Summary
Cysteine S-conjugate β-lyases are pyridoxal 5′-phosphate-containing enzymes that catalyze β-elimination reactions with cysteine S-conjugates that possess a good leaving group in the β-position. The end products are aminoacrylate and a sulfur-containing fragment. The aminoacrylate tautomerizes and hydrolyzes to pyruvate and ammonia. The mammalian cysteine S-conjugate β-lyases thus far identified are enzymes involved in amino acid metabolism that catalyze β-lyase reactions as non-physiological side reactions. Most are aminotransferases. In some cases the lyase is inactivated by reaction products. The cysteine S-conjugate β-lyases are of much interest to toxicologists because they play an important key role in the bioactivation (toxication) of halogenated alkenes, some of which are produced on an industrial scale and are environmental contaminants. The cysteine S-conjugate β-lyases have been reviewed in this journal previously [Cooper and Pinto, 2006]. Here we focus on more recent findings regarding: 1) the identification of enzymes associated with high-Mr cysteine S-conjugate β-lyases in the cytosolic and mitochondrial fractions of rat liver and kidney; 2) the mechanism of syncatalytic inactivation of rat liver mitochondrial aspartate aminotransferase by the nephrotoxic β-lyase substrate S-(1,1,2,2-tetrafluoroethyl)-L-cysteine (the cysteine S-conjugate of tetrafluoroethylene); 3) toxicant channeling of reactive fragments from the active site of mitochondrial aspartate aminotransferase to susceptible proteins in the mitochondria; 4) the involvement of cysteine S-conjugate β-lyases in the metabolism/bioactivation of drugs and natural products; and 5) the role of cysteine S-conjugate β-lyases in the metabolism of selenocysteine Se-conjugates. This review emphasizes the fact that the cysteine S-conjugate β-lyases are biologically more important than hitherto appreciated.
Keywords: cysteine S-conjugates; cysteine S-conjugate β-lyases; S-(1,2-dichlorovinyl)-L-cysteine; glutamine transaminase K; mitochondrial aspartate aminotransferase; S-(1,1,2,2-tetrafluoroethyl)-L-cysteine
Introduction – the mercapturate pathway
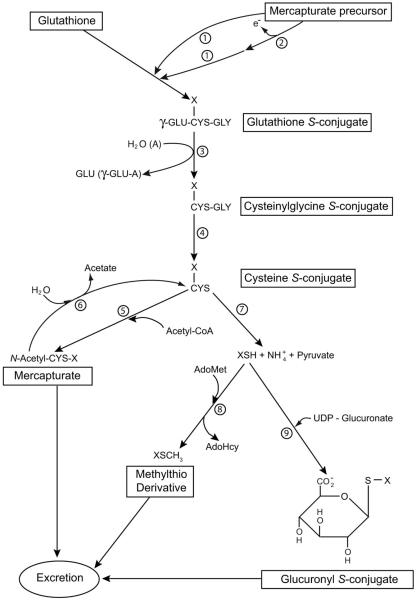
Many endogenous electrophiles (e.g. leukotrienes; unsaturated aldehydes) and exogenous electrophiles (e.g. many drugs) are detoxified by the mercapturate pathway. In this pathway the electrophile is consecutively converted to the glutathione S-conjugate, cysteinylglycine S-conjugate, cysteine S-conjugate and N-acetylcysteine S-conjugate (mercapturate) by the actions of glutathione S-transferases (which catalyze addition of glutathione (GSH) to electrophilic centers), γ-glutamyltransferase, dipeptidases and N-acetyltransferases, respectively (Fig. 1). The mercapturate is generally more polar and water soluble than is the unconjugated electrophile and is readily excreted in the urine and/or bile. Despite its biological importance there are surprisingly few reviews on the mercapturate pathway (Chasseaud, 1976; Stevens and Jones, 1989; Silbernagl and Heuner, 1993; Hinchman and Ballatori, 1994). In some cases, a toxic metabolite that is not an electrophilic substrate for glutathione S-transferases may be converted to an electrophile that can then be processed through the mercapturate pathway and the thiomethyl shunt (Bakke et al., 1982; Fig. 1) – see also below.
Fig. 1.
The mercapturate pathway and associated side reactions. If the mercapturate precursor contains an electrophilic center it may react directly with GSH (reaction 1). Alternatively, the precursor may be converted to a compound with an electrophilic center (reaction 2) prior to reaction with GSH. Reactions 1 through 5 represent the mercapturate pathway. Reactions 7, 8 and 9 are alternative reactions for elimination of the cysteine S-conjugate. Reactions 7 plus 8 denote the thiomethyl shunt. The thiomethyl compound (XSCH3) may be excreted unchanged or further oxidized to sulfoxide, sulfone or CO2 and sulfate, which are excreted. For some cysteine S-conjugates metabolism may also involve conversion to the α-keto acid, α-hydroxy acid, oxidatively decarboxylated product or sulfoxide (not shown). Enzymes: 1) glutathione S-transferases, 2) oxidases that generate an electrophilic center for attack by GSH (in some cases oxidation may be non-enzymatic); 3) γ-glutamyltransferase (GGT); 4) dipeptidases; 6) aminoacylases; 7) cysteine S-conjugate β-lyases; 8) thiomethyltransferase; 9) UDP-glucuronosyltransferases. In vivo the hydrolysis reaction of GGT predominates over the formation of γ-glutamyl amino acids. Abbreviations: AdoHcy, S-adenosyl-L-homocysteine; AdoMet, S-adenosyl-L-methionine; A, amino acid, dipeptide or GSH acceptor for the γ-glutamyltransferase reaction; γ-GLU-A, γ-glutamyl amino acid (or γ-glutamyldipeptide; γ-glutamylglutathione). Many potentially toxic xenobiotics and a few endogenous compounds are metabolized through the mercapturate pathway. Modified in part from Silbernagl and Heuner (1993) and Cooper and Pinto (2008). Reproduced from Cooper and Hanigan (2010) with permission.
Discovery of cysteine S-conjugate β-lyases – the thiomethyl shunt
Some xenobiotics are known to be transformed to mercaptan and methylmercaptan metabolites. For example, phenacetin, acetaminophen, N-hydroxy-2-acetylaminofluorene are thiomethylated in vivo (Tateishi et al., 1978a,b). Thiomethylation was originally assumed to involve a sulfonium compound derived from methionine. However, in 1965, Colucci and Buyske showed that benzothiazole-2-sulfonamide is converted in rats, rabbits and dogs in part to 2-mercaptobenzothiazine in which the sulfur of the mercaptan moiety is derived from GSH. Later, Chatfield and Hunter (1973) showed that 2-acetamido-4-chloromethylthiazole is converted in rats to 2-acetamido-4-methylthiomethylthiazole in part through the mercapturate pathway. Earlier, Anderson and Schultze (1965) showed that bovine liver and kidney extracts contain a “C-S” lyase that converts S-(1,2-dichlorovinyl)-L-cysteine (DCVC, the cysteine S-conjugate of trichloroethylene) to pyruvate, ammonia and a reactive chlorine-containing fragment.
Tateishi et al. (1978a) identified 6′-methylthiobromazepam as a metabolite of bromazepam in rat bile. These authors also showed that the thioether bonds of the cysteine S-conjugates of 2,4-dinitrobenzene and bromobenzene are readily cleaved by an enzyme in rat liver cytosol. Incubation of S-(2,4-dinitrophenyl)cysteine with the purified enzyme yielded pyruvate, ammonia and 2,4-dinitrobenzenethiol. The corresponding thiomethyl compound was obtained when the thiol product was incubated with a microsomal thiomethyltransferase and S-adenosylmethionine. Tateishi et al. (1978a,b) were the first to use the term “cysteine conjugate β-lyase” for an enzyme that catalyzes β elimination from a cysteine S-conjugate. The net cysteine S-conjugate β-lyase-catalyzed reaction is shown in Eq. (1). However, it should be noted that the actual enzyme-catalyzed products are XSH and aminoacrylate [CH2=C(NH3+)CO2−]. Based on the catalytic mechanism of the well studied β-lyase enzyme β-cystathionase (cystathionine β-lyase) (Clausen et al., 1997), the aminoacrylate formed at the active site of cysteine S-conjugate β-lyases is presumably bound in Schiff base linkage to PLP coenzyme. Protonation of the aminoacrylate and reverse transaldimination within the active site results in formation of β-iminopropionate [CH3C(=NH2+)CO2−] and enzyme-bound PLP. The reaction is completed upon hydrolysis of β-iminopropionate to pyruvate [CH3C(O)CO2−] and ammonium (Clausen et al., 1997).
| (1) |
The thiomethyl shunt (i.e. shunt away from the mercapturate pathway) may thus be depicted as: electrophile → glutathione S-conjugate → cysteinylglycine S-conjugate → cysteine S-conjugate → mercaptan (catalyzed by cysteine S-conjugate β-lyases) → thiomethyl compound (catalyzed by thiolmethyltransferases). The thiomethylated compound may be excreted intact or following oxidation to the sulfoxide or sulfone. The thiomethyl shunt can therefore act in conjunction with the mercapturate pathway for the detoxication and excretion of certain potentially toxic electrophiles (Jakoby and Stevens, 1984; Warrander et al., 1985; Fig. 1).
Cysteine S-conjugate β-lyases and glucuronidation
The mercaptan liberated from a cysteine S-conjugate by the action of cysteine S-conjugate β-lyases may be S-glucuronidated and excreted, as in the case of 2-benzothiazole derived from benzothiazolyl L-cysteine (BTC) (Elfarra and Hwang, 1990). As with the thiomethyl shunt, this mechanism acts in conjunction with the mercapturate pathway as a mechanism for the elimination of certain toxic metabolites (Fig. 1).
Generation of toxic, reactive sulfur-containing species from the cysteine S-conjugates derived from halogenated alkenes
The thiomethyl shunt and S-glucuronidation pathways are possible when the eliminated sulfur-containing fragment (XSH; Eq. (1)) is relatively stable. In those cases, the funneling of the cysteine S-conjugate away from the mercapturate by cysteine S-conjugate β-lyases is of little toxicological consequence because thiomethylation and S-glucuronidation act as alternative pathways for detoxication and excretion. However, there are many examples in which the eliminated sulfur-containing fragment is highly reactive, indeed much more so than the parent electrophile. In those cases, the cysteine S-conjugate β-lyase reaction is a bioactivation (toxication) mechanism. Moreover, since the glutathione S-conjugate, cysteinylglycine S-conjugate, mercapturate (as a result of acylase action [Uttamsingh and Anders, 1999]) can be converted to the cysteine S-conjugate in vivo, each S-conjugate of the mercapturate pathway is toxic. A large number of halogenated alkenes have been shown to be toxic to experimental animals at least in part through conversion to S-conjugates in the mercapturate pathway. However, most of the work in this area has been carried out with DCVC and TFEC [S-(1,1,2,2-tetrafluoroethyl)-L-cysteine, the cysteine S-conjugate of tetrafluoroethylene]. These compounds are toxic to liver, brain (in some cases) and especially to kidney. Within the kidney, the proximal tubules are vulnerable. The mitochondria are targets of halogenated cysteine S-conjugates.
Trichloroethylene and tetrachloroethylene are produced in large quantities industrially. Moreover, trichloroethylene is present in the majority of superfund sites in the US (http://www.atsdr.cdc.gov/tfacts19.html) and a large segment of the population has a body burden of trichloroethylene. Although the findings were disputed at first it now seems probable that there is an increased risk of several cancers, particularly kidney cancer, in humans exposed to very high levels of trichloroethylene (Scott and Chiu, 2006). Thus, it is important to identify the enzymes involved in the bioactivation of halogenated alkenes and the mechanisms involved (next section).
Since the toxicity of cysteine S-conjugates and other conjugates of the mercapturate pathway in relation to halogenated alkenes has been extensively reviewed (Dekant, 2003; Anders, 2004; 2008; Koob and Dekant, 1991; Cooper and Pinto, 2006; Nagelkerke and Boogard, 1991; Dekant et al., 1994; Anders et al., 1988; Cooper et al., 2002a) we will focus more heavily here on other important biological aspects of the cysteines S-conjugate β-lyase pathway.
Identification of cysteine S-conjugate β-lyases
Tateishi and coworkers (1978b) obtained a highly purified preparation of a cysteine S-conjugate β-lyase from rat liver. The same group also purified a cysteine S-conjugate β-lyase from human liver (Tomisawa et al., 1984). However, in neither case was the enzyme identified. In the mid 1980s, however, Stevens and colleagues identified kynureninase (Stevens, 1985) and glutamine transaminase K [GTK; identical to kynurenine aminotransferase isozyme I (KAT I)] (Stevens et al., 1986) as major cysteine S-conjugate β-lyases of rat liver and kidney cytosol, respectively (DCVC as substrate). Since that time a number of other pyridoxal 5′-phosphate (PLP)-containing enzymes have been identified with cysteine S-conjugate β-lyase activity toward DCVC (and TFEC) (Table 1). All are enzymes involved in amino acid metabolism that catalyze cysteine S-conjugate β-lyase reactions as secondary reactions as a result of the electron-withdrawing properties of the group attached at the sulfur.
Table 1.
Mammalian PLP-dependent enzymes with L-cysteine S-conjugate β-lyase activitya
| β-Lyase substrates | Syncatalytic inactivation |
Competing transamination |
Approximate specific activity (U/mgb) |
|||
|---|---|---|---|---|---|---|
| DCVC | TFEC | BTC | ||||
| Enzyme (cytosolic) | ||||||
| Kynureninase (R) | + | ND | + | + | ND | 0.25 |
| GTK/KAT I (R) | + | + | − | − | + | 0.6 – 6.4 |
| GTK/KAT I (H) c | + | + | + | − | + | 8 – 40 |
| cAAT (R) | + | + | ± | + | − | 0.04 – 0.16 |
| AlaAT (P) | + | + | + | + | − | 0.004 – 0.06 |
| BCATc (H) | + | + | + | + | − | 0.3 – 0.5 |
| Cystathionine γ-lyase (R) | − | + | − | − | − | 0.05 – 0.1 |
| High-Mr β-lyase (R)d | + | + | − | + | ~0.07-1.0 | |
| Enzyme (mitochondrial) | ||||||
| mAAT (R) | + | + | + | + | + | 0.8 – 2.3 |
| BCATm (H) | + | + | − | + | − | 0.2 – 0.5 |
| AGAT II (R) | + | + | + | + | + | 0.2 |
| GABA aminotransferase (P) | ND | + | ND | ND | ND | 0.016 |
| High-Mr β-lyase (R)e | + | + | + | ND | + | 0.1 – 1.2 |
This table is an update of that of Cooper and Pinto (2006) as modified by Cooper and Hanigan (2010). For original references see Cooper and Pinto (2006). A unit of enzyme activity (U) is defined as the amount of enzyme that catalyzes the formation of 1 μmol of pyruvate per min (usually at 37°C, but temperature was not always specified). The specific activities are from published data on highly purified enzymes. ND, not determined. Species abbreviations: R, rat; P, pig; H, human.
