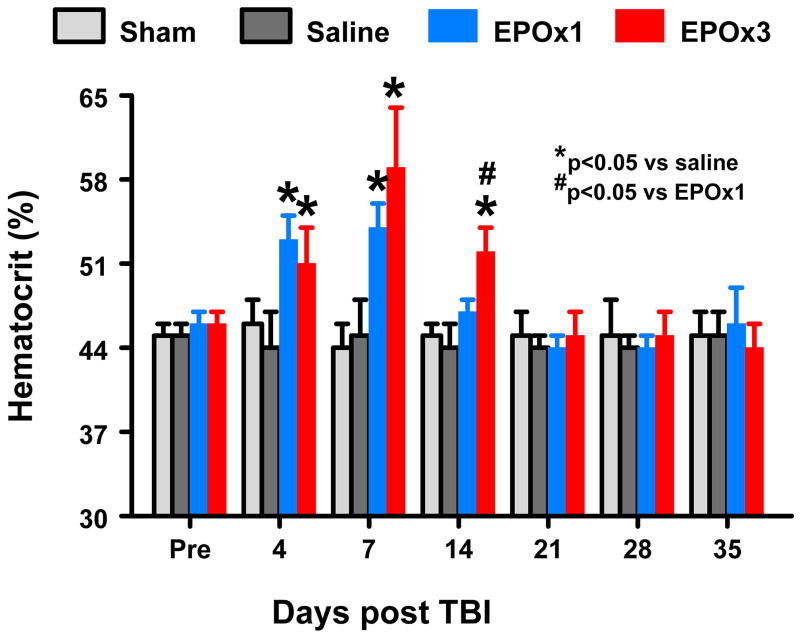
The baseline of hematocrit (HCT) was similar for all animals before injury or sham-surgery (Fig. 1). As compared to saline treatment, EPO treatment significantly increased HCT up to 1 week (EPOx1 group, p=0.003) and 2 weeks (EPOx3 group, p=0.026), which returned to normal thereafter. HCT was significantly higher at 2 weeks in the EPOx3 group than the EPOx1 group (p=0.031).
Fig. 1.
Changes in hematocrit before and after TBI. “Pre” represents preinjury level. *p < 0.05 vs. corresponding Pre. Data represent mean ± SD. #p < 0.05 vs. the EPOx1 group at Day 14 after injury. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
Lesion volume
Rats were sacrificed at 35 days post TBI for histological measurements. EPO treatment did not affect the lesion volume after TBI. The lesion volume was 16.9 ± 1.4%, 15.7 ± 0.5%, and 16.0 ± 1.5% for TBI rats treated with saline, EPOx1, and EPOx3, respectively.
Spatial Learning Test
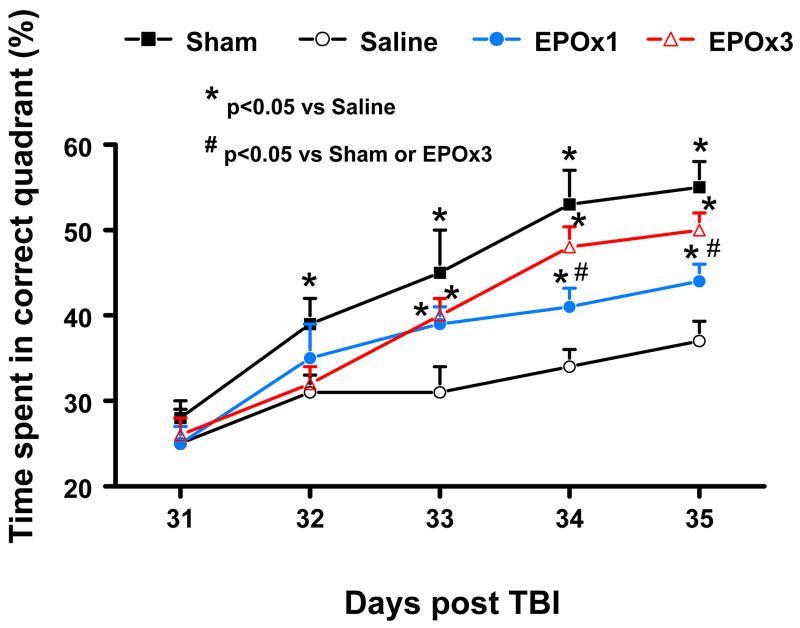
The water maze protocol in the present study was used to detect spatial learning deficits. To analyze day-by-day differences in the MWM, a repeated measures ANOVA was performed followed by Student-Newman-Keuls tests for multiple comparisons. As shown in Figure 2, the time spent in the correct quadrant (Northeast) by sham rats gradually increased from Days 31 to 35 after surgery. The saline-treated rats with TBI were significantly impaired compared to sham-operated rats at Days 32 (p = 0.033), 33 (p = 0.002), 34 (p = 0.001), and 35 (p = 0.000) after injury. As compared to saline treatment, EPO-treated rats with TBI showed significant improvement at both doses at Day 33 (p = 0.010 for EPOx1 and 0.013 for EPOx3), Day 34 (p = 0.033 for EPOx1 and 0.007 for EPOx3) and Day 35 (p = 0.039 for EPOx1 and 0.011 for EPOx3). However, as compared to the EPOx1 group, EPOx3 group showed a significant improvement in spatial learning (i.e., larger percentage of time spent in the correct quadrant) at Days 34 (p = 0.042) and 35 (p = 0.034).
