Abstract
MHC class I mismatched, but class II matched kidney transplants are tolerogenic in large animal models. CD4+ T regulatory cells specific for HLA-B*1501-derived peptide p37-MA (DSDAASPRMAPRAPWIEQ) developed in a long term (>12 yrs) tolerant patient who received a HLA-B*1501 mismatched, HLA class II closely matched renal allograft. We hypothesized that class II matching favored Treg development by allowing allopeptide presentation on either recipient [DR4/DQ7+] or donor [DR4/DQ8+] APC. Indirect pathway CD4+ T cell clones were generated from recipient PBMC by sorting antigen-stimulated, proliferating cells. Most clones responded to p37-MA-pulsed DQ7+B*1501− autologous, but not to DQ8+B*1501+ donor B-LCL. However, some clones responded to both; in fact, one responded even more strongly to donor B-LCL than to p37-MA-pulsed autologous B-LCL. P37-MA contained a DQ8-binding motif and induced strong TH1 responses from DQ8 but not DQ6 transgenic mice. Microchimerism,, was found to be enriched in the dendritic cells cultured from adherent PBMC. These data support the concept that, when the donor is MHC class II closely matched, a “hybrid” form of allorecognition (direct/indirect) occurs. This may favor the generation of beneficial form of indirect pathway alloreactivity, i.e. allopeptide-specific CD4 T regulatory cells, in the context of long term DC microchimerism.
Keywords: T regulatory cells, tolerance, allorecognition, Microchimerism
Introduction
T regulatory cells are an important component of immunologic tolerance. They have been demonstrated to be involved in autoimmunity, alloimmunity, infection, tumor immunity, allergy and asthma. In transplantation tolerance, the induced or adaptive T regulatory cells, which can be specific for either self or foreign antigens, might have better therapeutic potential than self-reactive CD4+CD25+ natural T regulatory cells [1]. Despite success in inducing allospecific T regulatory cells in animal models of tolerance, clinical transplantation tolerance is still rare and difficult to achieve. The mechanism of inducing alloantigen-specific adaptive T regulatory cells in transplant recipients is also not clear.
It has been known for a long time that HLA matching correlates with improved graft survival [2]. The degree of HLA-DR mismatch in particular, correlates with freedom from early acute rejection and long-term graft survival [3]. HLA-matching can clearly reduce the number of targets for alloantigen-specific antibodies and both direct and indirect pathway T effector cell responses. MHC-class II matching might also favor the activity of the natural T regulatory cells restricted by self class II, and the generation of adaptive T regulatory cells that inhibit alloreactive B and T cells. Another manifestation of donor-specific T regulatory cells, the bystander suppression of a recall response in the presence of donor antigen in trans-vivo DTH (Delayed-Type Hypersensitivity) assay has been shown to be associated with allograft acceptance in human and monkey kidney transplant recipients [4, 5]. The degree of HLA-DR matching was correlated with the detection of donor specific T regulatory cells in the bystander suppression assay, and with graft survival [6]. The HLA identical group had the best graft survival and uniformly exhibited DTH regulation [6]. Monkeys that received MHC class II matched kidney allografts had a better chance for drug-free tolerance and having adaptive CD4+TGF-β+ T regulatory cells in the peripheral blood and the renal allograft [5]. In the MHC class I mismatched inbred miniature swine renal transplantation model, long term tolerance has been induced by short term treatment with cyclosporine A only if donor and recipient MHC class II were matched [7]. The mechanism of tolerance was found to be mediated by the induction of CD4 T regulatory cells [8].
In contrast to the inbred miniature swine transplant model, human HLA class II match is often imprecise at the DNA and the protein level in kidney transplants between unrelated individuals. Yet HLA-DR imprecisely matched donors can still induce allo-cross reactive T cells in the recipient. For example, Frasca et al. [9] generated a series of T cell clones from a rejected kidney transplant. The clones were specific for a donor HLA-A2 (92–120)-derived peptide, presented by recipient HLA-DR2 (DRB1*1502) [9]. The HLA-A2-mismatched donor expressed a different DR2 subtype (DRB1*1501), yet the naturally processed and presented A2 peptide was recognized by one of the T cell clones, named EL26 [9], a CD4+. EL26 was later found to be a CD25+CTLA-4 (surface)+ TGF-β (surface)+ adaptive T regulatory cell [10].
We have previously reported a patient (K1) who discontinued all immunosuppression at 1.5 years post-transplant, yet has maintained excellent graft function for >12 years without any immunosuppressive drugs. He was found to have circulating CD4+ adaptive T regulatory cells that produce TGF-β in response to APC pulsed with either soluble donor HLA-B*1501 antigen, or HLA-B*1501/B*5701 derived allopeptide p37-MA (DSDAASPRMAPRAPWIEQ) [11]. We hypothesized that the p37-MA-specific T regulatory cells were generated by hybrid presentation, either by the closely matched DR4 (recipient: DRB1*0411; donor: DRB1*0407) or associated DQ3 (recipient DQ8, donor DQ7). In this paper, we show that, while rare, CD4+ T cell clone that can be activated by both direct (via donor DQ8) and indirect (probably by recipient DQ7) presentation of a class I-derived allopeptide does exist. The presence of donor dendritic cell microchimerism is also demonstrated, providing a mechanism for such T cells to persist long term during metastable tolerance.