Activity with DCVC and/or TFEC.
From Cooper et al. (2008a). Some GTK activity is also found in rat kidney and liver mitochondria, but the role of mitochondrial GTK as a cysteine S-conjugate β-lyase is uncertain.
Contains GTK activity
Contains mAAT activity
GTK has high inherent cysteine S-conjugate β-lyase activity and as a result the gene is listed in the rat and human genomes as CCBL1 (Cysteine Conjugate Beta-Lyase 1), even though the lyase activity is presumably not its major physiological role. Rat GTK/KAT1 was cloned and sequenced by Perry et al. (1993) and by Mosca et al. (1994). Shortly thereafter Abraham and Cooper (1996) also cloned and sequenced rat GTK. The deduced Mr was slightly different (~48,500) (misstated as 45,800 in the original publication) from the deduced Mr (~47,800) obtained by Perry et al. (1993) and Mosca et al. (1994), but was >90% identical. The full-length clone expressed in Cos1 cells had considerable GTK activity and some KAT-type activity (Abraham and Cooper, 1996). Relative expression of GTK mRNA was in accord with the relative specific activity of GTK in various rat tissues (i.e. kidney > liver > brain) (Abraham and Cooper, 1996). The gene identified by Perry et al. (1993) and Mosca et al. (1994) is now designated CCBL1-CRA-a in the rat, whereas that identified by Abraham and Cooper (1996) is designated CCBL1-CRA-b. Further work is required to evaluate the relative protein and message levels of the two expressed proteins, their genomic relationship, and the relative biological functions.
Most of the PLP-enzymes listed in Table 1 are aminotransferases. This raises the possibility that aminotransferase reactions might interfere with the elimination reaction. For example, if a half transamination reaction competes with the β-lyase reaction then the pyridoxamine 5′-phosphate (PMP) form of the enzyme will be generated. Since the PMP form cannot support a β-lyase reaction an α-keto acid (or PLP) must be present for maximal β-lyase activity to be sustained. Several aminotransferases, including GTK and mitochondrial aspartate aminotransferase (mAAT), require added α-keto acid substrate in order to efficiently catalyze β-elimination with DCVC and TFEC (Stevens et al., 1986; Cooper et al., 2002b; Table 1).
High-Mr cysteine S-conjugate β-lyases in rat kidney cytosol
With the exception of alanine glyoxylate aminotransferase II (AGAT II) the aminotransferases listed in Table 1 are homodimers with subunit Mr values of 40,000 – 50,000. AGAT II and cystathionine γ-lyase are homotetramers, with subunit Mr values of 53,000 and 40,000, respectively. For convenience, kynureninase and most aminotransferases (including GTK and mAAT) listed in Table 1 may be referred to as low-Mr cysteine S-conjugate β-lyases (Mr ≤100,000) and AGAT II and cystathionine γ-lyase may be regarded as intermediate-Mr cysteine S-conjugate β-lyases. There is a third class that we have termed high-Mr cysteine S-conjugate β-lyases (Mr ≥ 300,000). These were discovered when liver and kidney homogenates were subjected to non-denaturing (native) gel polyacrylamide electrophoresis and separately stained for GTK and cysteine S-conjugate β-lyase activities (Abraham and Cooper, 1991). Subsequently, high-Mr lyase activity was detected by activity staining in proximal tubule segments of rat kidney (Kim et al., 1997). A high-Mr β-lyase (Mr ~330,000) was purified about 55-fold from rat kidney cytosol using DCVC as substrate. The preparation contained at least two proteins with Mr values of ~50,000 and 70,000 (Abraham et al., 1995a). The β-lyase activity was stimulated by a number of α-keto acids, including α-keto-γ-methiolbutyrate. The complex was not immunopositive to polyclonal rabbit antibodies raised against rat kidney GTK. If the enzyme is truly a multi-heteromeric protein then the attractions among the various components are such that they remain intact during heat treatment (45°C) and chromatography on DE-52, hydroxylapatite and Sephadex G-200 columns (Abraham et al., 1995a). Recent work from our laboratory suggests that GTK is present in rat kidney cytosol in high-Mr (Mr ≥ 200,000) and low-Mr (Mr ~96,000) forms. Thus, when a portion of rat kidney cytosol is subjected directly to Sephadex G-200 column chromatography a fraction of the total cysteine S-conjugate β-lyase activity (TFEC as substrate) elutes in the void volume (Mr ≥ 200,000). GTK activity also elutes in the void volume (Table 2). Since the relative activity of rat kidney cytosolic GTK as a transaminase (phenylalanine – α-keto γ-methiolbutyrate assay) versus that as a β-lyase (TFEC as substrate) is about 2:1 (Abraham et al., 1995) the data in Table 2 indicate that GTK contributes substantially to the β-lyase activity in the high-Mr fraction in kidney cytosol. Whether this high-Mr species of GTK is a homopolymer (n ≥ 4) or contains associated proteins is unknown. It is also not known why GTK could not be detected immunologically in a cytosolic high Mr complex in previous work.
Table 2.
Relative specific activities of GTK and cysteine S-conjugate β-lyase in fractions obtained from rat kidney cytosol
| Fraction | GTK (mU/mg)a (A) |
Cysteine S-conjugate β- lyase (mU/mg)b (B) |
A/B |
|---|---|---|---|
| Cytosol (unfractionated)c | 11.2 | 6.1 | 1.84 |
| Cytosol (high-Mr fraction)d | 0.92 | 0.67 | 1.36 |
| Cytosol (low-Mr fraction)d | 17.7 | 14 | 1.26 |
The reaction mixture contained 5 mM α-keto-γ-methiolbutyrate, 10 mM L-phenylalanine and 100 mM potassium phosphate buffer (pH 7.4); 37°C. Phenylpyruvate formation was measured. One unit = one μmol/min.
The reaction mixture contained 10 mM TFEC, 0.1 mM α-keto-γ-methiolbutyrate, and 100 mM potassium phosphate buffer (pH 7.4); 37°C. Pyruvate formation was determined.
Kidney cytosol was prepared as described by Zhang et al. (2006).
A portion (1 ml) of the cytosol was subjected to Sephadex G-200 (1 × 30 cm) column chromatography and eluted with 5 mM potassium phosphate buffer (pH 7.4) at 4°C.
The presence of high-Mr lyase activity [S-(2-chloro-1,1,2-trifluoroethyl)-L-cysteine and Se-(4-methylbenzyl)-L-selenocysteine as substrates] in rat kidney cytosol has been verified by Commandeur et al. (2000) using high-resolution gel filtration chromatography. The major peak of cysteine S-conjugate β-lyase activity coincided with Mr species of ~300,000 on a gel filtration column, whereas the minor peak coincided with proteins of ~66,000-132,000. The proportion of cysteine S-conjugate β-lyase activity in the high-Mr fraction of rat kidney cytosol, however, is much higher (>70%) in the report of Commandeur et al. (2000) compared to that which we note in Table 2 (~8%). The apparent discrepancy may have been due to different substrates employed or to different methods for preparing and fractionating cytosol. Nevertheless, the presence of at least one high-Mr cysteine S-conjugate β-lyase species/complex in rat kidney cytosol is now well established.
High-Mr cysteine S-conjugate β-lyases in rat kidney mitochondria
Activity staining also demonstrated the presence of high-Mr β-lyase activity in rat kidney mitochondria (Abraham et al., 1995a,b; Cooper et al., 2001). In the study of Abraham et al. (1995b) very little cysteine S-conjugate β-lyase activity co-purified with mitochondrial GTK (mitGTK). Considerable activity co-purified with a high-Mr β-lyase. This lyase activity in the high-Mr fraction was stimulated by various α-keto acids including α-keto-γ-methiolbutyrate and most strongly by α-ketoglutarate (Abraham et al., 1995a,b). Amino acid sequence analyses showed that the high-Mr β-lyase purified from kidney liver mitochondria contained protein disulfide isomerase and mitHSP70 (Cooper et al., 2001). The enzyme responsible for the β-lyase activity in the mitochondrial cysteine S-conjugate β-lyase was not identified at the time. However, we now present evidence that the mitochondrial high-Mr β-lyase contains mAAT activity. Thus, when a rat kidney mitochondrial homogenate was subjected to Sephadex G-200 chromatography two peaks of cysteine S-conjugate β-lyase activity were observed. Most of the activity was found to be present in a low-Mr fraction (Mr ~93,000) corresponding to the Mr expected for native dimeric mAAT (Table 3). However, a small but significant amount (~3%) was also present in a well defined peak in the void volume (Mr >200,000). Interestingly, both peaks contained AspAT activity. The ratio of specific activity of highly purified rat liver mAAT as an aminotransferase (α-ketoglutarate – aspartate transaminase assay) versus that as a cysteine S-conjugate β-lyase (TFEC as substrate) is ~250 (Cooper et al., 2002b). The ratio in the rat kidney mitochondria is similar to this value indicating that mAAT is a major cysteine S-conjugate β-lyase of rat kidney mitochondria. Interestingly, the ratio is also similar in the high-Mr fraction. This finding indicates that the major cysteine S-conjugate β-lyase activity of the mitochondrial high Mr β-lyase is also mAAT. This conclusion is strongly supported by the finding that the β-lyase activities in both the low- and high-Mr fractions were strongly inhibited (>90%) by 20 mM L-aspartate.
Table 3.
Relative specific activities of mAAT and cysteine S-conjugate β-lyase in fractions obtained from rat kidney mitochondria
| Fraction | mAAT (mU/mg)a (A) |
Cysteine S-conjugate β- lyase (mU/mg)b (B) |
A/B |
|---|---|---|---|
| Mitochondria (unfractionated)c | 1316 | 5.4 | 244 |
| Mitochondria (high-Mr fraction)d | 51 | 0.23 | 222 |
| Mitochondria (low-Mr fraction)d | 2190 | 7.8 | 281 |
The reaction mixture contained 6 mM α-ketoglutarate, 10 mM L-aspartate and 100 mM potassium phosphate buffer (pH 7.4); 37°C. The rate of oxaloacetate formation was measured. One unit = one μmol/min.
The reaction mixture contained 10 mM TFEC, 0.1 mM α-ketoglutarate, and 100 mM potassium phosphate buffer (pH 7.4); 37°C. Pyruvate formation was determined.
Kidney mitochondria were prepared as described by Zhang et al. (2006) and disrupted by sonication.
Fractionated as noted in Table 2.
Previous work has shown that Hsc70 (a cytosolic HSP70) binds to the acid-unfolded precursor to mAAT (pmAAT) in vitro (Artigues et al., 2002). Hsc70 also binds to acid-unfolded inactive mAAT, but not to the active holoenzyme (Artigues et al., 2006). pmAAT bound to Hsc70 is imported into isolated mitochondria in a reticulocyte lysate-dependent manner, suggesting that Hsc70 together with cytosolic factors is important for the import of catalytically inactive pmAAT into mitochondria. In the mitochondria, pmAAT is processed to catalytically active mAAT by removal of the N-terminal 29 amino acid mitochondrial targeting sequence (Artigues et al., 2002). It is possible that once inside the mitochondrion pmAAT or mAAT can then bind to mitHSP70 to complete the maturation process.
The relative amount of the total cysteine S-conjugate β-lyase detected in rat kidney mitochondrial preparations on native polyacrylamide gels in a high-Mr fraction (Abraham et al., 1995b; Cooper et al. 2002b) appears to be much greater than that detected after Sephadex G-200 chromatography (present work). The reason for this difference may be due to greater stability of the complexes in the buffer used for native gel electrophoresis compared to that used for gel filtration. Nevertheless, the finding that mAAT can form high-Mr complexes that include mitHSP70 has important toxicological implications. The formation of a complex between mAAT and mitHSP70 explains, at least in part, the thioacylation of both mAAT and mitHSP70 in kidney after rats are administered TFEC (see below).
Syncatalytic inactivation of cysteine S-conjugate β-lyases by β-lyase substrates – general
Inactivation of PLP enzymes by product(s) of β-lyase side reactions (syncatalytic inactivation) has been known for many years. For example, both isozymes of aspartate aminotransferase are syncatalytically inactivated in the presence of β-lyase substrates, such as β-chloro-L-alanine, serine O-sulfate and phosphoserine. The inactivation of cAAT (or a bacterial homologue) by β-chloro-L-alanine, however, can be decreased by inclusion in the reaction mixture of a Michael acceptor such as thiosulfate (Cavallini et al., 1973) or β-mercaptoethanol (Adams et al., 2005). The products obtained are L-cysteine S-sulfonate and 3-(2-hydroxyethyl)-L-cysteine, respectively. The inactivating species is aminoacrylate either free in solution as suggested by Cavallini et al. (1973) or covalently bound to PLP coenzyme (Adams et al., 2005).
Morino et al. (1974) suggested that inactivation of cAAT by β-chloro-L-alanine is due to modification of a lysine residue by aminoacrylate. However, Ueno et al. (1982) showed that inactivation in the presence of serine O-sulfate is due to formation of the pyruvate-PLP aldol product by reaction of PLP with aminoacrylate. Rat liver mAAT is syncatalytically inactivated in the presence of β-chloroalanine and TFEC on average after ~3,850 and ~2,700 turnovers per enzyme monomer, respectively (Cooper et al., 2002b). This difference was ascribed to the production of one reactive species (aminoacrylate) in the case of β-chloroalanine, but two reactive species in the case of TFEC (aminoacrylate and −SCF2CF2H ion).
As noted above, the eliminated sulfur-containing fragment derived from TFEC is chemically very reactive. Previous work has shown that the ε-amino groups of lysine residues are thioacylated by the sulfur-containing fragment derived from TFEC (formation of RNHC(=S)CF2H, where R = lysine residue; Hayden and Stevens, 1990; Hayden et al., 1991; Harris et al., 1992; Fisher et al., 1993; see later). Curiously, both human BCATm and BCATc can catalyze β-elimination with TFEC, but both enzymes are rapidly inactivated. Inactivation occurs on average ~170-280 and 40-50 turnovers per subunit for BCATm and BCATc, respectively (Cooper et al., 2003). On the other hand, both rat kidney GTK and human GTK are resistant to inactivation by β-lyase substrates (Cooper et al., 2008a). Human GTK has an unusual “crown” of aromatic amino acid residues in the substrate binding pocket, which may account for the interaction with neutral amino acid substrates, including cysteine S-conjugates (Rossi et al., 2004). This arrangement may prevent access of reactive fragments to susceptible groups within the vicinity of the active site. Evidently, the relative ease of syncatalytic inactivation by β-lyase substrates varies greatly among the aminotransferases, and probably depends on ease of access of reactive fragments to susceptible residues in or near the active site.