Fig. 2.
Effect of EPO on spatial learning function 31–35 days after TBI. TBI significantly impaired spatial learning at Days 32–35 compared to sham controls (p < 0.05). Delayed treatment with EPO (EPOx1 and EPOx3) improves spatial learning performance measured by a recent version of the water maze test at Days 33–35 compared with the saline group (p < 0.05). However, the spatial learning performance at Days 34 and 35 in the EPOx3 group is better than that in the EPOx1 group (p < 0.05). Data represent mean ± SD. *p < 0.05 vs. Saline group. #p < 0.05 vs. sham controls or EPOx3 group. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
Foot fault Test
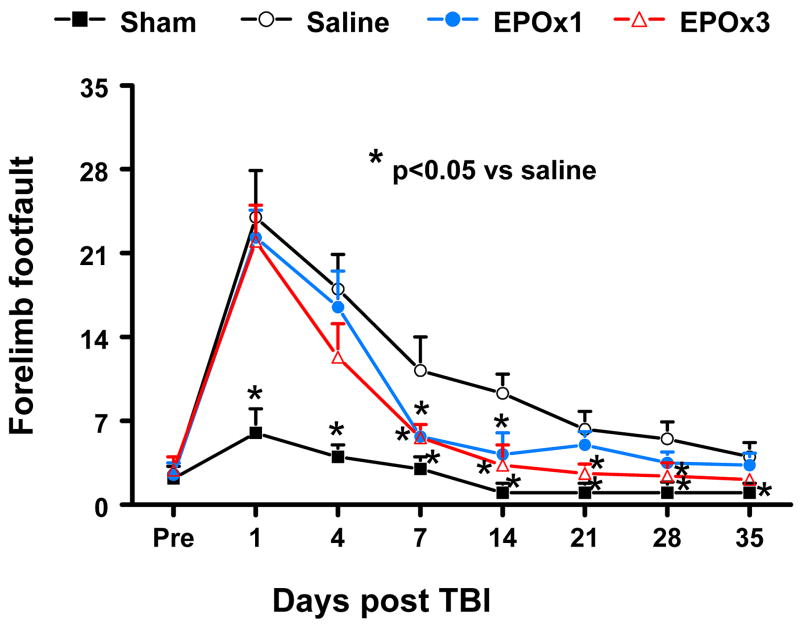
The incidence of forelimb footfaults during baseline (preoperatively) was about 4–5% (Fig. 3). Sham surgery alone mildly increased the frequency of footfaults at postoperative Days 1 and 4. TBI significantly increased the occurrence of right forelimb footfaults contralateral to the TBI at 1 to 35 days post injury as compared with the pre-injury baseline. Treatment with 3 doses of EPO (EPOx3 group) significantly reduced the number of contralateral forelimb footfaults at 7 to 28 days after TBI compared to treatment with saline (p = 0.015, 0.011, 0.024, and 0.035 for Days 7, 14, 21, and 28, respectively), while a single dose (EPOx1 group) showed benefits at Days 7 (p = 0.01) and 14 (p = 0.014).
Fig. 3.
Effect of EPO on sensorimotor function (forelimb footfault) before and after TBI. “Pre” represents pre-injury level. Delayed EPOx3 treatment significantly reduces forelimb foot faults at Days 7–28 while EPOx1 treatment significantly reduces them at Days 7 and 14 compared with the saline group (p < 0.05). Data represent mean ± SD. * p < 0.05 vs. EPOx1 or EPOx3. N (rats/group) = 6 (Saline); 6 (EPOx1); 7 (EPOx3).