Methods and Materials
Cell culture and T cell cloning
T cell cloning was done on antigen-specific proliferated cells with a protocol similar to that described by Mannering et al. [12]. PBMC (10 × 107/ml in 1.76ml PBS) from a leukapheresis obtained in 2002, 8.8 years after transplantation, was utilized as at this time point T effector cells predominated the anti-donor response in the peripheral blood [11]. Cells were labeled with CFSE (1μM, Invitrogen), in 37°C water bath for 5min, and treated with cold 10% fetal calf serum (FCS) in RPMI medium (10ml). After wash with PBS, cells were re-suspended with 2ml X-vivo 15 serum free medium (Lonza, Allendale, NJ), and plated at 8.8 × 105/100μl/well. The labeled cells were mixed with peptide p37-MA, p37-TE (both at 2mg/ml), or staphylococcal enterotoxin B (SEB, 0.5μg/ml, a gift from Dr. Stuart Knechtle in Department of Surgery, UW-Madison) and cultured for 8 days in 37°C, 5%CO2. Cells were harvested and stained for CD4-PE and CD3-APC (all from BD Pharmingen) and CFSElow cells were flow sorted into 1 cell per well in 96-well U-bottom plates (with 20μl medium/well) using flow cytometer FACS Vantage (BD Bioscience). Sorted cells were cultured with a mixture of γ-irradiated (6000 Rad) recipient and donor B-LCL (2 × 104/well for each), γ-irradiated allogenic PBMC (7 × 104/well), anti-CD3 (50ng/ml, clone OKT3), anti-CD28 (100ng/ml, clone ANC28.1/5D10; Ancell, Bayport, MN), and human IL-2 (20U/ml, Sigma) in RPMI 1640 medium plus 10% FCS If necessary, medium was replenished with human IL-2 every 3 days. The growing clones were transferred into 24-well-plates and maintained with mixture of γ-irradiated recipient and donor B-LCL and allogenic PBMC, human IL-2, and PHA (250ng/ml).
Immunization of animals
AB0DQ8 and AB0DQ6 mice were a gift from Dr. Chella S. David (Department of Immunology, Mayo Clinic, Rochester, MN). Transgenic mice with either DQ8 or DQ6 gene were generated in (B10 × SWR) F1 background, and then crossed with the class II knockout mouse from a B6 × 129 background. The founder mice were backcrossed to B10 mice. The resulting mice express human class II, either DQ6 or DQ8 without endogenous mouse class II [13].
AB0DQ8 and AB0DQ6 mice were immunized by injecting two rear footpads with peptides (50μg/25μl) emulsified with 25μl complete Freund’s adjuvant (CFA, Sigma). 11 days post-immunization, the cells from spleen and inguinal lymph node were harvested and re-challenged again for ELISPOT assays.
IFN-γ ELISA
EIA/RIA plate was coated with anti-human IFN-γ coating antibody (1μg/ml, BD Pharmingen) in 10mM Tris Buffer, pH=9. Human IFN-γ standard (Endogen), biotin mouse anti-human IFN-γ 2nd antibody (0.25μg/ml, Endogen), Horseradish Peroxidase-20 (1:20,000 dilutions; Research Diagnostics, Concord, MA) were diluted in 0.2% biotin-free BSA (Sigma) with 0.01% Tween-20. The ELISA was developed with Sure Blue TMB Microwell Substrate and Stop Solution (KPL, Gaithersburg, MD).
ELISPOT
ELISPOT assays were done with the protocols described elsewhere [14]. Polyvinylidene difluoride membrane ELISPOT plates (Whatman, Clifton, NJ) were coated with primary Ab and incubated overnight at 4°C. Plates were blocked for 2 h with 1% BSA in PBS (1% PBSA), washed in HL-1 serum free medium (Lonza, Allendale, NJ) supplemented with penicillin/streptomycin and L-glutamine. Mixtures of cells from spleen and inguinal lymph node from immunized mice (4×105/well) were plated with peptides (100μg/ml) in HL-1 medium. Plates were incubated at 37°C with 5% CO2. Twenty four (for IFN-γ) or 48 (for IL-2) hours later, plates were washed five times in PBS with 0.05% Tween-20 then five times in PBS. Secondary Abs were diluted in 1% PBSA and added to plates and incubated at 4°C overnight. Plates were washed and spot development was performed using ELISPOT Blue Development Module (R&D Systems) according to the manufacturer’s instructions. Spots were analyzed using an AID ELISPOT plate reader (AutoImmun Diagnostika). IFN-γ-coating Ab (4μg/ml), IFN-γ-biotinylated Ab (3μg/ml), IL-2-coating Ab (3μg/ml), and IL-2-biotinylated Ab (2μg/ml) were all from BD Biosciences.