Mechanism for the syncatalytic inactivation of mAAT by TFEC
We have investigated the mechanism of inactivation of mAAT by TFEC and preliminary reports have been published elsewhere (Cooper and Pinto, 2008; Villar et al., 2007). Rat liver mAAT was purified by the procedure of Mattingly et al. (1995). The protein concentration was estimated from the absorbance at 356 nm of the PLP cofactor (ε = 8,500 M−1 cm−1; Mr, 46,399). The purified protein was stored in 40 mM HEPES, 0.1 mM EDTA pH 7.4 (refolding buffer) containing 0.02 % (w/v) sodium azide. The homogeneity of the preparation and the Mr of the protein were verified by SDS-PAGE and electrospray ionization-mass spectrometry (ESI-MS). Aliquots of native mAAT (0.1 mg/ml) in either 10 mM sodium phosphate buffer, pH 7.5 or 50 mM HEPES, 0.1 mM EDTA, pH 7.5, were reacted with 10 mM TFEC in the presence of 10 mM α-ketoglutarate at 10°C. At different times after mixing, aliquots of the reaction mixture (5 μl) were analyzed for aspartate aminotransferase activity by a standard procedure (Mattingly et al. 1995).
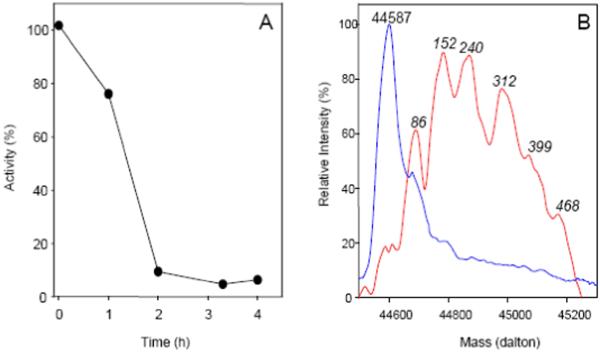
The TFEC-catalyzed loss of activity is shown in Fig. 2A. Inactivation is complete after 3 h of incubation. To determine the nature of the modification, these samples were subjected to mass spectrometric analysis. At different times after mixing, aliquots of the inactivation reactions (10 μl) were injected into a C8 reverse phase chromatography column (Zorbax C8SB Wide Pore Guard Column, Micro-Tech Scientific, 1 cm × 0.32 mm), which was connected online to a valve to direct the flow to either the waste or the mass spectrometer. After washing the column with 0.05 % (v/v) trifluoroacetic acid (TFA) at a flow of 100 μl/min, the flow was diverted to the mass spectrometer and the protein was eluted using a 0-54 % (v/v) acetonitrile gradient in 0.05 % (v/v) TFA, at a flow rate of 30 μl/min. The sample loop, reverse phase column, solvent delivery line, and injection and divert valves were included in a home-made cooling chamber and the temperature was maintained at 2.6°C. All ESI-MS data were acquired on a hybrid linear ion trap FT-ICR MS equipped with a 7 tesla magnet (LTQ-FT, ThermoFinnigan, San Jose, CA). As a control sample, an aliquot of mAAT kept under the same conditions, but without the addition of TFEC, was analyzed under identical conditions (Fig. 2B). This control sample shows a deconvoluted mass spectrum with a mass of 44,587 daltons, which is in agreement with the expected mass derived from the protein amino acid sequence. However, mAAT incubated with TFEC results in the formation of multiple forms of higher mass, indicating the covalent addition of multiple derivatives. Because of the complexity of the resulting mass spectra, the exact change in mass for each individual peak cannot be measured with absolute certainty. However, since the change in mass between consecutive mass peaks is not constant, one can assume that multiple adducts are formed.
Fig 2.
Syncatalytic inactivation of mAAT by TFEC. A. Inactivation of mAAT (0.1 mg/ml) in the presence of 10 mM sodium phosphate buffer (pH 7.5), 10 mM α-ketoglutarate and 10 mM TFEC; 10°C. Enzyme activities (●) are expressed as percent relative to the activity of a sample of native enzyme maintained under identical conditions, but in the absence of TFEC. B. Mass spectra of mAAT. Mass spectra were obtained before (blue line) and after 4 h of reaction with TFEC (red line). The protein aliquots were desalted in a C8 reverse phase column and injected into the mass spectrometer. Mass spectra were deconvoluted using the algorithm included in the Xcalibur software. For the covalently modified protein the masses are given as increment in mass relative to the mass of the mAAT polypeptide (44,587 daltons).
To determine the position of the amino acid residues modified and the exact composition of the TFEC modifications, the reacted protein was independently subjected to trypsin digestion and mass spectrometric analysis in duplicate. For this purpose, after 4 h of reaction, aliquots of control and reacted samples were dialyzed against three changes of 10 mM sodium phosphate buffer, pH 7.5, followed by three changes of water to remove excess of TFEC. After unfolding in 6 M guanidinium hydrochloride, the protein was reduced and alkylated. Then the protein sample was diluted six times in ammonium bicarbonate and digested with trypsin for 8 h at room temperature using a mAAT: trypsin ratio of 100:1 (w:w). The resulting peptides were pressure loaded onto an in-house packed C18 nano column (Michrom Bioresources Magic C18 AQ 300Å, 8 cm × 100 μm) and desalted with 0.05% formic acid at a flow rate of 250 nl/min for 20 min. Following desalting, the peptides were eluted with an acetonitrile gradient (0 to 90% in 10 minutes) at a flow rate of 250 nl/min, and directed to the mass spectrometer. The ESI source was operated at 1.8 kV. The mass spectrometer was controlled by Xcalibur software to perform continuously full mass scan analysis in the ICR cell, followed by MS2 scan analysis in the ion trap of the six most intense ions observed on the full scan. Dynamic exclusion of two repeat scans of the same ion was used, with a 30 sec repeat duration and 90 sec exclusion duration and a mass window of 4 daltons. Normalized collision energy for tandem mass spectrometry was set to 35%. Sequence assignment was made using the SEQUEST algorithm (Eng et al., 2008) included in Bioworks 3.1 (ThermoFinnigan) and the known sequence of rat liver precursor mAAT. To eliminate possible contaminating peptides, all MS2 scans of selected peptide ions were also searched against a protein database containing standard protein contaminants. The results of the search were filtered using the following set of criteria: minimum cross-correlation score (Xcorr) of 1.1, 1.7 and 2.3 for 1, 2 and 3 charged ions respectively and a delta correlation score (Δcorr) greater than 0.08.
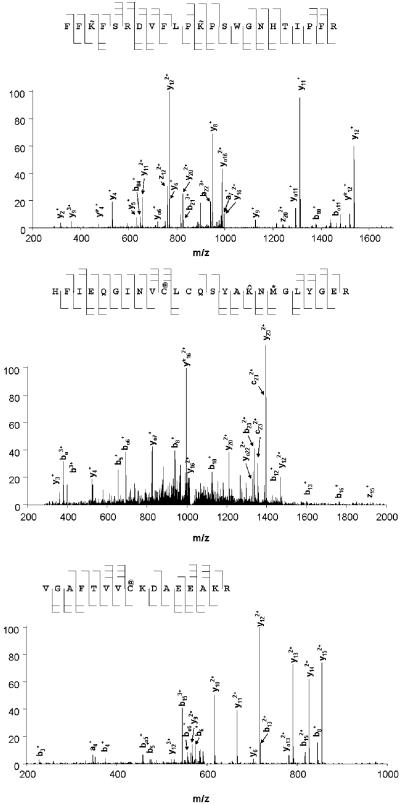
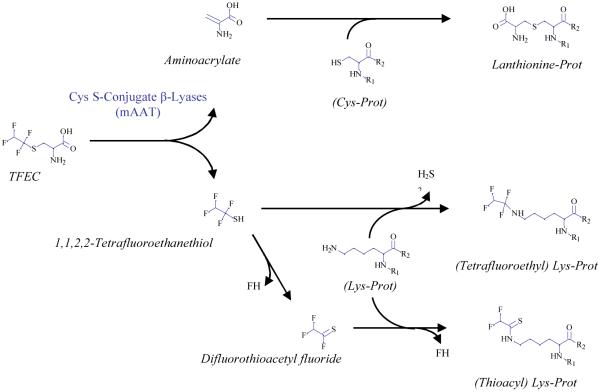
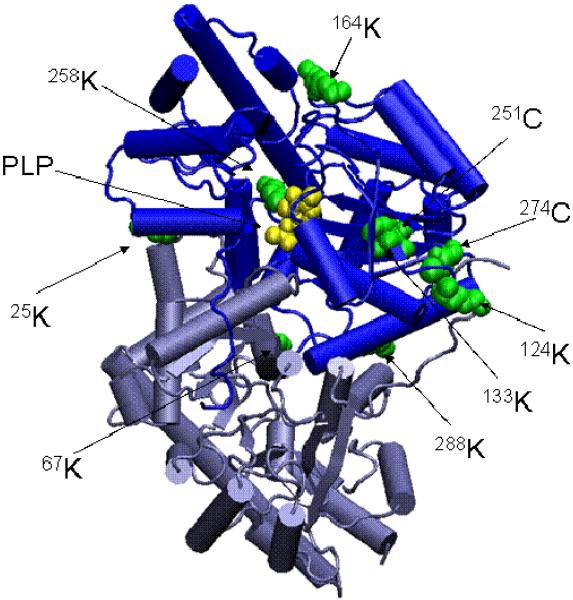
Several TFEC modified peptides were detected (Table 4; Fig. 3) these modifications are distributed over the entire length of the polypeptide chain, and, in general in solvent accessible regions. Three different kinds of modifications were detected. First, multiple K residues are modified by addition of a fragment that results in a net gain of 100 amus. This finding is consistent with a -CF2CF2H linkage to the ε-amino group of a lysyl residue. This linkage apparently results from direct attack of the ε-amino group of a lysine residue on tetrafluoroethanethiol with loss of hydrogen sulfide. This type of addition has not been described previously, and may have been obscured in previous studies with 35S-labeled TFEC as a result of loss of radiolabel as H2S. Second, two Cys residues denoted with the @ sign (on the peptides FIEQGINVC@LCQSYAK^NM*GLYGER and VGAFTVVC@KDAEEAKR) are modified by 87 amus. This finding is consistent with Michael addition of a cysteine thiolate residue to aminoacrylate generating a lanthionine moiety. Interestingly, in the peptide active site-K containing peptide (FIEQGINVC@LCQSYAK^NM*GLYGER) the K residue is modified by 96 amus, which is consistent with thioacylation (258K^). The corresponding tandem mass spectra of these peptides are shown in Fig. 3. Mechanisms whereby fragments derived from a β-lyase reaction on TFEC form covalent adducts with proteins are shown in Fig. 4.
Table 4.
Covalently modified tryptic peptides
| Sequencea | MH+ | Charge | XCorr |
|---|---|---|---|
| 122FF K~ FSRDVFLP K~ PSWGNHTIPF 148R | 3017.23 | 3 | 2.47 |
| 242H FIEQGINV C@ LCQSYA K^ N M* GLYGE 266R | 3077.29 | 3 | 2.95 |
| 267VGAFTVV C@ KDAEEAK 282R | 1808.91 | 3 | 3.12 |
| 55K AEAQIAGKNLD K~ EYLPIGGLADF C# 81K | 2945.47 | 3 | 4.03 |
| 64NLD K~ EYLPIGGLADF C# 81K | 2048.96 | 2 | 2.41 |
| 288K~ ILIRPLYSNNPLNGA 304R | 2730.51 | 3 | 2.53 |
| 283VESQL K~ ILIRPLYSNNPLNGA 304R | 2574.40 | 3 | 3.13 |
| 160YYDP K~ T C# GFDFSGALEDIS 179K | 2409.02 | 2 | 2.79 |
| 3ASSWWTHVEMGPPDPILGVTEAF 25K~ | 2907.30 | 3 | 3.35 |
Following trypsin treatment of a control (untreated) and TFEC-modified protein, the peptide mixture was analyzed on-line on a LTQ FT mass spectrometer, as indicated in the text. TFEC-modified residues are highlighted in bold face.
Fig. 3.
LC/ESI mass spectral analysis of covalently modified peptides. Tandem mass spectra of the triply-charged peptide-ions at m/z of 1006.4 (122FFK~FSRDVFLPK~ PSWGNHTIPF148R), 603.31 (242HFIEQGINVC@LCQSYAK^NM*GLYGE266R) and 981.30 (267VGAFTVVC@ KDAEEAK282R). Modified amino acid residues are indicated as in Table 4.
Fig. 4.
Reaction pathways of TFEC with mAAT. Aminoacrylate reacts with sulfhydryl groups of cysteine (Cys) residues resulting in the formation of lanthionine residues. The other product, tetrafluoroethanethiol, can react directly or after decomposition to difluorothioacetyl fluoride, with the ε-amino group of K residues. In the first case, loss of H2S results in the formation of a tetrafluoroethyl derivative. In the second case, loss of FH results in the formation of a thioacyl derivative.
mAAT is a homodimeric protein. Each subunit is structurally comprised of two domains, one small and one large, and one arm that extends from the large domain of one subunit and anchors in a hydrophobic pocket in the small domain of the other subunit. Each subunit has one catalytic site. The active sites are independent of each other and each contains one molecule of the coenzyme PLP in Schiff base bonding with the active site 254K. All of the modifications detected in this study are located in the large domain of mAAT. Significantly, four of the modifications are in residues located around the pocket where the arm of one subunit binds to the large domain of the other subunit (Fig. 5). These modifications may contribute to enzyme inactivation, since they may interfere with the conformational changes of the arm that occur during catalysis. However, the most likely cause of the irreversible inactivation of the enzyme is the modification of the 258K moiety. Modification of this active site K residue will render the protein unable to establish a Schiff base arrangement with the PLP coenzyme. It should be emphasized that the mechanisms of covalent inactivation of mAAT discussed in this section were obtained from in vitro studies. Thus far, as discussed in the next section, only thioacylation of mAAT (and five other mitochondrial proteins) has been detected in the kidneys of rats exposed to TFEC in vivo.