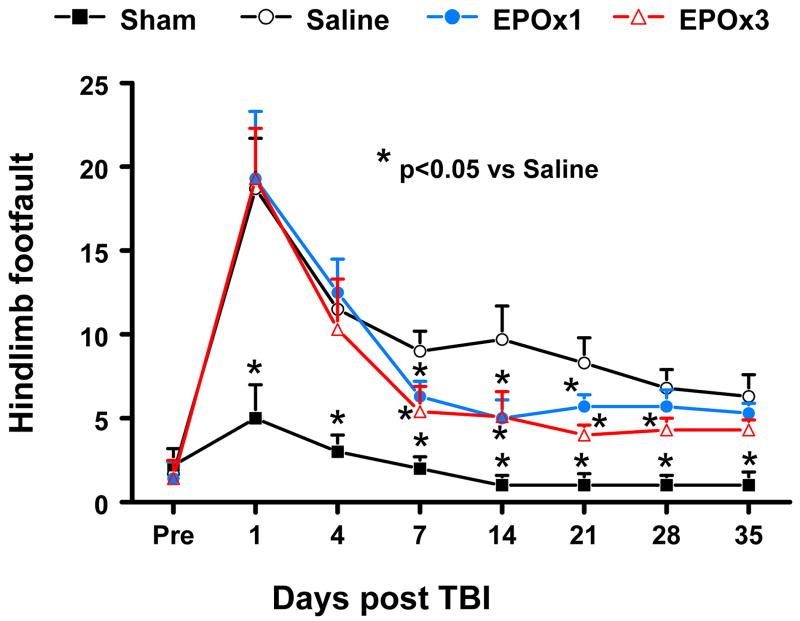
Similar results were found for the contralateral hindlimb (Fig. 4). As compared to preinjury baseline, TBI significantly increased the incidence of contralateral hindlimb footfaults at 1 to 35 days post-injury (p = 0.004, 0.013, 0.014, 0.008, 0.007, 0.001, and 0.016 for Days 1, 4, 7, 14, 21, 28, and 35, respectively). Treatment with 3 doses of EPO (EPOx3 group) significantly reduced the number of contralateral hindlimb footfaults at 7 to 28 days after TBI compared to treatment with saline (p = 0.031, 0.026, 0.020, and 0.042 for Days 7, 14, 21, and 28, respectively) while a single dose (EPOx1 group) showed benefits at Days 7–21 (p = 0.033, 0.028 and 0.044 for Days 7, 14, and 21, respectively).
Fig. 4.
Effect of EPO on sensorimotor function (hindlimb footfault) before and after TBI. “Pre” represents pre-injury level. Delayed EPOx3 treatment significantly reduces hindlimb foot faults at days 7–28 while EPOx1 treatment significantly reduces them at Days 7 and 21 compared with the saline group (p < 0.05). Data represent mean ± SD. * p < 0.05 vs. EPOx1 or EPOx3. N (rats/group) = 6 (Saline); 6 (EPOx1); 7 (EPOx3).
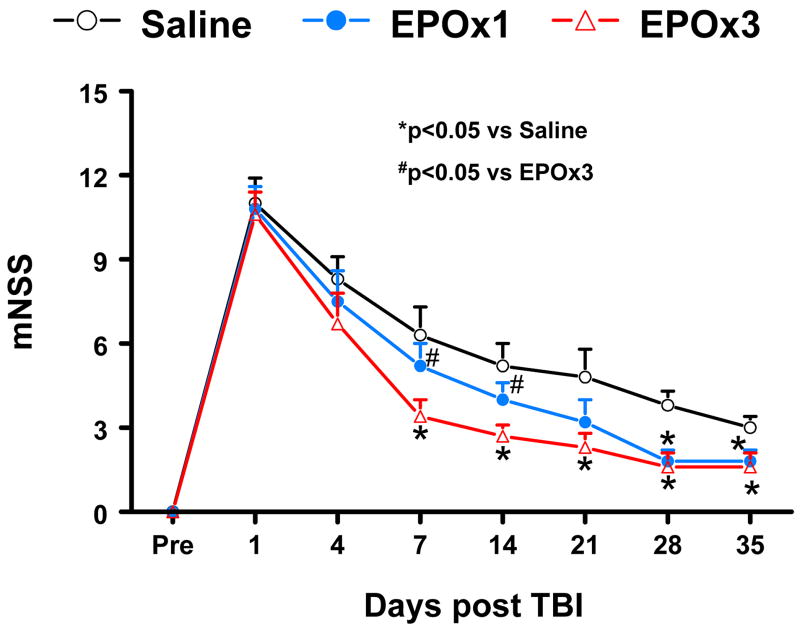
Neurological Severity Score
Figure 5 shows that there is no significant difference in the mNSS score among the saline, EPOx1 and EPOx3 groups at Day 4 post TBI. However, significantly improved scores were measured at Days 7–35 after TBI in the EPOx3 group compared saline treatment (p = 0.022, 0.030, 0.033, 0.037, and 0.039 for Days 7, 14, 21, 28 and 35, respectively). A single dose of EPO therapy had a tendency to reduce the mNSS score at Days 7–21 without reaching a significance, but it significantly reduced the mNSS score at Days 28 (p = 0.033) and 35 (p = 0.045) compared to saline therapy. Treatment with 3 doses showed significantly improved benefits at Days 7 (p = 0.014) and 14 (p = 0.044) compared to a single therapy.
