Abstract
Spinal metastatic disease is characterised by the preservation of the intervertebral disc structure, even after severe destruction of the vertebral body by neoplastic tissues. Anatomical features of the discs are thought to be the reason for the disc’s resistance to metastatic cancer. However, little is known about the biochemical mechanism to prevent or attenuate the local invasion of cancer cells into the discs. The purpose of this study was to investigate the hypothesis that Fas ligand (FasL) produced by nucleus pulposus cells can kill Fas-expressing cancer cells infiltrating into the discs by the activation of caspases. Fas-expressing MCF-7 breast cancer cells were cultured with (experimental group) and without (control group) supernatant of nucleus pulposus cells containing FasL (50 pg/ml) for 48 h. The apoptosis of MCF-7 breast cancer cells was determined by the TUNEL technique. In addition, the activation of caspase-8, -9 and -3 was investigated by Western blot analysis. After treatment with supernatant of the nucleus pulposus cells containing FasL, the apoptosis of MCF-7 breast cancer cells was significantly increased, along with the activation of caspase-8, -9 and -3 compared with those of the control group. Our results suggest that the Fas/FasL interaction of nucleus pulposus and cancer cells might be a potential mechanism of the disc’s resistance to metastatic cancer.
Résumé
Les lésions métastatiques de la colonne vertébrale sont caractérisées par la conservation du disque inter vertébral malgré d’importantes lésions du corps vertébral. Ces données anatomiques concernant le disque prennent en compte la résistance de ces derniers aux métastases. Cependant, nous ne connaissons que peu de choses pour prévenir ou atténuer l’invasion des disques par les cellules néoplasiques. Cette étude a pour but d’étudier l’hypothèse selon laquelle le FasL produit par le nucleus pulposus peut s’opposer à l’expression des cellules cancéreuses infiltrant ces disques en activant les caspases. Pour cela nous avons cultivé des cellules de cancer du poumon MCF-7 au contact des cellules contenant du FasL de nucleus pulposus (50 pg/ml) et ceci pendant 48 heures. L’apoptose des cellules de MCF-7 a été déterminée par TUNEL. Nous avons également étudié l’activation des caspase 8, 9 et 3. Après traitement contenant un supernageant de nucleus pulposus contenant du FasL, l’apoptose des cellules cancéreuses est significativement augmentée avec l’activation des caspases 8, 9 et 3 par rapport au groupe contrôle. Nos résultats nous permettent de penser que l’action du Fas/FasL du nucleus pulposus est un des mécanismes de résistance des disques aux métastases vertébrales.
Introduction
One of the characteristics of metastatic spinal tumour is the preservation of intervertebral disc structures, even after severe destruction of the vertebral body by cancer cells (Fig. 1) [1, 4, 9]. Anatomical features of the intervertebral disc, such as avascularity, high intradiscal pressure and abundant collagen content, have been proposed as the main factors of the disc’s resistance to metastatic cancer [2, 3, 25]. However, with increasing age, new vessel formation occurs around the intervertebral disc, which might serve as the haematogenous route for metastatic seeding [10, 16, 24]. In addition, metastatic cancer cells secrete matrix metalloproteinases, such as collagenase, that can destroy extracellular matrix components of the discs during metastasis [17]. The intervertebral disc is separated from the vertebral body by cartilaginous endplates. The cartilaginous endplates contain protease inhibitors and other substances that can inhibit tumour vascularisation [11]. Moreover, with the aging process, the cartilaginous endplates become sclerotic, calcific and bony plates that can inhibit vascular invasion of the cancer cells [8, 23]. However, the cartilaginous endplates do not cover the entire vertebral body. Therefore, direct infiltration of the cancer cells into the discs can occur from the rim of the vertebral body not covered by the cartilaginous plates [25]. Despite these findings, the intervertebral disc is still resistant to metastatic cancer, which suggests that there may be a mechanism other than the disc’s anatomical characteristics that confer its resistance to metastatic seeding.