Fig. 5.
Three dimensional structure of mAAT indicating residues modified by covalent attachment of TFEC fragments. The relative positions of the modified residues are shown in green on the native structure of mAAT. The PLP coenzyme is shown in yellow forming a Schiff base with the Lys 258. The diagram was generated using the program VMD (Theoretical Biophysics Group, University of Illinois at Urbana-Champaign) and the X-ray structures of mAAT (pyridoxal form, Protein Data Bank entry code 1TAT) from chicken heart mitochondria.
The mechanism of TFEC-mediated cell injury and death: From toxicant channeling and mitochondrial disruption to de novo protective responses
Recent work has clearly expanded on the toxicological significance of the cysteine S-conjugate β-lyase activities. The biochemical lesions produced by newer examples of β-lyase substrates (e.g. cisplatin, Zhang et al., 2006; see below) may closely resemble the toxicological actions of traditional halogenated aliphatic substrates. Nonetheless, TFEC still remains the best characterized example of a compound that undergoes β-lyase-mediated bioactivation and has been worthy of investigation for several reasons. For example, TFEC is a major metabolic product of the gas tetrafluoroethylene (TFE), which is a potential human carcinogen according to both the IARC and the National Toxicology Program. TFE itself is representative of many other halogenated gases with broad industrial and clinical uses that may also be bioactivated by similar mechanisms. The tissue damage produced by TFEC follows the “Covalent Binding Hypothesis” whereby a reactive intermediate of metabolism (in this case, 2,2-difluorothioacetyl fluoride) covalently modifies (i.e. thioacylates) lysyl ε-amino groups of neighboring proteins to produce “unnatural” difluorothioamidyl-lysine (DFTAL) modified proteins (for an excellent review see Nelson and Pearson, 1990). Earlier work by Bruschi and colleagues has largely defined the in vivo target proteins of DFTAL formation and it has been a revelation that these proteins are, without exception, mitochondrial. The six DFTAL-modified mitochondrial proteins were identified as 1) HSP60, 2) mitHSP70, 3) mAAT, 4) mitochondrial isoform of aconitase (mitACON), the 5) E2k (dihydrolipoamide succinyl transferase) enzyme component of the KGDHC, and 6) the E3 (dihydrolipoamide dehydrogenase) enzyme component of KGDHC (and possibly also of the branched-chain keto acid dehydrogenase complex; BCDHC) (Bruschi et al., 1993, 1994, 1998; James et al., 2002). More recently, additional covalent modifications have been observed using purified mAAT and in vitro TFEC incubations (previous section; Fig. 4). These observations may provide exciting new avenues for future mechanistic studies in vivo.
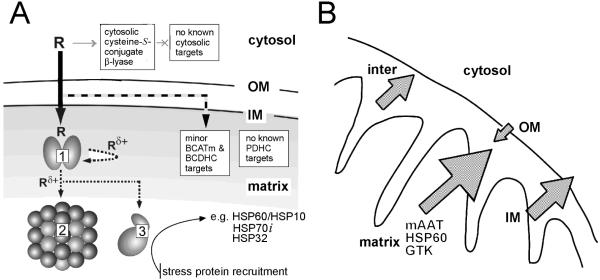
Our current knowledge of the initial events of TFEC-mediated intramitochondrial damage in vivo are summarized in Figure 6. Functional inactivation of a “metabolon” complex occurs when toxicants are atypically channeled between constituent proteins which normally function to channel metabolites (Srere, 1987; James et al., 2002). DFTAL-lysine formation and the disruption of protein conformation in metabolon targets and interactions signal intramitochondrial stress protein recruitment and other adaptive responses (Fig. 6A). Consequently, the close proximity of stress proteins results in their DFTAL modification and entrapment in partially ATP-reversible complexes (Bruschi et al., 1998). Furthermore, submitochondrial fractionation, flow cytometry and other biochemical studies confirm that mitochondria undergo a collapse of the inner mitochondrial membrane potential (Δψi), a permeability transition and cell death (Fig. 6B, James et al., 2002). Intriguingly, these events are preceded by a considerable increase in mitochondrial mass, indicating substantive cellular remodeling, and by a collapse in intramitochondrial NAD(P)H autofluorescence (Bruschi et al., unpublished data). Consistent with these findings, TFEC-mediated toxicity in a cell culture model [TGF transgenic murine hepatocytes (TAMH) cell line] have shown that over-expression of the antiapoptotic BCL2 family member, BCL-xL can very effectively protect against such induced mitochondrial pathologies (Ho et al., 2005). As a result, intramitochondrial pathophysiological processes serve to release matrix, inner membrane and intermembrane proteins to the periphery of the organelle and into the cytosol (Fig. 6B). Such releases are consistent with the numerous observations of traditionally inner mitochondrial proteins [and especially HSP60 and mitHSP70 (also known as “mortalin”)] in functional extramitochondrial complexes (e.g. the high Mr β-lyase; Cooper et al., 2001; see also Wadhwa et al., 2005). Most importantly, however, early mitochondrial releases likely function to signal de novo adaptive responses to injury (see below).
Fig. 6.
A. Mitochondrial toxicant channeling in vivo. TFEC (R) transport into the mitochondria results in conversion to the thioacylating agent (Rδ+) by β-lyases [e.g. mAAT (1), shown here as a homodimer]. Co-immunoprecipitation and biochemical studies confirm the close juxtaposition of other DFTAL target proteins – especially those of energy metabolism [KGDHC (2) and mitACON (3)]. Considerable evidence suggests that BCATm can form a metabolon with the branched-chain α-keto acid dehydrogenase complex (BCDHC) (Islam et al., 2010 and references quoted therein). Since BCATm can catalyze a β-lyase reaction with TFEC (Cooper et al., 2003) it is probable that subunits of the BCDHC are also inactivated by toxicant channeling. Other complexes not known to be associated with any aminotransferase/cysteine S-conjugate β-lyase activities [e.g. PDC] are not modified or inactivated by thioacylation. The curved arrow represents “self-thioacylation” of mAAT (syncatalytic inactivation). Positions of outer (OM) and inner (IM) mitochondrial membranes are indicated. Adapted from Cooper et al. (2002a). B. TFEC-induced mitochondrial pathophysiology. Submitochondrial fractionation studies confirm a unified movement of matrix, IM and inter-membrane space DFTAL-labeled and unmodified proteins to the periphery of rat renal mitochondria isolated from kidney tissue treated with TFEC. Such movements are consistent with a permeability transition and Δψi collapse as assessed by morphological changes, flow cytometry and biochemical inhibitor studies (see the text for details). Note that DFTAL covalent modifications of mAAT, KGDHC components, mitACON, HSP70, and HSP60 were detected in kidney mitochondria of rats exposed in vivo to TFEC by immunochemical methods. This finding does not, however, preclude the possibility of additional covalent modifications occurring in vivo as has been demonstrated for mAAT in vitro as shown in Fig. 4. Adapted from Bruschi et al. (1993).
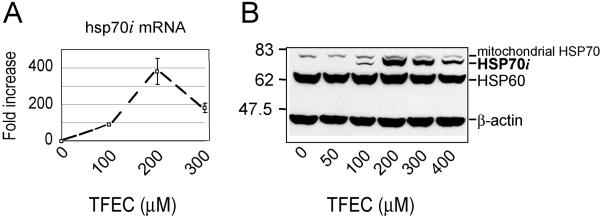
Early DFTAL protein modification events serve as the initiation of a cascade of cellular changes – part homeostatic and protective, part pathological. Genomic analyses confirm that mitochondrial permeability transitions induce de novo nuclear gene transcriptions, which facilitate mitochondrial and ER functions and a broader stress response (Ho et al., 2006). The inducible cytosolic HSP70 isoform (HSP70i) is a good example, but not the most prominent, of the many protective stress proteins preferentially upregulated in response to TFEC exposure (Fig. 7). The in vivo molecular events following TFEC exposure have been accurately modeled in vitro using TAMH cells (James et al., 2002). Following even moderate TFEC exposure in vitro HSP70i mRNA levels are markedly induced by nearly 400 fold (Fig. 7A) and the corresponding HSP70i protein levels in these cells are also substantially increased (Fig. 7B). The processes of interorganelle communications that function to link mammalian intramitochondrial perturbations, which in the case of DFTAL formation is both spatially and temporally restricted, to a multifaceted cellular response is currently unresolved.
Fig. 7.
De novo synthesis of stress responsive protein cytosolic HSP70i following TFEC exposure (100, 200 or 300 μM; 4h) to TAMH cells in cell culture. A. Quantitative real-time PCR determination of hsp70i mRNA with gene-specific primers. Data are represented as the fluorescence yield from successive PCR reaction cycles with the fold-induction indicated to the right (maximum of ≈ 400×). Data are the mean (± s.d.) from 3 independent experiments (n = 3-6 replicate plates per experiment). B. New synthesis of HSP70i protein also in TAMH cell cultures in vitro which paralleled hsp70i mRNA induction. Migration of Mr standards are shown to the left. Original and some additional (unpublished) data are from the study of Ho et al. (2006).
Role of cysteine S-conjugate β-lyases in drug metabolism
Methazolamide
The carbonic anhydrase inhibitor methazolamide is metabolized through the mercapturate pathway to the cysteine S-conjugate, which is a substrate of cysteine S-conjugate β-lyase(s) in bovine kidney and liver homogenates (Kishida et al., 2001). The β-elimination reaction may account for the binding of a metabolite of methazolamide to macromolecules in eye tissues and for the specific ocular toxicity (Kishida et al., 2001).
Cisplatin
Cisplatin is used to treat germ cell tumors, ovarian cancer, head and neck tumors and as a radiation sensitizer for cervical cancer. Unfortunately, it is ototoxic and renotoxic to proximal tubule cells (reviewed in [Zhang and Hanigan, 2003]). This limits its effectiveness particularly during tumor recurrence. DNA damage is the primary mechanism by which cisplatin kills tumor and other dividing cells. However, the renal proximal tubule cells are well-differentiated, non-dividing cells that are not killed by other DNA-damaging agents (Hanigan and Devarajan, 2003). Evidence has been presented by Hanigan and colleagues that damage to kidney cells is due to conversion of cisplatin to its glutathione S-conjugate and subsequently to its cysteine S-conjugate. The cysteine S-conjugate is then bioactivated by cysteine S-conjugate β-lyase(s) to generate a fragment containing a Pt-SH moiety (Eq. (2)). This Pt-SH fragment is proposed to react with macromolecules at thiophilic centers (Zhang and Hanigan, 2003; Hanigan and Devarajan, 2003; Zhang et al., 2006; Townsend et al., 2009).
| (2) |
In kidney cells, cisplatin is a mitochondrial toxicant. After mice were treated with cisplatin, kidney mitochondria proteins were more platinated than cytosolic proteins (Zhang et al., 2006). In mice pretreated with aminooxyacetate (AOA), an inhibitor of PLP enzymes, platination of renal proteins was decreased in the mitochondrial fraction, but not in the cytosolic fraction. The specific activities of mAAT, aconitase, and KGDHC were decreased in LLC-PK1 cells treated with cisplatin (Zhang et al., 2006). The specific activity of KGDHC was decreased even further in cisplatin-treated LLC-PK1 cells overexpressing mAAT. The data are consistent with the hypotheses that a) the cisplatin cysteine S-conjugate is a β-lyase substrate of mAAT, b) the released Pt-SH fragment reacts with proteins in kidney mitochondria including KGDHC, and c) the vulnerability of KGDHC may be another example of toxicant channeling from the active site of mAAT.
Busulfan
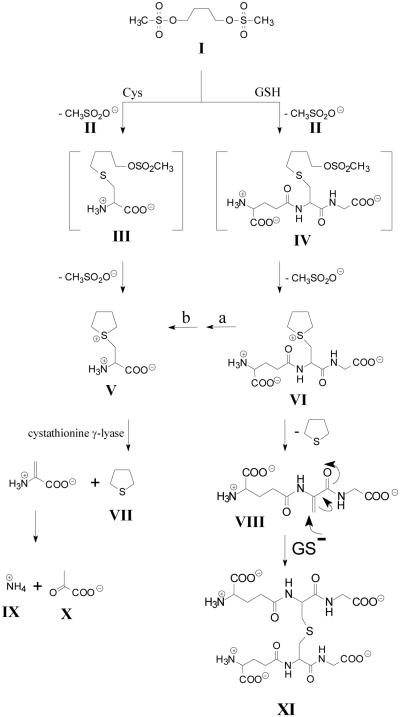
Busulfan is a bifunctional alkylating agent used for the treatment of hematological and other malignancies prior to stem cell transplantation (e.g. Iwamoto et al., 2004). Busulfan is converted to a glutathione S-conjugate [L-γ-glutamyl-β-(S-tetrahydrothiophenium)-L-alanylglycine; γ-E-THT-AG] by direct interaction with GSH (Ritter et al., 1999) and enzymatic catalysis by GSTs, especially GST A1-1 (Ritter et al., 1999, 2002; Czerwinski et al., 1996; Gibbs et al., 1996). The busulfan-glutathione adduct undergoes a base-catalyzed β-elimination reaction yielding tetrahydrothiophene (THT) (Fig. 8) (Roberts and Warwick, 1961). Oxidation products of THT make up the majority of identified busulfan metabolites.
Fig. 8.