PCR-SSOP (PCR-sequence specific oligonucleotides probe)
The genomic DNA was extracted from patient K1 whole PBMC, adherent or non-adherent cells or cultured dendritic cells (DC) extracted from the PBMC. The DC were cultured from adherent PBMC with IL-4 and GM-CSF following protocols described elsewhere [15, 16]. DNA (1μg) were amplified for Exons 2 and 3 with HLA-B locus-specific generic primers [17]: forward 1 (GGC GGG GGC GCA GGA CCT GA), forward 2 (CGG GGG CGC AGG ACC CGG), reverse (GAG GCC ATC CCC G(G/C)C GAC CAT T). GAPDH (forward CCA TGG AGA AGG CTGGGG and reverse GG TCA TCC ATG ACA ACT TTG) was amplified with protocols described [16]. PCR products were separated in 1% agarose gel at 90V for 2 hours and transferred to positively charged nylon membrane (Bio-Rad) for Southern blotting. The membrane was prehybridized firstly at 42°C for 1hour in hybridization buffer (50mM Tris-HCl, pH 8.0; 3M TMAC; 2mm EDTA; 5× Denhardt’s solution; 0.1% SDS; 100μg/ml herring sperm DNA). The probe (GCC CGT GAG GCG GAG CA) specific for donor HLA-B*1501 but not recipient HLA-B*3701 or HLA-B*4001 was labeled with γ32-P-ATP using T4 polynucleotide kinase and purified through Sephadex G-25 (Pharmacia). After hybridizing at 42°C for 18–20 hours, the membrane was washed (3× SSC, 1% SDS, 2 times: 1× SSC, 1% SDS, 1 time) and HLA-B*1501-specific signal was visualized by autoradiography for 18–48 hours at −70°C.
Results
Hybrid (direct/indirect)-presentation of allopeptide p37-MA to a T cell clone
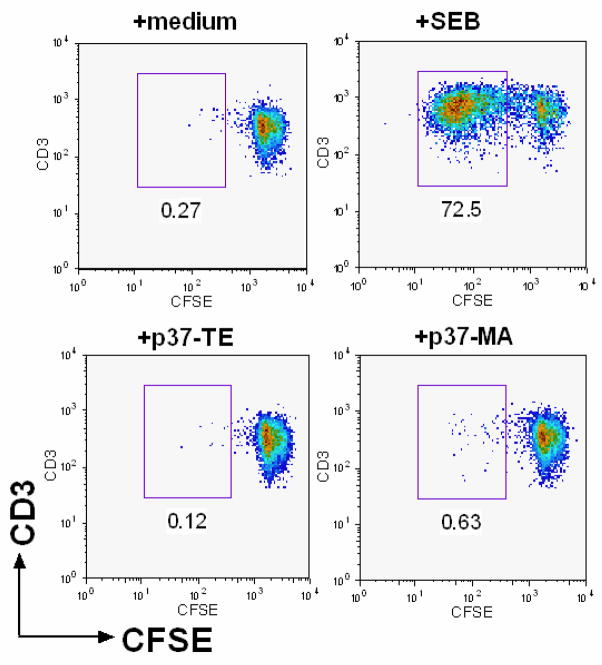
Patient K1 (A1,2, B37,40; DR4,6, DQ6,7) received a class II closely matched renal allograft (A1,2; B44,62[B*1501]; DR4,6, DQ6,8) from a deceased donor in 1993. In attempting to analyze the indirect pathway of alloreactivity in this patient, the main challenge for us was that the predominance of regulatory T cells during much of his clinical course. This made it difficult to culture alloreactive T cells from his PBMC. However, patient K1 lost his circulating adaptive T regulatory cells between years 7.5 and 9.5 post-transplant, regaining predominance of T regulatory specific for p37-MA at year 10 [11]. Using PBMC obtained at year 8.5 post-transplantation from patient K1, we applied a proliferation-based T cell cloning stratagem, similar to the one used to generate autoantigen-specific T cell clones from patients with type I diabetes [12, 18]. Allopeptide p37-MA (NH2-DSDAASPRMAPRAPWIEQ-COOH), control self peptide p37-TE (NH2-DSDAASPRTEPRAPWIEQ-COOH), and positive control super antigen staphylococcal enterotoxin B (SEB) were used to stimulate the CFSE labeled PBMC from patient K1. As shown in Fig. 1, a significant percentage (72.5%) of CD4+ T cells proliferated after 8 days culture in response to SEB and became CFSElow, while only 0.26% of CD4+ T cells proliferated in the culture with medium. There were more CFSElow, proliferated CD4+ T cells in response to allopeptide p37-MA (0.63%; Fig. 1, lower right panel) than self-peptide p37-TE (0.12%; Fig. 1, lower left panel). This is consistent with the previous data that there were far more IFN-γ producing T effector cells than TGF-β-producing T regulatory cells specific for allopeptide p37-MA at this timepoint [11].
Figure 1.
CD4+ T cells in patient K1’s PBMC proliferated specifically in response to allopeptide p37-MA. CFSE labeled PBMC from patient K1, were cultured with media, SEB, self-peptide (p37-TE) or donor allopeptide (p37-MA) for 8 days. Flow diagram shows the % of proliferated (CFSElow, number below box) cells within the CD3+CD4+ T cell gate after 8-day culture with specified antigens. The CD4+ T cells proliferating specifically to p37-MA were sorted into one cell/well for generating T cell clones.