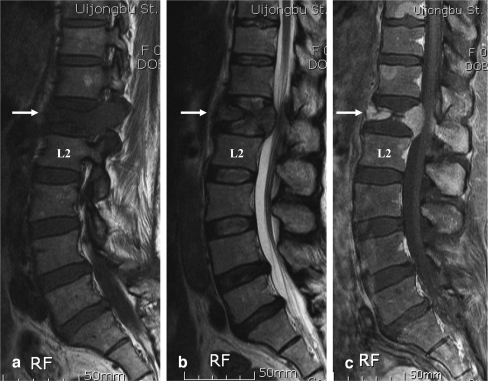
Fig. 1.
T1- (a), T2- (b) and gadolinium-enhanced (c) magnetic resonance images of a 51-year-old female patient with breast cancer shows the preservation of D12-L1 and L1-2 intervertebral disc structures, even after severe destruction of the L1 vertebral body by metastatic cancer
Fas ligand (FasL) is a well known death system that can induce apoptotic cell death in a variety of cells expressing Fas receptor by the activation of downstream caspases via intrinsic (mitochondrial) or extrinsic (death receptor) pathways [18, 21]. While the Fas receptor is expressed in a wide variety of cells, FasL expression is tightly restricted to activated T cells, natural killer cells and stromal cells of some immune-privileged sites, such as the eye and testis [6, 7]. In immune-privileged sites, stromal cells express FasL, which binds to the Fas receptor on the surface of immune cells that are infiltrating into the organs. This induces apoptosis of the infiltrating immune cells, so as to maintain the organ’s immune-privileged status. Recently, it has been reported that intervertebral disc cells, such as nucleus pulposus and annulus fibrosus cells, express FasL [14, 19]. Therefore, a similar mechanism may explain how the intervertebral discs resist infiltration by metastatic tumour cells that bear Fas receptor on their membranes. We therefore undertook this study to investigate the hypothesis that FasL produced by nucleus pulposus cells can kill Fas-bearing malignant tumour cells by the activation of caspases.
Materials and methods
Cell culture
Five lumbar intervertebral discs (L1–L6) were harvested from two male Sprague Dawley rats (age 4 weeks) immediately after sacrifice. We carefully dissected the discs under a microscope to obtain nucleus pulposus tissue and cultured them for 12 h. To isolate the cells, nucleus pulposus tissues were digested for 4 h, filtered and washed. After three-passages, the cells were trypsinised and subcultured into six-well plates at 1 × 106 cells/well. MCF-7 breast cancer cell line was purchased and cultured. After three-passages, the cells were trypsinised and subcultured into six-well plates at 1 × 106 cells/well.
Expression of Fas in MCF-7 breast cancer cells
The expression of Fas was determined in the lysate of MCF-7 breast cancer cells (1 × 106 cells/well) by Western blot analysis according to the manufacturer’s instructions. Primary antibody for Fas was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a dilution of 1:500. β-actin was used as an internal control for protein loading. Rat thymus was used as the positive control for Fas.
Expression of FasL in nucleus pulposus cells
The expression of FasL was determined in the lysate of nucleus pulposus (1 × 106 cells/well) by Western blot analysis according to the manufacturer’s instructions. Primary antibody for FasL was purchased from Santa Cruz Biotechnology and used at a dilution of 1:500. β-actin was used as an internal control for protein loading. Human breast tissue was used as the positive control for FasL.
For immunohistochemical staining for FasL, five lumbar intervertebral discs (L1–L6), including both cranial and caudal cartilaginous endplates, were harvested from two male Sprague Dawley rats (age 4 weeks) immediately after sacrifice. The specimens were fixed in 4% paraformaldehyde at 4°C for 24 h, decalcified in 20% EDTA (pH 7.4) for 4–6 weeks, dehydrated and embedded in paraffin. For immunohistochemistry, 4-μm-thickness midsagittal sections of the disc specimens were cut on a microtome and mounted on poly-L-lysine-coated slides. Primary antibody for FasL was purchased from Santa Cruz Biotechnology and used at a dilution of 1:500. The avidin–biotin–peroxidase complex method and a Histostain-plus SP kit (Zymed Laboratories, CA) were used according to the manufacturer’s instructions. The disc paraffin sections were deparaffinised in xylene and rehydrated. The endogenous peroxidase was subsequently blocked by 0.3% H2O2 for 30 min. After boiling in 10% citrate buffer (pH 6.0) for 15 min, the sections were incubated with primary antibody at 4°C for 16 h. Human breast tissue was used for the positive control.