Proposed mechanism for the metabolic conversion of busulfan to tetrahydrothiophene (THT) and to a glutathione adduct containing a lanthionine bridge. Facile elimination of a methanesulfonyl (II) group from busulfan (I) occurs by nucleophilic attack of cysteine (Cys) or glutathione (GSH), resulting in the formation of thioether conjugates [cysteine S-conjugate (III) and glutathione S-conjugate (IV), respectively]. Conversion of I to IV may occur non-enzymatically or may be accelerated by various GSTs. Structures III and IV spontaneously undergo elimination/ring closure to generate sulfonium conjugates [cysteine S-conjugate (V) and glutathione S-conjugate (VI), respectively]. Structure VI is converted to V by the action of γ-glutamyltransferase (a) and dipeptidases (b), both of which possess broad specificities. V undergoes a facile β-elimination reaction to yield THT (VII), ammonium (IX) and pyruvate (X), which may be catalyzed by OH-, PLP or several PLP-containing enzymes. THT (VII) may also be generated from γ-E-THT-AG (VI) by a non-enzymatic β-elimination reaction that results in the formation of γ-glutamyldehydroalanylglycine (EDAG) (VIII). Note that the sulfur in THT is not derived from busulfan, but rather from the sulfur of GSH. VIII contains an activated vinyl group and spontaneously undergoes a Michael reaction with GSH to form structure XI that contains a lanthionine bridge. Modified from Cooper et al. (2008b) and Younis et al. (2008)
An enzyme-catalyzed β-elimination of THT from a sulfonium cysteine S-conjugate has also been proposed as part of a drug metabolism pathway of busulfan (Fig. 8) (Cooper et al., 2008b). The sulfonium metabolite of busulfan [β-(S-tetrahydrothiophenium)-L-alanine, THT-A; Fig. 8, structure V] is a cysteine S-conjugate. Catalyzed by base, THT-A undergoes a non-enzymatic β-elimination reaction to yield THT, pyruvate and ammonia. THT-A contains such a good leaving group that this reaction occurs spontaneously even at pH 7.4. This reaction is, however, accelerated by a) rat liver, kidney and brain homogenates, b) isolated rat liver mitochondria, and c) PLP (Cooper et al., 2008b). Evidence was presented that several PLP-dependent enzymes can catalyze this reaction in rat tissues. One such enzyme in rat liver cytosol was identified as cystathionine γ-lyase. Evidently, enzyme-catalyzed β-elimination represents an alternate drug metabolism route for intermediates in the mercapturate pathway for drugs such methazolamide and busulfan.
The β-elimination of THT from the glutathione S-conjugate of busulfan (γ-E-THT-AG; Fig. 8, VI) yields a dehydroalanyl analogue of glutathione (γ-glutamyldehydroalanylglycine, EdAG; Fig.7, VIII). This reaction occurs readily in vitro at physiological pH and temperature (Younis et al., 2008). EdAG was identified as a metabolite of busulfan in a human liver cytosol fraction. EdAG condenses with GSH in a Michael addition reaction to produce a lanthionine-containing thioether (GSG), which is a non-reducible analog of glutathione disulfide (GSSG). EdAG was less cytotoxic than busulfan to C6 rat glioma cells. GSH and EdAG were equally effective in displacing a glutathione S-transferase isozyme (human GSTA1-1) from a GSH-Agarose column. The finding of an electrophilic metabolite of GSH suggests that alteration of cellular GSH concentrations, irreversible non-reducible glutathionylation of proteins, and interference with GST function may contribute to the toxicity of busulfan. For a discussion of the biomedical implications of non-reducible glutathionylation see Younis et al. (2008).
Occurrence of cysteine S-conjugate β-lyases in non-mammalian organisms and roles in the biotransformation of natural products
In addition to the enzymes identified as cysteine S-conjugate β-lyases in mammals (Table 1), many such enzymes have been described in non-mammalian organisms. Unlike those cysteine S-conjugate β-lyases in mammals where the activity is a secondary side reaction, some of the enzymes described in these non-mammalian organisms may have a primary function as a β-lyase.
Bacteria
Gut bacteria are known to catalyze biotransformations of certain xenobiotics, including conversion to the corresponding glutathione S-conjugates (Bakke et al., 1982). Several studies have shown that many enteric bacteria contain cysteine S-conjugate β-lyase activity (Saari and Schultze, 1965; Suzuki et al., 1982; Larsen and Bakke, 1983; Larsen and Stevens, 1986; Larsen et al., 1983; Tomizawa et al., 1984; Bernström et al., 1989; Schwiertz et al., 2008). The fact that cysteine S-conjugate β-lyase activity is widespread in enteric bacteria and that a major portion of cysteine S-conjugates are excreted into the bile suggests that the gut flora may be important for the incorporation of sulfur into some xenobiotics (Larsen and Stevens, 1986). If the cysteine S-conjugate is a precursor of a reactive sulfur-containing fragment the possibility exists that the mucosal cells of the gut will be damaged. The likelihood that these bile-derived cysteine S-conjugates (or those generated in situ from the glutathione S-conjugate) damage intestinal cells has not, however, been extensively studied. Although the cysteine S-conjugate β-lyase reactions catalyzed by the enteric bacteria thus far investigated are probably side reactions of PLP enzymes involved in amino acid housekeeping, the β-lyase reaction may be of physiological relevance under certain circumstances. For example, a cysteine S-conjugate β-lyase isolated from the gut bacterium was shown to catalyze a β-elimination reaction with leukotriene E4 (a cysteine S-conjugate), a reaction that may be of physiological importance (Bernström et al., 1989).
Several species of enteric bacteria (Bacteroides distasonis, Bacteroides vulgatus, Enterococcus faecalis, Enterococcus faecium) exhibit high β-lyase activity toward Se-p-methoxybenzylselenocysteine, S-methylcysteine and Se-methylselenocysteine (Schwiertz et al., 2008). It was suggested that the β-lyase activity associated with human intestinal bacteria might play a key role in the bioactivation and chemopreventive activity of selenocysteine conjugates (Schwiertz et al., 2008). The chemopreventive properties of naturally occurring Se-methyl-L-selenocysteine are discussed below.
Bacteria are also responsible for some aspects of human body odor. The odor of human sweat may be enhanced by the action of skin bacteria. 3-Sulfanylhexan-1-ol, along with 2-methyl-3-sulfanylbutan-1-ol, 3-sulfanylpentan-1-ol and 3-methyl-3-sulfanylhexan-1-ol, have been identified as odiferous compounds in human sweat (Emter and Natsch, 2008; Natsch et al., 2004; Starkenmann et al., 2005). It was suggested that the non-odiferous precursors of the sulfanylalkanols are cysteine S-conjugates. In agreement with this hypothesis, Natsch et al. (2004) showed that cysteine S-conjugates of sulfanylalkanols are substrates of a cysteine S-conjugate β-lyase cloned from the skin bacterium Corynebacterium striatum Ax20 present in human axillary secretions. The enzyme responsible was identified as cystathionine β-lyase.
It has been suggested that each human being has a distinct odor profile (despite the use of soaps and deodorants!). Indeed, it is well known that dogs (whose olfactory prowess is generally tens of thousands of times greater than that of humans) can identify individual humans by odor profiles, and dogs are often used forensically to identify human scent clues. Since the range of sulfanyl (mercapto) compounds potentially available through β-lyase reactions on cysteine S-conjugates is presumably very large, each person’s odor profile may depend in large part on the types of S-conjugates excreted, the constituent skin bacterial flora, body area (e.g. armpits, genital area), and enzymatic repertoire in the skin bacteria (including enzymes of the mercapturate pathway and cysteine S-conjugate β-lyases). Whether machines can replace dogs in forensic analysis of human odor signatures remains to be determined. For a recent, entertaining discussion of this pungent subject see Amato (2009). One possibility for the design of deodorants for human use might be to include an inhibitor of bacterial cystathionine β-lyase or even a general PLP antagonist such as aminooxyacetate or canaline.
Fungi
Fungi are known to contain GST activity (Dowd and Sheehan, 1999) and cysteine S-conjugate β-lyase activity (Hafsah et al., 1987; Shimomura et al., 1992). For example, Mucor circinelloides (formerly M. javanicus) converts 2,4-dichloro-1-nitrobenzene to the corresponding glutathione S-conjugate, cysteine S-conjugate, and mercapturate as well as to 5-chloro-2-nitrobenzenethiol (Hafsah et al., 1987; Shimomura et al., 1992). Evidently, the role of fungi in transforming environmental xenobiotics through the mercapturate and β-lyase pathways and their possible role in bioremediation needs to be further evaluated. Given the complex biochemical pathways present in many fungi it seems probable that the mercapturate and cysteine S-conjugate β-lyase pathways will be found to contribute to the formation of complex mercaptans as has been described in other organisms (see below).
Helminths
Three species of parasitic helminths have been shown to contain cysteine S-conjugate β-lyase activity (Adcock et al., 1999). A cysteine S-conjugate β-lyase was purified from the tapeworm Moniezia expansa and shown to co-purify with an enzyme that exhibited aspartate aminotransferase activity (Adcock et al., 2000). The possible biological significance of this reaction to the parasite remains to be elucidated, but may represent a possible target for anti-helminthics (Adcock et al., 2000).
Plants
Recent work has established that a multitude of alkyl mercaptans impart organoleptic properties to plants. For example, 3-mercaptohexanol is an odor detected in passion fruit and in Sauvignon blanc wines (Wakabayashi et al., 2004). 3-Mercaptohexanol was suggested to be derived from the cysteine S-conjugate of E-2-hexenal (Wakabayashi et al., 2004). A β-lyase reaction with this compound will release 3-mercaptohexenal, which may be reduced to the corresponding alcohol. An alternative route may occur, namely reduction of the cysteine S-conjugate of E-2-hexenal to the cysteine S-conjugate of 2-hexenol, followed by a cysteine S-conjugate β-lyase-catalyzed elimination to yield directly 3-mercaptohexenol. Both pathways are feasible. A series of alkanoates of 3-methyl-3-sulfanylbutan-1-ol [(CH3)2C(SH)CH2CH2OC(O)R] and a series of alkanoates of 3-sulfanylhexan-1-ol [CH3CH2CH2CH(SH)CH2CH2OC(O)R] have recently been detected in the fruit peel of Poncilius trifiliata (a close relative of citrus) (Starkenmann et al., 2007). The authors suggested that the origin of these volatile sulfur-containing compounds is via β-lyase reactions on cysteine S-conjugates. The same group also reported the presence of 3-sulfanylhexanal and sulfanylhexan-1-ol in a member of the Polygonium family (Starkenmann et al., 2006) and eighteen sulfanyl (thiol) compounds in the plant Ruta chalepensis (Escher et al., 2006). These compounds also presumably arose through the action of a cysteine S-conjugate β-lyase. An interesting observation from this group is the finding that odorless cysteine S-conjugates such as S-3-(1-hexanol)-L-cysteine in wine, S-(1-propyl)-L-cysteine in onions and S-(2-heptyl)-L-cysteine in bell peppers are transformed into volatile thiols by mouth flora (Starkenmann et al., 2008). The authors noted that “the mouth acts as a reactor, adding another dimension to odor perception, and saliva modulates flavors by trapping free thiols”.
Alliinase (EC 4.4.1.4), a PLP-dependent enzyme found in garlic and other Allium vegetables (onion, shallots, leeks), catalyzes β-elimination reactions with various S-alk(en)yl-L-cysteine sulfoxides (references quoted in Musah et al., 2009). In the case of the cysteine S-conjugate sulfoxide, L-alliin, the eliminated species is allylsulfenic acid (Eq. (3)). Allylsulfenic acid is extremely reactive and forms the anhydride allicin (a thiosulfinate) even in an aqueous medium (Eq. (4)) (Cooper and Pinto, 2005 and references quoted therein). Allicin may be depicted as RS(O)SR and is regarded as a symmetrical thiosulfinate. However, condensation between two different alk(en)yl sulfenic acids is possible, in which case the resulting thiosulfinate is regarded as an asymmetrical thiosulfinate [RS(O)SR’].
| (3) |
| (4) |
Allicin reacts with cysteine to form a series of alkyl cysteine S-conjugates. Several of these cysteine S-conjugates are β-lyase substrates of cystathionine γ-lyase (Cooper and Pinto, 2005). In some cases the eliminated product is a persulfide, which may be a source of sulfane sulfur [one S attached to another S (S1) or positioned between two S atoms (S0)], contributing to some of the anti-cancer properties of garlic (Pinto et al., 2006).
A novel alliinase has recently been isolated and partially characterized from the root of Petiveria alliacea, a perennial shrub that grows in tropical areas of Africa, South America and Central America, and long used in folk medicine (Musa et al., 2009). Although the enzyme exhibits activity analogous to that observed in previously identified cysteine S-conjugate sulfoxide lyases (alliinases) from Allium plants its substrate specificity and enzyme structure are entirely different. The preferred substrates of P. alliacea alliinase are the naturally occurring sulfoxides, S-benzyl-L-cysteine sulfoxide (petiveriin) and S-2-hydroxyethyl-L-cysteine sulfoxide. [Note that since the α-carbon is in the L-configuration and the sulfoxide may be in either the S- or R-configuration these compounds exist in diastereoisomeric forms, named petiveriin A and B, and 2-hydroxythiin A and B, respectively]. P. alliacea alliinase mediates the formation of thiosulfinates from these substrates to produce symmetric sulfinates, namely S-benzyl phenylmethanethiosulfinate (petivericiin; eqs. (5) and (6)) and S-(2-hydroxyethyl)-2-hydroxyethanethiosulfinate, respectively (Musah et al., 2009). Formation of asymmetric thiosulfinates is possible when both petiveriin and 2-hydroxythiin are simultaneous substrates. Thus, P. alliacea alliinase has the potential of catalyzing the formation of symmetrical and asymmetrical sulfinates that contain an aromatic moiety. By contrast, alliinases from Allium plants thus far characterized catalyze the formation of symmetrical and asymmetrical thiosulfinates only from aliphatic cysteine S-conjugate sulfoxides, such as S-methyl-, S-propyl-, S-(1-propenyl)- and S-(2-propenyl)-L-cysteine sulfoxides (Musah et al., 2009).
| (5) |
| (6) |
In summary, the intrinsic value of many of the small molecular organosulfur compounds resides in their broad range of flavorant, aromatic, and therapeutic properties. Alterations in the organosulfur constituents of foods and wines can have a profound influence on qualities of taste and smell. In addition, several organosulfur constituents of plants have been shown to have biological activity that includes anticancer, immunostimulant, antiinflammatory, antimicrobial, and hypoglycemic activities. For example, following tissue damage to certain plants such as allium species and P. alliacea, a number of S-benzyl- and/or S-alk(en)yl cysteine sulfoxides are converted to thiosulfinates by the action of alliinases. In addition to inducing cell cycle arrest and apoptosis in cancer cells and acting as cardiovascular protectants, these compounds can induce detoxification enzymes, such as glutathione S-transferases and quinone reductases, as well as the glutathione synthesizing enzyme, γ-glutamylcysteine synthetase. The chemical diversity of organosulfur metabolites in the context of their biochemical and pharmacological mechanisms has been the topic of numerous reviews (e.g. Pinto et al., 2001; Nagini, 2008; Iciek et al., 2009).