The cells which proliferated in response to p37-MA were sorted to generate T cell clones. It should be noted that these T cells encountered only p37-MA-pulsed autologous APC during the initial proliferation stage of culture prior to sorting. In the end, 110 out of 400 cultured cells (27.5%) grew into T cell clones. The T cell clones were CD3+CD4+TCR(αβ)+. Seventeen of 110 (15.5%) clones proliferated significantly more in response to recipient B-LCL plus p37-MA allopeptide than recipient B-LCL alone. However, one of these clones (#101) proliferated significantly better in response to the donor B-LCL than to recipient B-LCL plus p37-MA allopeptide.
To confirm the specificity of each T cell clone, type 1 cytokine production was tested using an IFN-γ ELISA. As shown in Fig. 2, three types of T cell clones were found. Clone #65 was a typical self-reactive T cell clone, which responded similarly to recipient B-LCL with or without allopeptide p37-MA and didn’t respond to donor B-LCL. Clones #35, #40, #55, #76, #90, #103 were typical alloreactive indirect pathway CD4 T cells. They responded significantly better (p<0.05) to recipient B-LCL plus allopeptide p37-MA than recipient B-LCL alone, but didn’t respond to donor B-LCL. Clone #101 was also an indirect pathway T cell, responding to allopeptide p37-MA-pulsed recipient B-LCL (p<0.05 vs. recipient B-LCL alone). In addition, it also responded to unpulsed donor APC. Most convincing in this regard is that clone #101 produced 8 times more IFN-γ in response to donor B-LCL (p<0.01) than to recipient B-LCL plus allopeptide p37-MA;i.e. it exhibited both indirect and direct pathway alloreactivity,
Figure 2.
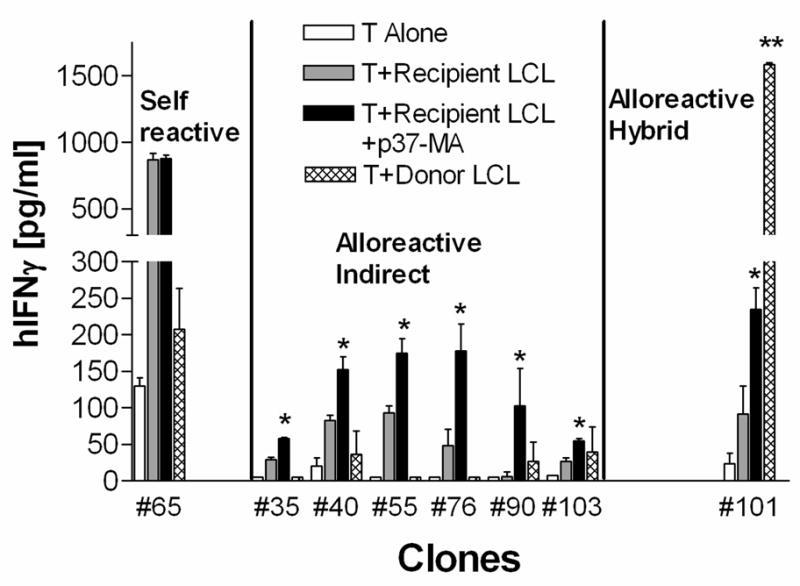
There are three types of T cell clones. Culture supernatant from T cell clones stimulated with media (open bars), recipient LCL (grey bars), recipient LCL pulsed with donor peptide p37-MA (black bars) or donor LCL (stippled bar) were tested by ELISA for IFN-γ production. The three different types of clones were grouped. *p< 0.05 vs. T+ recipient B-LCL, **p<0.01 vs. T+ recipient B-LCL + p37-MA, in student’s T test.