Fig. 5.
The plot shows the functional improvement detected on the modified neurological severity scores (mNSS). EPOx3 treatment significantly lowers mNSS scores at Days 7–35 compared to saline group (*p < 0.05). However, the functional recovery (lowered mNSS score) at Days 7 and 14 in the EPOx3 group is better than that in the EPOx1 group (#p < 0.05). N (rats/group) = 6 (Saline); 6 (EPOx1); 7 (EPOx3).
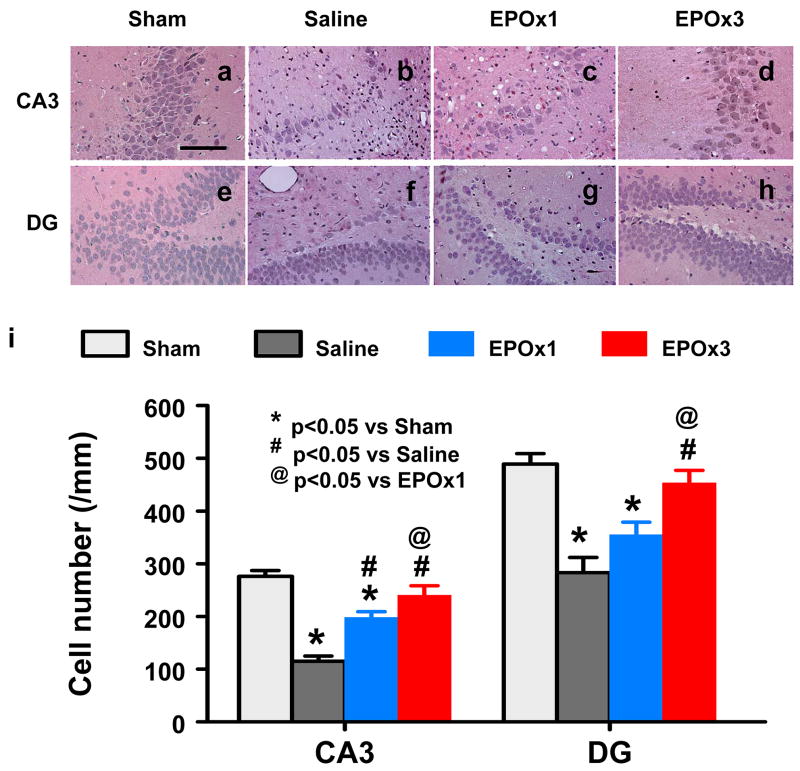
Cell Loss in the CA3 and DG
When examined at 35 days post TBI (Fig. 6), the neuron counts in the ipsilateral CA3 (p = 0.003) and DG (p = 0.010) had significantly decreased after TBI (Fig. 6b and 6f) compared to sham controls (Fig. 6a and 6e). As compared to saline controls, a single dose of EPO treatment significantly increased the neuron counts in the CA3 (p = 0.035) but not in the DG. Three doses of EPO treatment had significantly higher neuron counts as compared to a single dose group in the CA3 (Fig. 6d vs 6c; p = 0.044) and DG (Fig. 6h vs 6g, p = 0.035).
Fig. 6.