Chemoprevention by naturally occurring selenocysteine Se-conjugates
Several naturally occurring selenocysteine Se-conjugates have been shown to be chemopreventive in experimental carcinogenesis (Ip et al., 1999, 2000, 2002; Zhu et al., 2000; Dong et al., 2003; Suzuki et al., 2007). These compounds, which are especially enriched in garlic and other seleniferous Allium species, include Se-methyl-L-selenocysteine, Se-propyl-L-selenocysteine, and Se-allyl-L-selenocysteine. It was suggested that part of the chemopreventive action of these selenocysteines is due to the action of selenocysteine Se-conjugate β-lyase activity (equation (7)).
| (7) |
In the rat, the selenocysteine Se-conjugate β-lyase activity is especially pronounced in liver and kidney (Ip et al., 1999). Given our findings that cystathionine γ-lyase can catalyze β-elimination reactions with S-allyl-L-cysteine and other similar cysteine S-conjugates (Cooper and Pinto, 2005) and that this enzyme is active in rat cytosol (less so in kidney cytosol), it is probable that a portion of the selenocysteine Se-conjugate β-lyase activity in rat liver and kidney homogenates is due to cystathionine γ-lyase. However, it is also likely that the activity is catalyzed by other PLP-enzymes, especially GTK. In this regard, Commandeur et al. (2000) have shown that a highly purified rat kidney GTK preparation catalyzes both transamination and β-elimination reactions with selenocysteine Se-conjugates, in most cases at least ten times more rapidly than with the corresponding cysteine S-conjugates. The authors suggested that greater reactivity of the selenocysteine Se-conjugates relative to the cysteine S-conjugates is due to the weaker C-Se bond than the C-S bond and to the greater lability of the α C-H bond in the selenocysteine Se-conjugates (Commandeur et al., 2000). See also [Wessjohann et al., 2007].
The chemopreventive activity of Se-methyl-L-selenocysteine has been extensively studied and assumed to be due in part to the formation of methylselenol (CH3SeH) catalyzed by β-lyase(s) (Ohta and Suzuki, 2008; Ip et al., 1999, 2000, 2002; Zhu et al., 2000; Dong et al., 2003; Suzuki et al., 2006, 2007; Tsuji et al., 2009). The methylselenol is extremely difficult to detect in vitro owing to its high vapor pressure and extremely low water solubility. However, its formation in vivo in the rat can be inferred from tracer studies with 76Se-labeled Se-methyl-L-selenocysteine in which the label is found in selenoprotein P (a protein unusually rich in selenocysteine), trimethylselenonium ion and selenosugars (Suzuki et al., 2006). The chemopreventive properties of Se-methylselenocysteine may be due in part to its alteration of redox properties of key proteins (Suzuki et al., 2007). Posttranslational modification of redox-sensitive signaling proteins has been suggested to occur through the action of the highly reactive methylselenol. Since many of these proteins harbor free sulfhydryl domains that may be targeted by methylselenol, it has been proposed that the antiproliferative and pro-apoptotic control over cancer cells observed by organoselenium is achieved through these control proteins within signal pathways (Gundimeda et al., 2008). However, we have suggested an alternative explanation for the efficacy of Se-methyl-L-selenocysteine as a chemopreventive agent. As noted above, this compound is a transaminase substrate of human GTK and the resulting α-keto acid is a potent histone deacetylase (HDAC) inhibitor (Nian et al., 2009; Lee et al., 2009). A review of the inherent chemopreventive properties of seleno α-keto acid metabolites in cell signaling pathways and HDAC inhibition can be found in this issue of the journal (Pinto et al., 2010).
Selenocysteine Se-conjugates and tellurocysteine Te-conjugates – potential drug delivery vehicles?
The possibility that selenocysteine Se-conjugates may be designed as useful prodrugs has been suggested by Vermeulen and colleagues (Andreadou et al., 1996a,b; Commandeur et al., 2000, 2001; Rooseboom et al., 2000, 2002a,b). This proof-of-concept has already been shown in the case of cysteine S-conjugates. Thus, Elfarra and colleagues showed that S-(6-purinyl)-L-cysteine and S-(guaninin-6-yl)-L-cysteine are excellent prodrugs of the anti-cancer drugs 6-mercaptopurine and 6-thioguanine, respectively, in rats (Elfarra and Hwang, 1993; Elfarra et al., 1995). The anticancer drugs were found to bioaccumulate in the rat kidney as a result in part of the high renal cysteine S-conjugate β-lyase activity toward the prodrugs. Given the much better leaving group propensity of RSe- versus RS- and the excellent characteristics of many selenocysteine Se-conjugates as β-lyase substrates it seems reasonable that selenocysteine Se-conjugates can be designed to act as delivery vehicles for chemopreventive selenols to kidney, liver and other organs with high cysteine S(Se)-conjugate β-lyase activity (Rooseboom et al., 2000, 2002a,b). Indeed, as discussed above, the naturally occurring selenocysteine Se-conjugate, Se-methyl-L-selenocysteine, has already been shown to be chemopreventive in cancer cell models and to readily undergo β-elimination reactions.
Rooseboom et al. have also synthesized one tellurocysteine Te-conjugate, namely Te-phenyl-L-tellurocysteine (Rosseboom et al., 2002c). Not unexpectedly, this compound was a β-lyase substrate of purified rat kidney GTK. The authors suggested that the tellurocysteine Te-conjugates might be an interesting novel class of prodrugs for the formation of biologically active tellurols.
Evidently, the possibility of designing selenocysteine Se-conjugates and tellurocysteine Te-conjugates as prodrugs of pharmacologically active selenols and tellurols, respectively, is an attractive area for future research.
Conclusion
Although much of the early work on cysteine S-conjugate β-lyases was related to the bioactivation of halogenated alkenes, recent studies have greatly expanded the scope of cysteine S-conjugate β-lyase reactions. Indeed, we show in this review that the cysteine S-conjugate β-lyase reaction is more important in the metabolism of chemotherapeutic agents and natural products than has hitherto been realized.
Acknowledgements
Part of the work cited from the authors’ laboratories was supported by NIH grants RO1 ES8421 (AJLC), CA111842 (JTP), GM51916 (SAB) National Institute of Justice Grant IJ-CX-K014 (PSC),University of Kansas Medical center ROV10525 (AA).
Abbreviations used
- AlaAT
alanine aminotransferase
- AGAT II
alanine-glyoxylate aminotransferase isoenzyme II
- BCATc
cytosolic branched-chain aminotransferase
- BCATm
mitochondrial branched-chain aminotransferase
- BCDHC
branched-chain α-keto acid dehydrogenase complex
- BTC
S-(2-benzothiazolyl)-L-cysteine
- cAAT
cytosolic aspartate aminotransferase
- DCVC
S-(1,2-dichlorovinyl)-L-cysteine
- DFTAL
difluorothioamidyl-lysine
- EDAG
γ-glutamyldehydroalanylglycine
- ESI-MS
electrospray ionization mass spectrometry
- GSH
glutathione
- GST
glutathione S-transferase
- GTK
glutamine transaminase K
- HSP60
heat shock protein 60 kDa
- HSP70i
heat shock protein 70 kDa (inducible isoform)
- Hsc70
cytosolic HSP70
- KAT I
kynurenine aminotransferase isoenzyme I
- KGDHC
α-ketoglutarate dehydrogenase complex
- mAAT
mitochondrial aspartate aminotransferase
- mitACON
mitochondrial aconitase
- mitHSP70
heat shock protein 70 kDa (mitochondrial isoform)
- mitGTK
mitochondrial GTK
- pmAAT
precursor to mAAT
- PDHC
pyruvate dehydrogenase complex
- PLP
pyridoxal 5′-phosphate
- PMP
pyridoxamine 5′-phosphate
- SAC
S-allyl-L-cysteine
- SAMC
S-allylmercapto-L-cysteine
- TCA cycle
tricarboxylic acid cycle
- TFA
trifluoroacetic acid
- TFEC
S-(1,1,2,2-tetrafluoroethyl)-L-cysteine
- THT
tetrahydrothiophene
- THT-A
β-(S-tetrahydrothiophenium)-L-alanine
References
- Abraham DG, Cooper AJL. Glutamine transaminase K and cysteine S-conjugate β-lyase activity stains. Anal Biochem. 1991;197:421–427. doi: 10.1016/0003-2697(91)90414-o. [DOI] [PubMed] [Google Scholar]
- Abraham DG, Cooper AJL. Cloning and expression of a rat kidney cytosolic glutamine transaminase K that has strong sequence homology to kynurenine-pyruvate aminotransferase. Arch Biochem Biophys. 1996;335:311–320. doi: 10.1006/abbi.1996.0512. [DOI] [PubMed] [Google Scholar]
- Abraham DG, Patel PP, Cooper AJL. Isolation from rat kidney of a high molecular weight cysteine S-conjugate β-lyase with activity toward leukotriene E4. J Biol Chem. 1995a;270:180–188. doi: 10.1074/jbc.270.1.180. [DOI] [PubMed] [Google Scholar]
- Abraham DG, Thomas RJ, Cooper AJL. Glutamine transaminase K is not a major cysteine S-conjugate β-lyase of rat kidney mitochondria: Evidence that a high-molecular-weight enzyme fulfills this role. Mol Pharmacol. 1995b;48:855–860. [PubMed] [Google Scholar]
- Adams B, Lowpetch K, Thorndycroft F, Whyte SM, Young DW. Stereochemistry of reactions of the inhibitor/substrates L- and D-chloroalanine with β-mercaptoethanol catalysed by L-aspartate aminotransferase and D-amino acid aminotransferase respectively. Org Biomol Med. 2005;3:3357–3364. doi: 10.1039/b508199h. [DOI] [PubMed] [Google Scholar]
- Adcock HJ, Brophy PM, Teesdale-Spittle PH, Bucknberry LD. Cysteine conjugate β-lyase in three species of parasitic helminth. Int J Parasitol. 1999;29:543–548. doi: 10.1016/s0020-7519(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Adcock HJ, Brophy PM, Teesdale-Spittle PH, Bucknberry LD. Purification and characterisation of a novel cysteine conjugate β-lyase from the tapeworm Moniezia expansa. Int J Parasitol. 2000;30:56–71. doi: 10.1016/s0020-7519(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Amato I. You smell. Chemical Engineering News. 2009;87:50–54. [Google Scholar]
- Anders MW. Glutathione-dependent bioactivation of haloalkanes and haloalkenes. Drug Metab Rev. 2004;36:583–594. doi: 10.1081/dmr-200033451. [DOI] [PubMed] [Google Scholar]
- Anders MW. Chemical toxicology of reactive intermediates formed by the glutathione-dependent bioactivation of halogen-containing compounds. Chem Res Toxicol. 2008;21:145–159. doi: 10.1021/tx700202w. [DOI] [PubMed] [Google Scholar]
- Anders MW, Lash L, Dekant W, Elfarra AA, Dohn DR. Biosynthesis and biotransformation of glutathione S-conjugates to toxic metabolites. Crit Rev Toxicol. 1988;18:311–341. doi: 10.3109/10408448809037470. [DOI] [PubMed] [Google Scholar]
- Anderson PM, Schultze MO. Cleavage of S-(1,2-dichlorovinyl)-L-cysteine by an enzyme of bovine origin. Arch Biochem Biophys. 1965;111:593–602. doi: 10.1016/0003-9861(65)90240-7. [DOI] [PubMed] [Google Scholar]
- Andreadou I, Menge WMPB, Commandeur JNM, Worthington EA, Vermeulen NPE. Synthesis of novel Se-substituted selenocysteine derivatives as potential kidney selective prodrugs of biologically active selenol compounds: evaluation of kinetics of β-elimination reactions in rat renal cytosol. J Med Chem. 1996a;39:2040–2046. doi: 10.1021/jm950750x. [DOI] [PubMed] [Google Scholar]
- Andreadou I, van de Water B, Commandeur JNM, Nagelkerke FJ, Vermeulen NPE. Comparative cytotoxicity of 14 novel selenocysteine Se-conjugates in rat renal proximal tubular cells. Toxicol Appl Pharmacol. 1996b;141:278–287. [PubMed] [Google Scholar]
- Artigues A, Iriarte A, Martinez-Carrion M. Identification of Hsc70 binding sites in mitochondrial aspartate aminotransferase. Arch Biochem Biophys. 2006;450:30–38. doi: 10.1016/j.abb.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Artigues A, Iriarte A, Martinez-Carrion M. Binding to chaperones allows import of a purified mitochondrial precursor into mitochondria. J Biol Chem. 2002;277:25047–25055. doi: 10.1074/jbc.M203474200. [DOI] [PubMed] [Google Scholar]
- Bakke JE, Bergman ÅL, Larsen GL. Metabolism of 2,4′,5-trichlorobiphenyl by the mercapturic acid pathway. Science. 1982;217:645–647. doi: 10.1126/science.6806905. [DOI] [PubMed] [Google Scholar]
- Bernström K, Larsen GL, Hammerström S. Metabolism of leukotriene E4 to 5-hydroxy-6-mercapto-7,9-trans-11,14-cis-eicosatetraenoic acid by microfloral cysteine conjugate β-lyase and rat cecum contents. Arch Biochem Biophys. 1989;275:531–539. doi: 10.1016/0003-9861(89)90399-8. [DOI] [PubMed] [Google Scholar]
- Bruschi SA, West KA, Crabb JW, Gupta RS, Stevens JL. Mitochondrial HSP60 (P1 protein) and HSP70-like protein (mortalin) are major targets for modification during S-(1,1,2,2-tetrafluoroethyl)-L-cysteine-induced toxicity. J Biol Chem. 1993;268:23157–23161. [PubMed] [Google Scholar]
- Bruschi SA, Crabb JW, Stevens JL. The E3 subunit of 2-oxoglutarate, branched-chain α-keto acid, and malate dehydrogenase are adducted during nephrotoxic cysteine-conjugate injury. Toxicologist. 1994;14:428. Abstract. [Google Scholar]
- Bruschi SA, Lindsay JG, Crabb JW. Mitochondrial stress protein recognition of inactivated dehydrogenases during mammalian cell death. Proc Natl Acad Sci USA. 1998;95:13413–13418. doi: 10.1073/pnas.95.23.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini D, Federici G, Bossa F, Granata F. The protective effect of thiosulfate upon the inactivation of aspartate aminotransferase by aminoacrylic-acid-producing substrates. Eur J Biochem. 1973;39:301–304. doi: 10.1111/j.1432-1033.1973.tb03127.x. [DOI] [PubMed] [Google Scholar]