Donor DQ8 (DQA*0301, DQB*0302) can present p37-MA
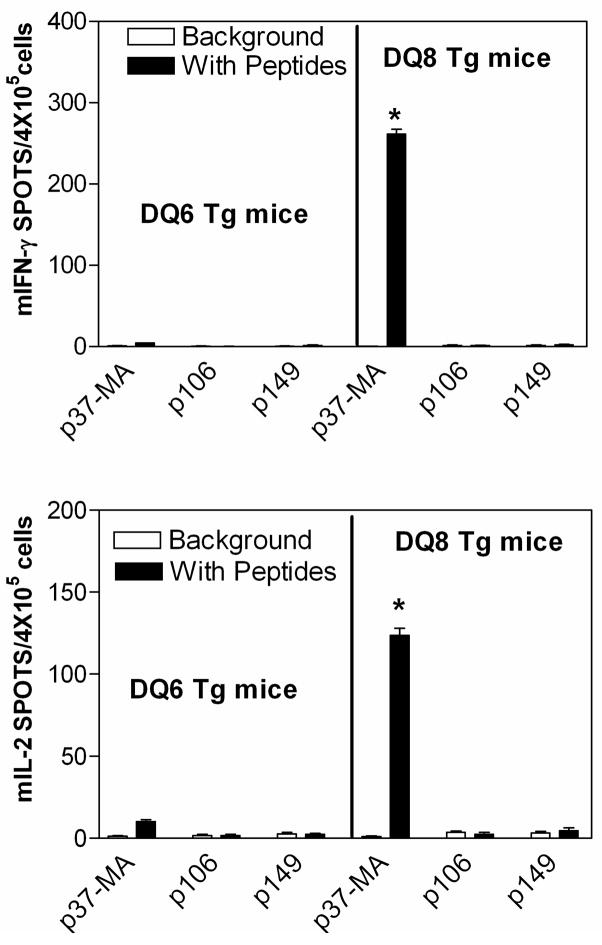
We surmised, based on a limited immunogenetic analysis of responses in whole PBMC from transplant recipients to donor B*1501-derived allopeptides [11], that either DR4 or a DR4-associated DQ3 (DQ8 in donor, DQ7 in recipient) might be able to present the allopeptide p37-MA. Using the RANKPEP program (http://bio.dfci.harvard.edu/RANKPEP/) [19], p37-MA was predicted to bind to DQ8 but not DR4. To test the hypothesis that donor-type DQ8 could indeed present the allopeptide p37-MA, we took advantage of I-Anull DQ8 transgenic mice (AB0DQ8), in which the HLA-DQ8 α and β transgenes (DQA*0301/DQB*0302) are the only expressed class II genes. The HLA-DQ6 (DQA*0103/DQB*0601) transgenes expressed in DQ6 transgenic mice (AB0DQ6) is similar to the DQ6 (DQA*0103/DQB*0603) found in association with DR6 (DRβ1*1301) in patient K1 and his donor, and these transgenic mice were used as a control. Mice were immunized with allopetide p37-MA, or HLA-B*1501 derived control allopeptide p106 (DGRLLRGHDQSAYDGKDY), p149 (AAREAEQWRAYLEGLCVE). Spleen and lymph node cells were harvested and re-challenged again in vitro with the immunizing peptide. As shown in Figure 3, lymphocytes from p37-MA immunized DQ8 transgenic mice produced ELISPOTs for IFN-γ (261±7,) or IL-2 (124±5,) when cultured in vitro with p37-MA peptide. This did not happen with p37-MA immunized DQ6 transgenic mice, or mice immunized with control allopeptides, such as p106 or p149, which do not stimulate a significant response in patient K1 [11].
Figure 3.
Donor DQ8 (DQA*0301, B*0302) can present p37-MA. DQ8 (DQA*0301, B*0302) or DQ6 (DQA*0103, B*0601) transgenic mice (DQ8 or DQ6 Tg mice) were immunized with allopeptide p37-MA, control allopeptide p106 or p149. Cells from the spleen and lymph node of the immunized mice were harvested and challenged again in vitro either with (black bars) or without (blank bars, background) the immunizing peptide mIFN-γ (upper panel) or mIL-2 (lower panel) in ELISPOT. The data are shown as the mean ± SE (n=4) of spots per 4 × 105 cells. * p<0.01 vs. the background in student’s T test.
Donor DC microchimerism in patient K1
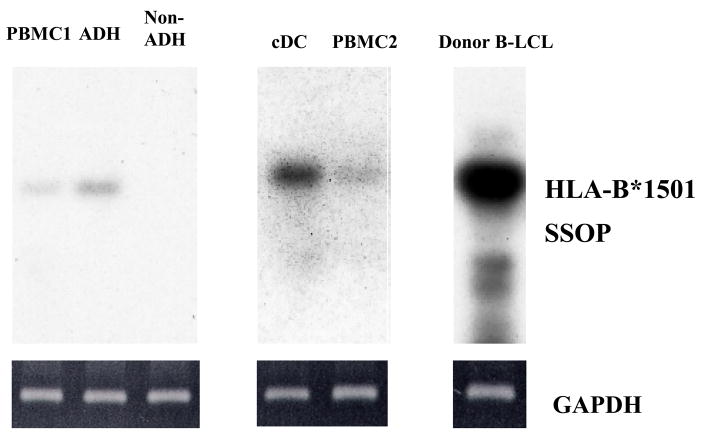
If DQ8 from the kidney donor is capable of directly presenting the allopeptide p37-MA to some indirect pathway CD4+ T cells, this pathway of allorecognition would be relevant to tolerance only if class II+ donor-derived APC persist in vivo. To test this hypothesis, we studied the PBMC from patient K1 obtained at year 4.5 post-transplantation, a time when the donor alloantigen-specific T regulatory cells predominated over T effector cells specific for the same donor alloantigen [11]. Using a PCR-SSOP specific for donor HLA-B*1501, donor cell microchimerism was detected in whole PBMC (lanes PBMC1 & 2), and in the adherent fraction, but not in the non-adherent lymphocyte fraction (Fig. 4). When dendritic cells (DC) were generated in vitro by culturing adherent cells with GM-CSF and IL-4, the signal for donor HLA-B*1501 DNA was enriched (cDC lane). Donor B-LCL (right lane) were used as a positive control for HLA-B*1501 specific PCR-SSOP. The PCR amplifications of GAPDH (Fig. 4, bottom) were used as control to show that similar amounts of genomic DNA were used in each sample. These data indicate that donor leukocytes are indeed circulating in the peripheral blood of tolerant renal transplantation recipient K1. The microchimerism was enriched in the professional antigen-presenting cells, the dendritic cells, suggesting that antigen-presentation by HLA class II+ donor (DQ8/direct) cells is possible in vivo.