Quantification of FasL in the supernatant of nucleus pulposus cells
The presence of FasL in the supernatant of cultured nucleus pulposus cells (1 × 106 cells/well) was determined and quantified by an enzyme-linked immunosorbent assay (ELISA) kit with an antibody that recognises rat FasL (R and D Systems, Minneapolis, MN) according to the manufacturer’s instructions. For calibration, we used natural rat FasL provided by the supplier to construct a standard curve and to obtain absolute values. The concentration of FasL was measured three times in each sample and the average of the three measurements was considered to be the final concentration.
Apoptosis of MCF-7 breast cancer cells
MCF-7 breast cancer cells were subcultured into six-well plates at 1 × 106 cells/well and treated with 300 μl of supernatant obtained from cultured nucleus pulposus cells (1 × 106 cells/well) for 48 h. The apoptosis of MCF-7 breast cancer cells was determined by staining cells with 15 μl of APOPercentage dye incubated for 30 min. After syringing off the culture medium and dye mixture, and gently washing the cells twice with 500 μl/well PBS, the cells were transferred to an inverted microscope and photographs were obtained. MCF-7 breast cancer cells that were cultured for 48 h by themselves were used as the control.
Activation of caspase-8, -9 and -3 in MCF-7 breast cancer cells
Western blot analysis was performed to investigate the expression of caspase-8, -9 and -3 in the lysate of MCF-7 breast cancer cells treated with and without 300 μl of supernatant of nucleus pulposus cells containing FasL for 48 h according to the manufacturer’s instructions. Primary antibodies for caspase-8, -9 and -3 were purchased from Lab Vision and Cell Signalling companies and used at a dilution of 1:500, 1:500 and 1:1,000, respectively. Human stomach and tonsil cells were used as the positive controls for caspase-8, -9 and -3, respectively.
Results
Expression of Fas in MCF-7 breast cancer cells
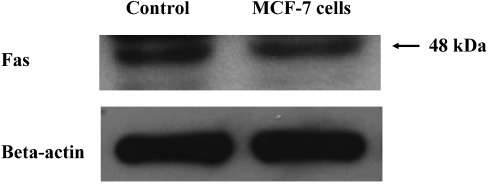
Western blot analysis clearly demonstrated the expression of Fas in the lysate of MCF-7 breast cancer cells (Fig. 2).
Fig. 2.
The expression of Fas by MCF-7 breast cancer cells: Western blot analysis shows the expression of Fas in the lysate of MCF-7 breast cancer cells. Rat thymus was used as the positive control for Fas
Expression of FasL in nucleus pulposus cells
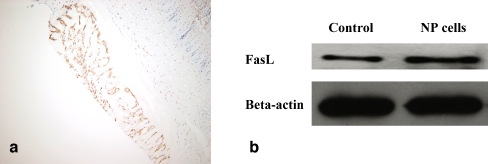
Immunohistochemical staining shows the expression of FasL on nucleus pulposus cells of the intervertebral disc tissue (Fig. 3a). In addition, Western blot analysis clearly demonstrated the expression of FasL in the lysate of nucleus pulposus cells (Fig. 3b). The mean concentration of FasL in the supernatant of nucleus pulposus cells (1 × 106 cell/well) was 49.7 ± 5.3 pg/mL (mean±standard deviation).
Fig. 3.