- Chasseaud LF. Conjugation with glutathione and mercapturic acid excretion. In: Arias IM, Jakoby WB, editors. Glutathione: Metabolism and Function. Raven Press; New York: 1976. pp. 77–114. [Google Scholar]
- Chatfield DH, Hunter WH. The metabolism of acetamidothiazoles in the rat. 2-Acetamido-4-chloromethylthiazole. Biochem J. 1973;134:879–884. doi: 10.1042/bj1340879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Huber R, Messerschmidt A, Pohlenz HD, Laber B. Slow-binding inhibition of Escherichia coli cystathionine beta-lyase by L-aminoethoxyvinylglycine: a kinetic and X-ray study. Biochemistry. 1997;36:12633–12643. doi: 10.1021/bi970630m. [DOI] [PubMed] [Google Scholar]
- Colucci DF, Buyske DA. The biotransformation of a sulfonamide to a mercaptan and to mercapturic acid and glucuronide conjugates. Biochem Pharmacol. 1965;14:457–466. doi: 10.1016/0006-2952(65)90218-2. [DOI] [PubMed] [Google Scholar]
- Commandeur JNM, Andreadou I, Rooseboom M, Out M, de Leur LJ, Groot E, Vermeulen NPE. Bioactivation of selenocysteine Se-conjugates by a highly purified rat renal cysteine conjugate β-lyase/glutamine transaminase K. J Pharmacol Exp Ther. 2000;294:753–761. [PubMed] [Google Scholar]
- Commandeur JNM, Rooseboom M, Vermeulen NPE. Chemistry and biological activity of novel selenium-containing compounds. Adv Exp Med Biol. 2001;500:105–112. doi: 10.1007/978-1-4615-0667-6_11. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT. Aminotransferase, L-amino acid oxidase and β-lyase reactions involving L-cysteine S-conjugates found in allium extracts. Relevance to biological activity? Biochem Pharmacol. 2005;69:209–220. doi: 10.1016/j.bcp.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT. Cysteine S-conjugate β-lyases. Amino Acids. 2006;30:1–15. doi: 10.1007/s00726-005-0243-4. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT. Role of cysteine S-conjugate β-lyases in the bioactivation of renal toxicants. In: Elfarra AA, editor. Biotechnology: Pharmaceutical Aspects. Advances in Bioactivation Research. New York Springer; New York: 2008. pp. 323–346. [Google Scholar]
- Cooper AJL, Hanigan MH. Enzymes involved in processing of glutathione conjugates. In: McQueen CA, Guengerich FP, editors. Comprehensive Toxicology 2nd Edition: Volume 4. Biotransformations. Elsevier Press; Oxford: 2010. in press. [Google Scholar]
- Cooper AJL, Wang J, Gartner CA, Bruschi SA. Co-purification of mitochondrial HSP70 and mature protein disulfide isomerase with a functional rat kidney high-Mr cysteine S-conjugate β-lyase. Biochem Pharmacol. 2001;62:1345–1353. doi: 10.1016/s0006-2952(01)00802-4. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Bruschi SA, Anders MW. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem Pharmacol. 2002a;64:553–564. doi: 10.1016/s0006-2952(02)01076-6. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Bruschi SA, Iriarte A, Martinez-Carrion M. Mitochondrial aspartate aminotransferase catalyses cysteine S-conjugate β-lyase reactions. Biochem J. 2002b;368:253–261. doi: 10.1042/BJ20020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Bruschi SA, Conway M, Hutson SM. Human mitochondrial and cytosolic branched-chain aminotransferases are cysteine S-conjugate β-lyases, but turnover leads to inactivation. Biochem Pharmacol. 2003;65:181–192. doi: 10.1016/s0006-2952(02)01513-7. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Pinto JT, Krasnikov BF, Niatsetskaya ZV, Han Q, Li J, Vauzour D, Spencer JPE. Substrate specificity of human glutamine transaminase K as an aminotransferase and as a cysteine S-conjugate β-lyase. Arch Biochem Biophys. 2008a;474:72–81. doi: 10.1016/j.abb.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Younis IR, Niatsetskaya ZV, Krasnikov BF, Pinto JT, Petros WP, Callery PS. Metabolism of the cysteine S-conjugate of busulfan involves a β-lyase reaction. Drug Metab Dispos. 2008b;36:1546–1552. doi: 10.1124/dmd.108.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases α, μ, and π. Drug Metab Dispos. 1996;24:1015–1019. [PubMed] [Google Scholar]
- Dekant W. Biosynthesis of toxic glutathione conjugates from halogenated alkenes. Toxicol Lett. 2003;144:49–54. doi: 10.1016/s0378-4274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Dekant W, Vamvakas S, Anders MW. Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: Cysteine conjugate β-lyase pathway. Adv Pharmacol. 1994;27:115–162. doi: 10.1016/s1054-3589(08)61031-5. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang H, Hawthorn L, Ganther HE, Ip C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Cancer Res. 2003;63:52–59. [PubMed] [Google Scholar]
- Dowd CA, Sheehan D. Variable expression of glutathione S-transferase isoenzymes in the fungus, Mucor circinelloides. FEMS Microbiol Lett. 1999;170:13–17. doi: 10.1111/j.1574-6968.1999.tb13349.x. [DOI] [PubMed] [Google Scholar]
- Escher S, Niclass Y, van de Waal M, Starkenmann C. Combinatorial synthesis by nature: volatile organic sulfur-containing constituents of Ruta chalepensis L. Chem Biodivers. 2006;3:943–957. doi: 10.1002/cbdv.200690103. [DOI] [PubMed] [Google Scholar]
- Elfarra AA, Hwang IY. In vivo metabolites of S-(2-benzothiazolyl)-L-cysteine as markers of in vivo cysteine conjugate β-lyase and thiol glucuronosyl transferase activities. Drug Metab Dispos. 1990;18:917–922. [PubMed] [Google Scholar]
- Elfarra AA, Hwang IY. Targeting of 6-mercaptopurine to the kidneys. Metabolism and kidney-selectivity of S-(6-purinyl)-L-cysteine analogs in rats. Drug Metab Dispos. 1993;21:841–845. [PubMed] [Google Scholar]
- Elfarra AA, Duescher RJ, Hwang IY, Sicuri AR, Nelson JA. Targeting 6-thioguanine to the kidney with S-(guanin-6-yl)-L-cysteine. J Pharmacol Exp Ther. 1995;274:1298–1304. [PubMed] [Google Scholar]
- Emter R, Natsch A. The sequential action of a dipeptidase and a β-lyase is required for the release of the human body odorant 3-methyl-3-sulfanylhexan-1-ol from a secreted Cys-Gly-(S) conjugate by Corynebacteria. J Biol Chem. 2008;25:20645–20652. doi: 10.1074/jbc.M800730200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JK, Fischer B, Grossmann J, Maccoss MJ. A fast SEQUEST cross correlation algorithm. J Proteome Res. 2008;7:4598–4602. doi: 10.1021/pr800420s. [DOI] [PubMed] [Google Scholar]
- Escher S, Niclass Y, van de Waal M, Starkenmann C. Combinatorial synthesis by nature: volatile organic sulfur-containing constituents of Ruta chalepensis L. Chem Biodivers. 2006;3:943–957. doi: 10.1002/cbdv.200690103. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Hayden PJ, Bruschi SA, Dulik DM, Yang Y, Ward AJ, Stevens JL. Formation, characterization, and immunoreactivity of lysine thioamide adducts from fluorinated nephrotoxic cysteine conjugates in vitro and in vivo. Chem Res Toxicol. 1993;6:223–230. doi: 10.1021/tx00032a012. [DOI] [PubMed] [Google Scholar]
- Gibbs JP, Czerwinski M, Slattery JT. Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res. 1996;56:3678–3681. [PubMed] [Google Scholar]
- Gundimeda U, Schiffman JE, Chhabra D, Wong J, Wu A, Gopalakrishna R. Locally generated methylseleninic acid induces specific inactivation of protein kinase C isoenzymes: relevance to selenium-induced apoptosis in prostate cancer cells. J Biol Chem. 2008;283:34519–34531. doi: 10.1074/jbc.M807007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafsah Z, Tahara S, Mizutani J. Microbiol metabolism of chlorinated nitrobenzenes. IV. Metabolic pathways of 2,4-dichloro-1-nitrobenzene in Mucor javanicus. J Pesticide Sci. 1987;12:617–625. [Google Scholar]
- Gundimeda U, Schiffman JE, Chhabra D, Wong J, Wu A, Gopalakrishna R. Locally generated methylseleninic acid induces specific inactivation of protein kinase C isoenzymes: relevance to selenium-induced apoptosis in prostate cancer cells. J Biol Chem. 2008;283:34519–34531. doi: 10.1074/jbc.M807007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- Harris JW, Dekant W, Anders MW. In vivo detection and characterization of protein adducts resulting from bioactivation of haloethene cysteine S-conjugates by 19F NMR: chlorotrifluoroethene and tetrafluoroethene. Chem Res Toxicol. 1992;5:34–41. doi: 10.1021/tx00025a007. [DOI] [PubMed] [Google Scholar]
- Hayden PJ, Stevens JL. Cysteine conjugate toxicity, metabolism and binding to macromolecules in isolated rat kidney mitochondria. Mol Pharmacol. 1990;37:468–476. [PubMed] [Google Scholar]
- Hayden PJ, Ichimura T, McCann DJ, Pohl LR, Stevens JL. Detection of cysteine conjugate metabolite adduct formation with specific mitochondrial proteins using antibodies raised against halothane metabolite adducts. J Biol Chem. 1991;266:18415–18418. [PubMed] [Google Scholar]
- Hinchman CA, Ballatori N. Glutathione conjugation and conversion to mercapturic acids can occur as an intrahepatic process. J Toxicol Environ Health. 1994;41:387–409. doi: 10.1080/15287399409531852. [DOI] [PubMed] [Google Scholar]
- Ho HK, Hu Z-H, Tzung S-P, Hockenbery DM, Fausto N, Nelson SD, Bruschi SA. BCL-xL overexpression effectively protects against tetrafluoroethylcysteine-induced intramitochondrial damage and cell death. Biochem Pharmacol. 2005;69:147–157. doi: 10.1016/j.bcp.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Ho HK, Jia Y, Coe KJ, Gao Q, Doneanu CE, Hu ZH, Bammler TK, Beyer RP, Fausto N, Bruschi SA, Nelson SD. Cytosolic heat shock proteins and heme oxygenase-1 are preferentially induced in response to specific and localized intramitochondrial damage by tetrafluoroethylcysteine. Biochem Pharmacol. 2006;72:80–90. doi: 10.1016/j.bcp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Iciek M, Kwiecień I, Włodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen. 2009;50:247–265. doi: 10.1002/em.20474. [DOI] [PubMed] [Google Scholar]
- Ip C, Zhu Z, Thompson HJ, Lisk D, Ganther HE. Chemoprevention of mammary cancer with Se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Res. 1999;19:2875–2880. [PubMed] [Google Scholar]
- Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- Ip C, Dong Y, Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002;21:281–289. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- Islam MM, Nautiyal M, Wynn RM, Mobley JA, Chunag DT, Hutson SM. Branched-chain amino acid metabolon. Interaction of glutamate dehydrogenase with mitochondrial branched-chain aminotransferase (BCATm) J Biol Chem. 2010;285:265–276. doi: 10.1074/jbc.M109.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Hiraku Y, Oikawa S, Mizutani H, Kojima M, Kawanishi S. DNA intrastrand cross-link at the 5′-GA-3′ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004;95:454–458. doi: 10.1111/j.1349-7006.2004.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby WB, Stevens J. Cysteine conjugate lyase and the thiomethyl shunt. Biochem Soc Trans. 1984;12:33–35. doi: 10.1042/bst0120033. [DOI] [PubMed] [Google Scholar]
- James EA, Gygi SP, Adams ML, Pierce RH, Fausto N, Aebersold RH, Nelson SD, Bruschi SA. Mitochondrial aconitase modification, functional inhibition, and evidence for a supramolecular complex of the TCA cycle by the renal toxicant S-(1,1,2,2-tetrafluoroethyl)-L-cysteine. Biochemistry. 2002;41:6789–6797. doi: 10.1021/bi020038j. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cha SH, Abraham DG, Cooper AJL, Endou H. Intranephron distribution of cysteine-S-conjugate β-lyase activity and implication for hexachloro-1,3-butadiene-induced nephrotoxicity in rats. Arch Toxicol. 1997;71:131–141. doi: 10.1007/s002040050367. [DOI] [PubMed] [Google Scholar]
- Kishida K, Saida N, Yamamura N, Iwai Y, Sasabe T. Cysteine conjugate of methazolamide is metabolized by β-lyase. J Pharm Sci. 2001;90:224–233. doi: 10.1002/1520-6017(200102)90:2<224::aid-jps13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Koob M, Dekant W. Bioactivation of xenobiotics by formation of toxic glutathione conjugates. Chem Biol Interact. 1991;77:107–136. doi: 10.1016/0009-2797(91)90068-i. [DOI] [PubMed] [Google Scholar]
- Larsen GL, Bakke JE. Metabolism of mercapturic acid-pathway metabolites of 2-chloro-N-isopropylacetanilide (propachlor) by gastrointestinal bacteria. Xenobiotica. 1983;13:115–126. doi: 10.3109/00498258309052245. [DOI] [PubMed] [Google Scholar]
- Larsen GL, Stevens JL. Cysteine conjugate β-lyase in the gastrointestinal bacterium Eubacterium limosum. Mol Pharmacol. 1986;29:97–103. [PubMed] [Google Scholar]
- Larsen GL, Larson JD, Gustafsson J-Å. Cysteine conjugate β-lyase in the gastrointestinal bacterium Fusobacterium necrophorum. Xenobiotica. 1983;13:689–700. doi: 10.3109/00498258309052230. [DOI] [PubMed] [Google Scholar]
- Lee J-I, Nian H, Cooper AJL, Sinha R, Dai J, Bisson WH, Dashwood RH, Pinto JT. α-Keto acid metabolites of naturally occurring organoselenium compounds as inhibitors of histone deacetylase in human prostate cancer cells. Cancer Prev Res. 2009;2:683–693. doi: 10.1158/1940-6207.CAPR-09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly JR, Jr, Iriarte A, Martinez-Carrion M. Homologous proteins with different affinities for groEL. The refolding of the aspartate aminotransferase isozymes at varying temperatures. J Biol Chem. 1995;270:1138–1148. doi: 10.1074/jbc.270.3.1138. [DOI] [PubMed] [Google Scholar]