Effect of EPO on cell loss in the ipsilateral DG and CA3 region at 35 days after TBI. H&E staining: a–h. Delayed treatment with EPOx1 (c, g) and EPOx3 (d, h) significantly reduced cell loss as compared with the saline group (b, f) (p < 0.05). As compared to EPOx1 group, the cell number in the EPOx3 group was significantly higher (p < 0.05). The cell number in the DG and CA3 region is shown in (i). Data represent mean ± SD. Scale bar = 50μm (a–h). *p < 0.05 vs. corresponding sham. #p < 0.05 vs. the saline group. @ p < 0.05 vs EPOx1 group. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
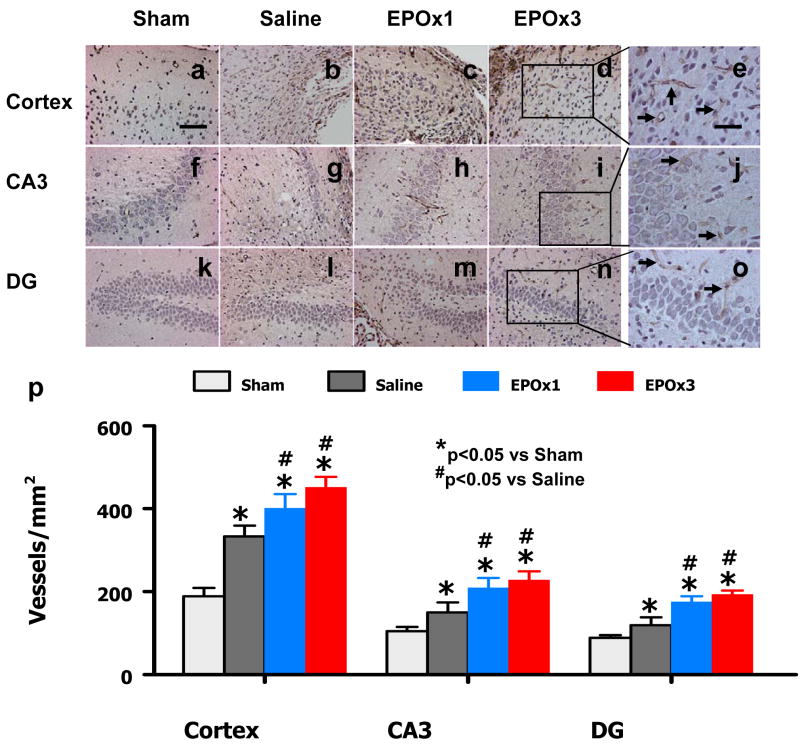
Angiogenesis
vWF-staining has been used to identify vascular structure in the brain after TBI.23 TBI alone significantly increased the density of vessels in the cortex (p = 0.018), CA3 (p = 0.035) and DG (p = 0.044) of the ipsilateral hemisphere compared to sham controls (Fig. 7). EPO treatment significantly increased the vascular density in the cortex (p = 0.047 for EPOx1 and 0.039 for EPOx3), CA3 (p = 0.038 for EPOx1 and 0.044 for EPOx3) and DG (p = 0.042 for EPOx1 and 0.022 for EPOx3) compared to saline treatment (Fig. 7). There was no significant difference in the vascular density between the EPOx1 and EPOx3 groups.
Fig. 7.
Effect of EPO on vWF-staining vascular structure in the injured cortex, ipsilateral DG and CA3 region 35 days after TBI. TBI alone (d, g, and i) significantly increased the vascular density in these regions compared to sham controls (p < 0.05). EPO treatment (EPOx1 and EPOx3) further enhanced angiogenesis after TBI compared to saline groups (p < 0.05). The density of vWF-stained vasculature is shown in (p). Data represent mean ± SD. Scale bar = 50 μm (a); 25 μm (e). *p < 0.05 vs. Sham. #p < 0.05 vs. the saline group. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
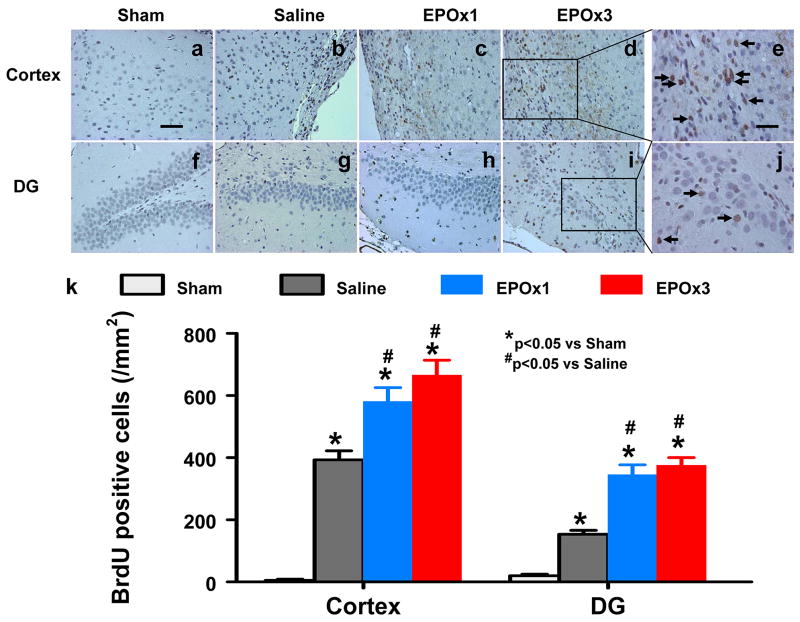
Cell Proliferation
BrdU, an analog of thymidine, is commonly used to detect proliferating cells in living tissues. BrdU can be incorporated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle, substituting for thymidine during DNA replication. The number of BrdU-positive cells found in the ipsilateral cortex (p = 0.008), and DG (p = 0.006) areas was significantly increased at 35 days after TBI, compared with sham controls (Fig. 8). However, EPO treatment further increased the number of BrdU-positive cells in the cortex (p = 0.015 for EPOx1 and 0.012 for EPOx3) and DG (p = 0.010 for EPOx1 and 0.018 for EPOx3) after TBI compared to saline controls (Fig. 8). There was no significant difference in the number of BrdU-positive cells in these regions between the EPOx1 and EPOx3 groups.