Figure 4.
Donor cell microchimerism was enriched in DC. Two different PBMC (PBMC1 and PBMC2) obtained from patient K1 between year 4–5 post-transplantation, PBMC1-derived adherent (ADH) or non-adherent (Non-ADH) cells, or cultured DCs (cDC) from PBMC2-derived monocytes were tested for the presence of donor HLA-B*1501+ cells using HLA-B*1501 specific PCR-SSOP. The donor B-LCL was used as the positive control. Ethidium bromide staining of GAPDH–specific PCR products from the amplification of the genomic DNA samples is shown in the lower panels.
Discussion
We found that the majority of CD4+ effector T cell clones responding to allopeptide stimulation were true “indirect pathway” T cells restricted to host APC-presentation. Similar results were reported by Frasca et al. [9], who found the majority of T cell clones derived from a rejected HLA-A2-mismatched kidney allograft with a closely matched HLA-DR only recognized an A2-derived allopeptide presented by recipient-type DRB1*1502 and not by DRB1*1501 in the donor.
Interestingly, one clone (#101) generated by indirect antigen recognition on recipient APC was also able to be strongly activated by direct presentation via donor APC. The T cell clones were generated with flow sorting cells into a single cell per well, so theoretically they should be “true” clones. However we don’t know if the dual reactivity is due to a mixture of two types of T cell clones with different TCRs in this case one could imagine that a “donor-reactive” T cell that proliferated in response to allopeptide and autologous APC had perhaps adhered to a T cell with conventional indirect pathway alloreactivity during flow-sorting, and had grown out in subsequent culture (in which donor APC were included as feeder cells). This would explain the data but still would not account for why the directly alloreactive T cell would have proliferated in absence of donor APC in the first place. Secondly, #101 could indeed be a pure T cell clone, either one with a single TCR having dual reactivity for p37-MA peptide presented by either DQ8 or the similar DQ7, or, one that might express a single TCR Vβ, but two different kinds of TCR Vα chains. The last scenario might favor the generation of T regulatory cells since dual TCR T cells were found to be enriched more in human natural CD4+CD25+ T regulatory cells than non-T regulatory CD4+CD25− T cells in the peripheral blood and thymus [20]. A detailed TCR analysis might help answer the question.
If it is truly a clone, #101 would be an example of hybrid-allorecognition (Fig. 5 & [21]), which happens when the MHC class I is mismatched and class II is identical or closely matched between the organ donor and recipient. Clone EL26 [9, 10] would be another example of this unusual type of alloreactivity. As shown in Figure 5, such CD4 T cells can recognize both the direct native presentation of the mismatched MHC class I antigen-derived allopeptide by donor antigen presenting cells (APC), as well as the indirect presentation of the same class I allopeptide by recipient APC that ingest soluble donor HLA class I.
Figure 5.
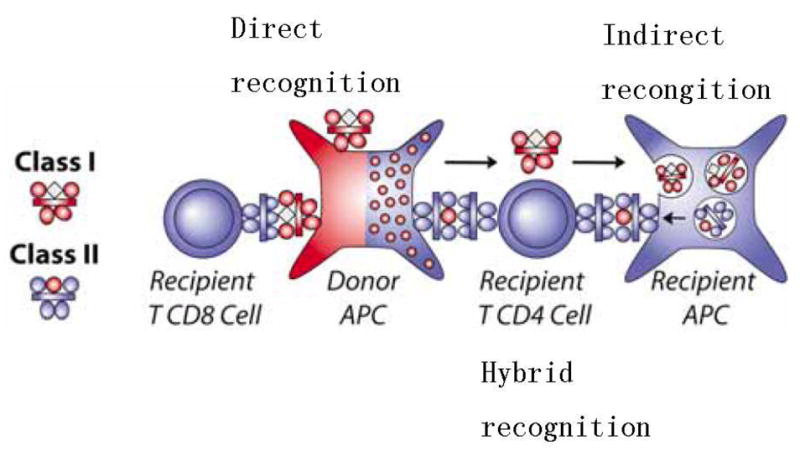
Three pathways for allo-recognition. The donor and recipient are matched for MHC class II and mismatched for MHC class I. MHC class I alloantigens (red) can be recognized with their native peptides (white diamond) on donor APC by recipient CD8+ T cells (direct recognition, left). The recipient CD4+ T cell can recognize the allopeptide (red circle) derived from shed HLA class I of the donor, processed and presented by a recipient APC’s MHC class II (shaded blue, indirect recognition, right). The recipient’s indirect pathway CD4+ T cells can also recognize a shared MHC class II alloantigens containing a native “allo” peptide (red circle) derived from the mismatched MHC class I (hybrid recognition, center). Reprinted by permission, from Burlingham & Torrealba [21].