- Morino Y, Osman AM, Okamoto M. Formate-induced labeling of the active site of aspartate aminotransferase by β-chloro-L-alanine. J Biol Chem. 1974;249:6684–6692. [PubMed] [Google Scholar]
- Mosca M, Cozzi L, Breton J, Speciale C, Okuno E, Schwarcz R, Benatti L. Molecular cloning of rat kynurenine aminotransferase: identity with glutamine transaminase K. FEBS Lett. 1994;353:21–24. doi: 10.1016/0014-5793(94)01003-x. [DOI] [PubMed] [Google Scholar]
- Musah RA, He Q, Kubec R, Jadhav A. Studies of a novel cysteine sulfoxide lyase from Petiveria alliacea: The first heteromeric alliinase. Plant Physiol. 2009;151:1304–1316. doi: 10.1104/pp.109.142430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke JF, Boogaard PJ. Nephrotoxicity of halogenated alkenyl cysteine-S-conjugates. Life Sci. 1991;49:1769–1776. doi: 10.1016/0024-3205(91)90477-s. [DOI] [PubMed] [Google Scholar]
- Nagini S. Cancer chemoprevention by garlic and its organosulfur compounds – panacea or promise? Anticancer Agents Med Chem. 2008;8:313–321. doi: 10.2174/187152008783961879. [DOI] [PubMed] [Google Scholar]
- Natsch A, Schmid J, Flachsmann F. Identification of odiferous sulfanylalkanols in human axilla secretions and their formation through cleavage of cysteine precursors by a C-S lyase isolated from axilla bacteria. Chem Biodiv. 2004;1:1058–1072. doi: 10.1002/cbdv.200490079. [DOI] [PubMed] [Google Scholar]
- Nelson SD, Pearson PG. Covalent and noncovalent interactions in acute lethal cell injury caused by chemicals. Annu Rev Pharmacol Toxicol. 1990;30:169–195. doi: 10.1146/annurev.pa.30.040190.001125. [DOI] [PubMed] [Google Scholar]
- Nian H, Bisson WH, Dashwood WM, Pinto JT, Dashwood RH. α-Keto acid metabolites of organoselenium compounds inhibit histone deacetylase activity in human colon cancer cells. Carcinogenesis. 2009;30:1416–1423. doi: 10.1093/carcin/bgp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Suzuki KT. Methylation and demethylation of intermediates selenide and methylselenol in the metabolism of selenium. Toxicol Appl Pharmacol. 2008;226:169–177. doi: 10.1016/j.taap.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Lapsia S, Shah A, Santiago H, Kim G. Antiproliferative effects of garlic-derived and other allium related compounds. Adv Exp Med Biol. 2001;492:83–106. doi: 10.1007/978-1-4615-1283-7_8. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Krasnikov BF, Cooper AJL. Redox-sensitive proteins are potential targets of garlic derived mercaptocysteine derivatives. J Nutr. 2006;136:S835–S841. doi: 10.1093/jn/136.3.835S. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Lee JI, Sinha, Cooper AJL. Chemopreventive mechanisms of α-keto acid metabolites of naturally occurring organoselenium compounds. Amino Acids. 2010 doi: 10.1007/s00726-010-0578-3. this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Schofield MA, MacFarlane M, Lock EA, King LJ, Gibson GG, Goldfarb PS. Isolation and expression of a cDNA coding for rat kidney cytosolic cysteine conjugate β-lyase. Mol Pharmacol. 1993;43:660–665. [PubMed] [Google Scholar]
- Ritter CA, Bohnenstengel F, Hofmann U, Kroemer HK, Sperker B. Determination of tetrahydrothiophene formation as a probe of in vitro busulfan metabolism by human glutathione S-transferase A1-1: use of highly sensitive gas chromatographic-mass spectrometric method. J Chromatog B Biomed Sci Appl. 1999;730:25–31. doi: 10.1016/s0378-4347(99)00170-x. [DOI] [PubMed] [Google Scholar]
- Ritter CA, Sperker B, Grube M, Dressel D, Kunert-Keil C, Kroemer HK. Overexpression of glutathione S-transferase A1-1 in ECV 304 cells protects against busulfan mediated G2-arrest and induces tissue factor expression. Br J Pharmacol. 2002;137:1100–1106. doi: 10.1038/sj.bjp.0704972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JJ, Warwick GP. The mode of action of alkylating agents. III. The formation of 3-hydroxytetrahydrothiophene-1:1-dioxide from 1:4-dimethanesulphonyloxybutane (myleran), S-β-L-alanyl-tetrahydrothiophenium mesylate, tetrahydrothiophene and tetrahydrothiophene-1:1-dioxide in the rat, rabbit and mouse. Biochem Pharmacol. 1961;6:217–227. [Google Scholar]
- Rooseboom M, Vermeulen NPE, Andreadou I, Commandeur JNM. Evaluation of the kinetics of β-elimination reactions of selenocysteine Se-conjugates in human renal cytosol: possible implications for the use as kidney selective prodrugs. J Pharmacol Exp Ther. 2000;294:762–769. [PubMed] [Google Scholar]
- Rooseboom M, Vermeulen NPE, Groot EJ, Commandeur JNM. Tissue distribution of cytosolic β-elimination reactions of selenocysteine Se-conjugates in rat and human. Chem Biol Interact. 2002a;140:243–264. doi: 10.1016/s0009-2797(02)00039-x. [DOI] [PubMed] [Google Scholar]
- Rooseboom M, Vermeulen NPE, Commandeur JNM. Enzymatic pathways of β elimination of chemopreventive selenocysteine Se conjugates. Methods Enzymol. 2002b;348:191–200. doi: 10.1016/s0076-6879(02)48638-7. [DOI] [PubMed] [Google Scholar]
- Rooseboom M, Vermeulen NPE, Durgut F, Commandeur JNM. Comparative study on the bioactivation, mechanisms and cytotoxicity of Te-phenyl-L-tellurocysteine, Se-phenyl-L-selenocysteine and S-phenyl-L-cysteine. Chem Res Toxicol. 2002c;15:1610–1618. doi: 10.1021/tx020034f. [DOI] [PubMed] [Google Scholar]
- Rossi F, Han Q, Li J, Li J, Rizzi M. Crystal structure of human kynurenine aminotransferase I. J Biol Chem. 2004;279:50214–50220. doi: 10.1074/jbc.M409291200. [DOI] [PubMed] [Google Scholar]
- Saari JC, Schultze MO. Cleavage of S-(1,2-dichlorovinyl)-L-cysteine by Escherichia coli B. Arch Biochem Biophys. 1965;109:595–602. doi: 10.1016/0003-9861(65)90405-4. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Deubel S, Birringer M. Bioactivation of selenocysteine derivatives by β-lyases present in common gastrointestinal bacterial species. Int J Vitam Nutr Res. 2008;78:169–174. doi: 10.1024/0300-9831.78.45.169. [DOI] [PubMed] [Google Scholar]
- Scott CS, Chiu WA. Trichloroethylene cancer epidemiology: a consideration of select issues. Environ Health Perspect. 2006;114:1471–1478. doi: 10.1289/ehp.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura N, Honma M, Chiba S, Tahara S, Mizutani J. Cysteine-conjugate β-lyase from Mucor javanicus. Biosci Biotech Biochem. 1992;56:963–964. doi: 10.1271/bbb.56.963. [DOI] [PubMed] [Google Scholar]
- Silbernagl S, Heuner A. Renal transport of mercapturic acids and their precursors, the S-conjugates of glutathione and cysteine. In: Anders MW, Dekant W, Henschler D, Oberleithner H, Silbernagl S, editors. Renal Disposition and Nephrotoxicity of Xenobiotics. Academic Press, Inc.; San Diego: 1993. pp. 135–154. [Google Scholar]
- Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Starkenmann C, Niclass Y, Troccaz M, Clark AJ. Identification of the precursor of (S)-3-methyl-3-sulfanylhexan-1-ol, the sulfury malodour of human axilla sweat. Chem Biodivers. 2005;2:705–716. doi: 10.1002/cbdv.200590048. [DOI] [PubMed] [Google Scholar]
- Starkenmann C, Luca L, Niclass Y, Praz E, Roguet D. Comparison of volatile constituents of Persicaria odorata (Lour.) Soják (Polygonum odoratum Lour.) and Persicaria hydropiper L. Spach (Polygonum hydropiper L.) J Agric Food Chem. 2006;54:3067–3071. doi: 10.1021/jf0531611. [DOI] [PubMed] [Google Scholar]
- Starkenmann C, Niclass Y, Escher S. Volatile organic sulfur-containing constituents in Poncirus trifoliata (L.) Raf. (Rutaceae) J Agric Food Chem. 2007;55:4511–4517. doi: 10.1021/jf063453h. [DOI] [PubMed] [Google Scholar]
- Starkenmann C, Le Calvé B, Niclass Y, Cayeux I, Beccucci S, Troccaz M. Olfactory perception of cysteine-S-conjugates from fruits and vegetables. J Agric Food Chem. 2008;56:9575–9580. doi: 10.1021/jf801873h. [DOI] [PubMed] [Google Scholar]
- Stevens JL. Isolation and characterization of a rat liver enzyme with both cysteine conjugate β-lyase and kynureninase activity. J Biol Chem. 1985;260:7945–7950. [PubMed] [Google Scholar]
- Stevens JL, Jones DP. The mercapturic acid pathway: Biosynthesis, intermediary metabolism, and physiological disposition. In: Dolphin D, Poulson R, Avramović O, editors. Glutathione: chemical, biochemical and medicinal aspects. Wiley; New York: 1989. pp. 45–84. Part B. [Google Scholar]
- Stevens JL, Robbins JD, Byrd RA. A purified cysteine conjugate β-lyase from rat kidney cytosol. Requirement for an α-keto acid or an amino acid oxidase for activity and identity with soluble glutamine transaminase K. J Biol Chem. 1986;261:15529–15537. [PubMed] [Google Scholar]
- Suzuki S, Tomisawa H, Ichihara S, Fukuzawa M, Tateishi M. A C-S bond cleavage enzyme of cysteine conjugates in intestinal microorganisms. Biochem Pharmacol. 1982;31:2137–2140. doi: 10.1016/0006-2952(82)90437-3. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Doi C, Suzuki N. Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol Appl Pharmacol. 2006;217:185–195. doi: 10.1016/j.taap.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Kurasaki K, Suzuki N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim Biophys Acta. 2007;1770:1053–1061. doi: 10.1016/j.bbagen.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Tateishi M, Suzuki S, Shimizu H. The metabolism of bromazepam in the rat – identification of mercapturic acid and its conversion in vitro to methylthiobromazepam. Biochem Pharmacol. 1978a;27:809–810. doi: 10.1016/0006-2952(78)90529-4. [DOI] [PubMed] [Google Scholar]
- Tateishi M, Suzuki S, Shimizu H. Cysteine conjugate β-lyase in rat liver. A novel enzyme catalyzing formation of thiol-containing metabolites of drugs. J Biol Chem. 1978b;253:8854–8859. [PubMed] [Google Scholar]
- Tomisawa H, Suzuki S, Ichihara S, Fukuzawa H, Tateishi M. Purification and characterization of a C-S lyase from Fusobacterium varium. A C-S cleavage enzyme of cysteine conjugates and some S-containing amino acids. J Biol Chem. 1984;259:2588–2593. [PubMed] [Google Scholar]
- Tsuji Y, Suzuki N, Suzuki K, Ogra Y. Selenium metabolism in rats with long-term ingestion of Se-methylselenocysteine using enriched stable isotopes. J Toxicol Sci. 2009;34:191–200. doi: 10.2131/jts.34.191. T. [DOI] [PubMed] [Google Scholar]
- Townsend DM, Tew KD, He L, King JB, Hanigan MH. Role of glutathione S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed Pharmacother. 2009;63:79–85. doi: 10.1016/j.biopha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H, Likos JJ, Metzler DE. Chemistry of the inactivation of cytosolic aspartate aminotransferase by serine O-sulfate. Biochemistry. 1982;21:4387–4393. doi: 10.1021/bi00261a030. [DOI] [PubMed] [Google Scholar]
- Uttamsingh V, Anders MW. Acylase-catalyzed deacetylation of haloalkene-derived mercapturates. Chem Res Toxicol. 1999;12:937–942. doi: 10.1021/tx990090p. [DOI] [PubMed] [Google Scholar]
- Villar MT, Niatsetskaya ZV, Cooper AJL, Artigues A. Inactivation of rat liver mitochondrial aspartate aminotransferase by a halogenated cysteine S-conjugate. Mol Cell Proteomics. 2007;6(Supplement 1):40. Abstract #B5. [Google Scholar]
- Wadhwa R, Takano S, Kaur K, Aida S, Yaguchi T, Kaul Z, Hirano T, Taira K, Kaul SC. Identification and characterization of molecular interactions between mortalin/mtHsp70 and HSP60. Biochem J. 2005;391:185–190. doi: 10.1042/BJ20050861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H, Wakabayashi M, Eisenreich W, Engel KH. Stereochemical course of the generation of 3-mercaptohexanol by β-lyase-catalyzed cleavage of cysteine conjugates. J Agric Food Chem. 2004;52:110–116. doi: 10.1021/jf0305478. [DOI] [PubMed] [Google Scholar]
- Warrander A, Allen JM, Andrews RS. Incorporation of radiolabelled amino acids into the sulphur-containing metabolites of paracetamol by the hamster. Xenobiotica. 1985;15:891–897. doi: 10.3109/00498258509045042. [DOI] [PubMed] [Google Scholar]
- Wessjohann LA, Schneider A, Abbas M, Brandt W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem. 2007;388:997–1006. doi: 10.1515/BC.2007.138. [DOI] [PubMed] [Google Scholar]
- Younis IR, Elliott M, Peer CJ, Cooper AJL, Pinto JT, Konat GW, Kraszpulski M, Petros WP, Callery PS. Dehydroalanine analog of glutathione: an electrophilic busulfan metabolite that binds to human glutathione S-transferase A1-1. J Pharmacol Exp Ther. 2008;327:770–776. doi: 10.1124/jpet.108.142208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hanigan MH. Role of cysteine S-conjugate β-lyase in the metabolism of cisplatin. J Pharmacol Exp Ther. 2003;306:988–994. doi: 10.1124/jpet.103.052225. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cooper AJL, Krasnikov BF, Xu H, Bubber P, Pinto JT, Gibson GE, Hanigan MH. Cisplatin-induced toxicity is associated with platinum deposition in mouse kidney mitochondria in vivo and with selective inactivation of α-ketoglutarate dehydrogenase complex in LLC-PK1 cells. Biochemistry. 2006;45:8959–8971. doi: 10.1021/bi060027g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Jiang W, Ganther HE, Ip C, Thompson HJ. In vitro effects of Se-allylselenocysteine and Se-propylselenocysteine on cell growth, DNA integrity, and apoptosis. Biochem Pharmacol. 2000;60:1467–1473. doi: 10.1016/s0006-2952(00)00461-5. [DOI] [PubMed] [Google Scholar]