Fig. 8.
Effect of EPO on BrdU-positive cells in the injured cortex and ipsilateral DG 35 days after TBI. The cells with BrdU (brown stained) that clearlylocalized to the nucleus (hematoxylin stained) were counted as BrdU-positive cells (arrows in e and j). TBI alone (b, g) significantly increased the number of BrdU-positive cells in the ipsilateral cortex and DG compared to sham controls (p < 0.05). EPO treatment (EPOx1 and EPOx3) significantly increased the number of BrdU-positive cells in these regions (c, d, h, and i) compared to saline groups (p < 0.05). The number of BrdU-positive cells is shown in (k). Data represent mean ± SD. Scale bar = 50μm (a); 25μm (e). *p < 0.05 vs. Sham. #p < 0.05 vs. the saline group. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
Neurogenesis
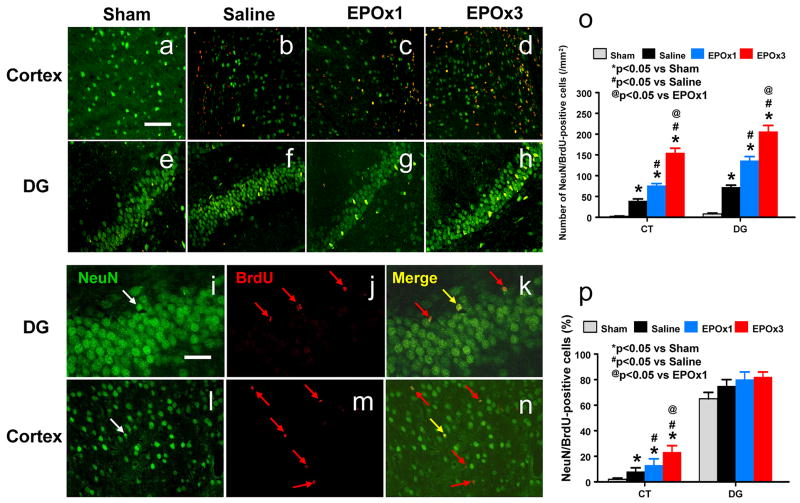
Newly generated neurons were identified by double labeling for BrdU (proliferating marker) and NeuN (mature neuronal marker). The number of NeuN/BrdU-colabeled cells (newborn neurons) was significantly higher in the injured cortex (p = 0.014) and DG (p = 0.010) after TBI compared to sham controls (Fig. 9b vs 9a; Fig. 9f vs 9e). EPO treatment significantly increased the number of newborn neurons in the injured cortex (p = 0.027 for EPOx1 and 0.022 for EPOx3) and DG (p = 0.019 for EPOx1 and 0.021 for EPOx3) (Fig. 9c, d, g, h) compared to saline controls. The number of newborn neurons in the injured cortex (p = 0.031) and DG (p = 0.041) was significantly higher in the EPOx3 group than in the EPox1 group (Fig. 9c vs 9d; Fig. 9g vs 9h). EPO treatment significantly increased the percentage of NeuN/BrdU-colabeled cells in the injured cortex (p = 0.047 for EPOx1 and 0.034 for EPOx3) (Fig. 9p) compared to saline controls. The percentage of newborn neurons in the injured cortex was significantly higher in the EPOx3 group than in the EPOx1 group (p = 0.044). However, there is no significant difference in the percentage of newborn neurons in the DG among sham, saline- and EPO-treated groups.
Fig. 9.