There are relative few detailed human studies about the indirect pathway T cells [9, 22–24], most of which are associated with HLA-DR, not HLA-DQ. We recently analyzed indirect pathway responses in three transplant recipients (K1, K3, L1), all of whom received HLA-B62 mismatched allografts. All 3 had HLA-B62-specific CD4+ T cell responses in their PBMC detectable by trans-vivo DTH assay. However, patient K1 (DR4, 6; DQ3, 6) and K3 (DR4, 15; DQ3, 6), which share the DR4/DQ3 haplotype, had T cell responses to p37-MA, while T cells in the DR15/DQ6-homozygous patient L1 recognized p149 but not p37-MA [11]. This data suggests strongly that HLA-DR4 or associated DQ3 presents the allopeptide p37-MA. Evidence from computer-based analysis of peptide motifs suggested, and studies in DQ8 transgenic mice confirmed that HLA-DQ8 (DQA*0301/DQB*0302) can present p37-MA to T cells. Due to the similarity between DQ7 (DQA*0301/DQB*0301) and DQ8 (DQA*0301/DQB*0302), we speculate that the DQ7 (DQA*0301/DQB*0301) might be the HLA class II that presents p37-MA in the recipient.
Indirect pathway T cells specific for HLA-DR-derived allopepeitdes can contribute to chronic rejection of HLA-DR-mismatched heart allograft, where hybrid recognition by the CD4 T cells does not occur [24]. Donor microchimerism promoted rejection rather than tolerance when HLA class II are mismatched [16] However, MHC class II close matching, which favors hybrid presentation, helps achieving tolerance of renal allograft in miniature swine [7], monkey [5], and human [6]. This indicates that hybrid presentation can make indirect pathway beneficial for tolerance, probably by inducing alloreactive T regulatory cells and confining alloreactive B cells and T effector cells.
Is donor microchimerism really necessary for the induction CD4+ T regulatory cells specific for mismatched HLA class I antigen? Many solid organs express HLA class I, which can be presented by either the direct or indirect pathway. Normal human renal peritubular and glomerular capillaries also constitutively expressed HLA-DR and -DQ, but without co-stimulation molecular CD86/80 or CD40 [25]. These cells might be tolerogenic and can help generate T regulatory cells by either direct, or hybrid (direct/indirect) presentation of allopeptides. Recipient DCs can also acquire donor antigen by intercellular exchanges of membrane patches (trogocytosis) and therefore present intact donor MHC to direct pathway T cells (“semi direct” presentation) [22]. However, studies in animal models demonstrated that tolerance induction was more dependent on the indirect pathway, rather than the direct pathway T regulatory cells [26]. Nonetheless, recent data showed that systematic donor antigen and systemic chimerism favored donor specific tolerance, while localized donor antigen and lacking of systemic chimerism would promote active immunity [27]. This suggests that although an allograft itself, such as kidney, can be a good source for donor antigen, without donor microchimerism, the transplanted organs might not facilitate induction of donor antigen specific T regulatory cells.
At the peptide level, HLA molecules might not be as polymormphic as once thought. The HLA-B*1501-derived allopeptide p37-MA is also found in most of the HLA-B62 family and all of HLA-B46 and HLA-B57 family. In fact, p37-MA specific T regulatory cells were found in another tolerant renal transplantation K2, which received a HLA-B57 mismatched allograft [11]. This indicates that HLAs might share some common T cell epitopes among them and the total number of the epitopes might be limited. A possible T cells epitope library might be useful to predict the alloreactive T cells in a recipient in response to his donor candidates.
Acknowledgments
We thank Dr. Chella S. David from Mayo clinic for providing the DQ transgenic mice, Dr. Sidney, J., and Sette, A. from La Jolla Institute for Allergy and Immunology for solid phase peptide binding assay, Drs. Huaizhong Hu, and Debbie Bloom for helping T cell cloning, and Lynn D. Haynes for reading and providing nice comment for the manuscript.
Nonstandard Abbreviations used
- B-LCL
B-lymphoblastoid cell lines
- ELISPOT
Enzyme-linked ImmunoSPOT
Footnotes
This work was supported in part by R21-AI49900-01, R01-AI/HL 48624-01, K02-AI01452 (W.J.B.), the University of Wisconsin Medical Foundation (J.L.), University of Wisconsin Graduate School (Q.X., 135G144). There is no conflict of interest.