Double fluorescent staining for BrdU (red) and NeuN (green) to identify newborn neurons (yellow after merge, k and n) in the injured cortex (a-d) and the ipsilateral DG (e-h) at 35 days after TBI (b, f) and EPO treatment (c, d, g, and h). Newborn BrdU-positive cells (red, j and m) differentiate into neurons expressing NeuN (yellow, k and n). The total number and percentage of NeuN/BrdU-colabeled cells are shown in (o) and (p), respectively. Data represent mean ± SD. Scale bars = 50 μm (a); 25 μm (i). *p < 0.05 vs. corresponding sham. #p < 0.05 vs. the saline group. @ p < 0.05 vs EPOx1 group. N (rats/group) = 6 (Sham); 6 (Saline); 6 (EPOx1); 7 (EPOx3).
Discussion
The main findings of the present study are: 1) delayed (24-hours post injury) EPO treatment after TBI provides long-term behavioral benefits, as reflected in improvements in sensorimotor functional recovery and spatial learning, as evaluated by MWM test, footfault test and mNSS score, respectively; 2) the improvements in spatial learning and sensorimotor function may derive from the effect of EPO on reducing hippocampal cell loss and increasing angiogenesis and neurogenesis; and 3) although a single dose of EPO shows substantial benefits after TBI, the three-dose paradigm provides a better outcome in terms of reducing hippocampal cell loss, enhancing neurogenesis, and improving spatial learning and sensorimotor functional recovery.
There are several major differences between the present work and earlier studies. Our previous study has demonstrated that delayed EPO treatment significantly increases neurogenesis and improves spatial memory when the EPO was given daily for 14 days starting 24 hours after TBI in rats.21 The sensorimotor function and spatial learning were not examined in that short-term survival (14 days) study.21 The present study of 35-day survival after CCI in rats demonstrates that delayed treatment with EPO not only significantly improves the spatial learning function (acquisition) at Days 33–35 after CCI but also promotes sensorimotor functional recovery (reduced footfaults and mNSS score).
EPO given subcutaneously within 6 hours of TBI reduces lesion volume and cell loss in the CA1 and CA3 region of the hippocampus (functional outcome not measured in that study).8 It should be noted that intraperitoneal injection yielded a faster and greater peak concentration of plasma EPO than subcutaneous injection.34 This may partially explain why the present delayed (24 hours) EPO treatment (intraperitoneal) provides substantial benefits while the 12-hour EPO treatment (subcutaneous) does not show significant neuroprotection (without data on 24-hour injection).8 Many of the treatments including EPO in experimental TBI were given immediately or within 6 hours after TBI.8,16,18,20 The translation of early TBI therapies from the laboratory to the clinic (Phase III) has not been successful.29 Our previous study has demonstrated that apoptotic cells in the ipsilateral cortex and hippocampus were observed as early as 2 hours after the impact, peaked at 2 days, and gradually tapered off afterward. 19 Our present findings demonstrated that delayed EPO therapy reduces hippocampal cell loss, enhances angiogenesis and neurogenesis and improves functional outcomes after TBI, further indicating that the therapeutic time window may not be limited to early hours and can be extended to later time periods.
Our previous studies have shown that early (6 hours) EPO therapy does not affect angiogenesis in mice after TBI 41 and delayed (24 hours) EPO treatment significantly increases angiogenesis after stroke in rats.40 Our present study demonstrates for the first time that delayed EPO treatment increases angiogenesis in the ipsilateral hippocampus and cortex in rats after TBI. Under physiological conditions, neurogenesis in the subgranular zone of the DG and subventricular zone (SVZ) takes place within an angiogenic microenvironment.48 In vivo, neurogenesis and angiogenesis are highly interdependent and work together to promote brain remodeling and subsequent improvement of neurological functional after brain injury.48 Our present data support the coupling of angiogenesis and neurogenesis, which is shown by the increased number of NeuN/BrdU-positive cells in the injured cortex and hippocampus where angiogenesis is also enhanced after TBI. The present study demonstrated that EPO treatment further increased angiogenesis and neurogenesis. In the present study, EPO significantly increased the total number and percentage of newborn neurons found in the injured cortex, suggesting the EPO might induce SVZ-derived neurogenesis, i.e., migration of newborn blasts from the SVZ into the injured cortex where some of SVZ-derived newborn blasts differentiate into mature neurons.13,36,45,47 Although EPO therapy significantly increased the total number of newborn neurons in the DG, it did not change the percentage (approximately 80% in this study) of newborn neurons, indicating that most of the BrdU-positive cells in the DG differentiate into granule neurons and that cell-lineage commitment patterns do not change in the DG after TBI and EPO therapy. This finding is in agreement with the report that the majority of BrdU-positive cells in the DG become granule neurons after TBI and these neurons exhibit extensive anatomical integration into the CA3 region at the time cognitive recovery is observed.35 Our present data suggest that newborn neurons may participate in brain repair and functional recovery.