References
- 1.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003 Mar;3(3):253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 2.Opelz G, Wujciak T, Dohler B. Is HLA matching worth the effort? Collaborative Transplant Study. Transplant Proc. 1999 Feb–Mar;31(1–2):717. doi: 10.1016/s0041-1345(98)01620-0. [DOI] [PubMed] [Google Scholar]
- 3.Pirsch JD, D’Alessandro AM, Sollinger HW, Hoffmann RM, Roecker E, Voss BJ, Lorentzen D, Knechtle SJ, Reed A, Kalayoglu M, et al. The effect of donor age, recipient age, and HLA match on immunologic graft survival in cadaver renal transplant recipients. Transplantation. 1992 Jan;53(1):55. doi: 10.1097/00007890-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 4.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier RP, Orosz CG. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000 Jul;106(1):145. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrealba JR, Katayama M, Fechner JH, Jr, Jankowska-Gan E, Kusaka S, Xu Q, Schultz JM, Oberley TD, Hu H, Hamawy MM, Jonker M, Wubben J, Doxiadis G, Bontrop R, Burlingham WJ, Knechtle SJ. Metastable Tolerance to Rhesus Monkey Renal Transplants Is Correlated with Allograft TGF-{beta}1+CD4+ T Regulatory Cell Infiltrates. J Immunol. 2004;172(9):5753. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez DS, Jankowska-Gan E, Haynes LD, Leverson G, Munoz A, Heisey D, Sollinger HW, Burlingham WJ. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am J Transplant. 2004;4(4):537. doi: 10.1111/j.1600-6143.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 7.Rosengard BR, Ojikutu CA, Guzzetta PC, Smith CV, Sundt TM, 3rd, Nakajima K, Boorstein SM, Hill GS, Fishbein JM, Sachs DH. Induction of specific tolerance to class I-disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54(3):490. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Mezrich J, Yamada K, Sachs DH, Madsen JC. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery. 2004;135(5):473. doi: 10.1016/j.surg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Frasca L, Amendola A, Hornick P, Brookes P, Aichinger G, Marelli-Berg F, Lechler RI, Lombardi G. Role of donor and recipient antigen-presenting cells in priming and maintaining T cells with indirect allospecificity. Transplantation. 1998 Nov 15;66(9):1238. doi: 10.1097/00007890-199811150-00020. [DOI] [PubMed] [Google Scholar]
- 10.Roelen DL, van Bree S, van Hulst P, van Beelen E, Claas FH. Regulatory functions of human CD4(+) T cells recognizing allopeptides in the context of self-HLA class II. Hum Immunol. 2002;63(10):902. doi: 10.1016/s0198-8859(02)00453-6. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, Lee J, Jankowska-Gan E, Schultz J, Roennburg DA, Haynes LD, Kusaka S, Sollinger HW, Knechtle SJ, VanBuskirk AM, Torrealba JR, Burlingham WJ. Human CD4+CD25low Adaptive T Regulatory Cells Suppress Delayed-Type Hypersensitivity during Transplant Tolerance. J Immunol. 2007;178(6):3983. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 12.Mannering SI, Dromey JA, Morris JS, Thearle DJ, Jensen KP, Harrison LC. An efficient method for cloning human autoantigen-specific T cells. J Immunol Methods. 2005;298(1–2):83. doi: 10.1016/j.jim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169(10):5595. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 14.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, Burlingham WJ. Developmental Exposure to Noninherited Maternal Antigens Induces CD4+ T Regulatory Cells: Relevance to Mechanism of Heart Allograft Tolerance. J Immunol. 2007;179(10):6749. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham WJ. Minor H Antigen HA-1-specific Regulator and Effector CD8+ T Cells, and HA-1 Microchimerism, in Allograft Tolerance. J Exp Med. 2004;199(7):1017. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell PJ, Burlingham WJ. Donor dendritic cell persistence in organ allograft recipients in the absence of immunosuppression. J Leukoc Biol. 1999 Aug;66(2):301. doi: 10.1002/jlb.66.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Cereb N, Maye P, Lee S, Kong Y, Yang SY. Locus-specific amplification of HLA class I genes from genomic DNA: locus-specific sequences in the first and third introns of HLA-A, -B, and -C alleles. Tissue Antigens. 1995;45(1):1. doi: 10.1111/j.1399-0039.1995.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 18.Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP, Kay TWH, Rossjohn J, Falk BA, Nepom GT, Purcell AW. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005;202(9):1191. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reche PA, Glutting JP, Zhang H, Reinherz EL. Enhancement to the RANKPEP resource for the prediction of peptide binding to MHC molecules using profiles. Immunogenetics. 2004;56(6):405. doi: 10.1007/s00251-004-0709-7. [DOI] [PubMed] [Google Scholar]
- 20.Tuovinen H, Salminen JT, Arstila TP. Most human thymic and peripheral-blood CD4+ CD25+ regulatory T cells express 2 T-cell receptors. Blood. 2006;108(13):4063. doi: 10.1182/blood-2006-04-016105. [DOI] [PubMed] [Google Scholar]
- 21.Burlingham WJ, Torrealba J. Immunologic tolerance as taught by allografts. In: Wilkes D, Burlingham W, editors. Immunobiology of Organ Transplantation. New York: Plenum/Kluwer Press; 2004. [Google Scholar]
- 22.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16(5):550. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Salama AD, Najafian N, Clarkson MR, Harmon WE, Sayegh MH. Regulatory CD25+ T Cells in Human Kidney Transplant Recipients. J Am Soc Nephrol. 2003;14(6):1643. doi: 10.1097/01.asn.0000057540.98231.c1. [DOI] [PubMed] [Google Scholar]
- 24.Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101(2):398. doi: 10.1172/JCI1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muczynski KA, Ekle DM, Coder DM, Anderson SK. Normal Human Kidney HLA-DR-Expressing Renal Microvascular Endothelial Cells: Characterization, Isolation, and Regulation of MHC Class II Expression. J Am Soc Nephrol. 2003;14(5):1336. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Fueyo A, Domenig CM, Mariat C, Alexopoulos S, Zheng XX, Strom TB. Influence of direct and indirect allorecognition pathways on CD4+CD25+ regulatory T-cell function in transplantation. Transpl Int. 2007;20(6):534. doi: 10.1111/j.1432-2277.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]