To our knowledge, the present study for the first time compared the efficacy of a single dose with that of triple doses of delayed EPO treatment for TBI. TBI upregulated the expression of EPO and EPOR in rats.20 However, EPO upregulation is transient (3 days) while increased EPOR expression lasts at least 7 days.20 These findings suggest that transiently increased endogenous EPO level does not match prolonged increased EPOR, and therefore may not provide enough neuroprotection. In addition, prolonged EPOR upregulation provides a platform for exogenous EPO treatment and suggests multiple doses may be required. In the present study, a single dose of delayed EPO therapy shows substantial benefits (i.e., significantly reducing hippocampal cell loss, increasing angiogenesis and neurogenesis, and improving functional recovery), while three doses of EPO provide better outcomes.
In conclusion, delayed (24 hours) EPO therapy for TBI significantly reduces hippocampal cell loss, enhances angiogenesis and neurogenesis, and improves sensorimotor function and spatial learning recovery, suggesting that delayed treatment with EPO is both neuroprotective and neurorestorative. Our data also demonstrate that a multiple dosing protocol of EPO provides better efficacy than a single EPO dose for treatment initiated at 24 hours post TBI.
Acknowledgments
Sources of financial support: NINDS grants RO1 NS62002 (Ye Xiong) and PO1 NS42345 (Asim Mahmood, Michael Chopp).
This work was supported by NIH grants RO1 NS62002 and PO1 NS42345.
References
- 1.Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- 2.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 3.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerami A. Beyond erythropoiesis: novel applications for recombinant human erythropoietin. Semin Hematol. 2001;38:33–39. doi: 10.1016/s0037-1963(01)90128-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- 9.Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q. 2000;23:1–13. doi: 10.1097/00002727-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 12.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 13.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 15.Grasso G, Sfacteria A, Erbayraktar S, Passalacqua M, Meli F, Gokmen N, et al. Amelioration of spinal cord compressive injury by pharmacological preconditioning with erythropoietin and a nonerythropoietic erythropoietin derivative. J Neurosurg Spine. 2006;4:310–318. doi: 10.3171/spi.2006.4.4.310. [DOI] [PubMed] [Google Scholar]
- 16.Grasso G, Sfacteria A, Meli F, Fodale V, Buemi M, Iacopino DG. Neuroprotection by erythropoietin administration after experimental traumatic brain injury. Brain Res. 2007;1182:99–105. doi: 10.1016/j.brainres.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 17.Grasso G, Sfacteria A, Passalacqua M, Morabito A, Buemi M, Macri B, et al. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56:821–827. doi: 10.1227/01.neu.0000156493.00904.7e. discussion 821–827. [DOI] [PubMed] [Google Scholar]
- 18.Hartley CE, Varma M, Fischer JP, Riccardi R, Strauss JA, Shah S, et al. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J Neurosurg. 2008;109:708–714. doi: 10.3171/JNS/2008/109/10/0708. [DOI] [PubMed] [Google Scholar]
- 19.Kaya SS, Mahmood A, Li Y, Yavuz E, Goksel M, Chopp M. Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res. 1999;818:23–33. doi: 10.1016/s0006-8993(98)01204-9. [DOI] [PubMed] [Google Scholar]
- 20.Liao ZB, Zhi XG, Shi QH, He ZH. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion 602–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Qu C, Goussev A, Jiang H, Lu C, Schallert T, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 27.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 29.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okutan O, Turkoglu OF, Gok HB, Beskonakli E. Neuroprotective effect of erythropoietin after experimental cold injury-induced vasogenic brain edema in rats. Surg Neurol. 2008;70:498–502. doi: 10.1016/j.surneu.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 31.Royo NC, Schouten JW, Fulp CT, Shimizu S, Marklund N, Graham DI, et al. From cell death to neuronal regeneration: building a new brain after traumatic brain injury. J Neuropathol Exp Neurol. 2003;62:801–811. doi: 10.1093/jnen/62.8.801. [DOI] [PubMed] [Google Scholar]
- 32.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Sundholm-Peters NL, Yang HK, Goings GE, Walker AS, Szele FG. Subventricular zone neuroblasts emigrate toward cortical lesions. J Neuropathol Exp Neurol. 2005;64:1089–1100. doi: 10.1097/01.jnen.0000190066.13312.8f. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 38.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 39.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 41.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, et al. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 44.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 45.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, et al. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]