The expression of Fas ligand (FasL) on nucleus pulposus cells: immunohistochemical staining (a, ×100) and Western blot analysis (b) show the expression of FasL on nucleus pulposus cells. Human breast was used as the positive control for FasL
Apoptosis of MCF-7 breast cancer cells with the activation of caspase-8, -9 and -3
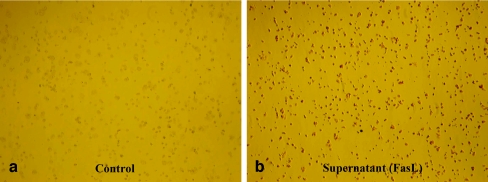
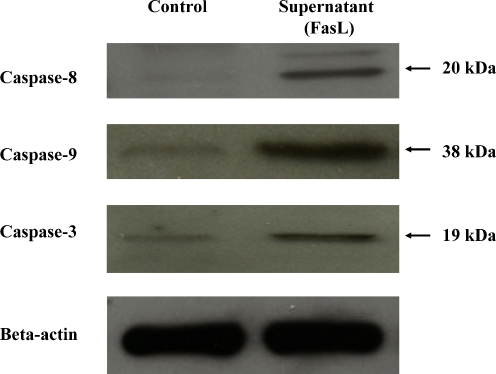
TUNEL assay clearly demonstrated significantly increased apoptotic cell death of MCF-7 breast cancer cells treated with the supernatant of nucleus pulposus cells containing FasL compared with that of controls (Fig. 4). Western blot analysis showed significantly increased expression of caspase-8, -9 and -3 in the lysates of MCF-7 breast cancer cells treated with the supernatant of nucleus pulposus cells containing FasL compared with those of controls (Fig. 5).
Fig. 4.
After treatment of the supernatant of nucleus pulposus cells containing FasL for 48 h, TUNEL shows a significantly increased apoptotic cell death (red staining) of MCF-7 breast cancer cells (b) compared with that of controls (a) (×40)
Fig. 5.
After treatment of the supernatant of nucleus pulposus cells containing FasL for 48 h, Western blot analysis shows increased expression of cleaved caspase-8, -9 and -3 in the lysates of MCF-7 breast cancer cells compared with that of controls. Human stomach and tonsil cells were used as the positive controls for caspase-8, -9 and -3, respectively
Discussion
Cancer cells have been found to exhibit de novo expression of FasL, which plays an important role in local tissue destruction, metastatic spread and immune escape [12, 15]. The expression of FasL by cancer cells enables them to kill Fas-bearing immune cells or stromal cells of the target organs or tissues. Therefore, FasL expression by cancer cells has been thought to facilitate the establishment of distant metastasis in organs, such as the liver and lungs. In the spine, however, the anatomical structure of the disc is often preserved, even in the face of severe destruction of the vertebral body by cancer cells. According to Fujita et al. [5], although many cancer cells are frequently found at the outer surface of the disc, none of the metastatic cancer cells are found at the inner portion of the discs. The intervertebral disc cells is known to express FasL, a prerequisite of a immune-privileged site, so that is able to kill Fas-bearing immune or cancer cells [14, 19]. We therefore undertook this study to investigate the hypothesis that FasL produced by nucleus pulposus cells kill Fas-bearing cancer cells infiltrating into the discs by the activation of caspases so as to resist metastatic seeding.
Two major pathways of Fas-mediated apoptosis have been identified [18, 21]. In the death receptor (extrinsic) pathway, caspase-8 directly activates caspase-3, an executioner of apoptosis, whereas in the mitochondrial (intrinsic) pathway, caspase-3 is activated by caspase-9 via mitochondria. In our study, we found a significantly increased apoptosis of Fas-expressing MCF-7 breast cancer cells by FasL produced by nucleus pulposus cells. In addition, the activation of two initiator caspases, caspase-8 and caspase-9, and an executioner caspase, caspase-3, of Fas-mediated apoptosis were identified. These results suggest that both pathways (death receptor and mitochondrial) of the Fas receptor are involved in the apoptosis of Fas-expressing cancer cells induced by FasL of nucleus pulposus cells. The human FasL is known to be highly homologous to that of the rat, so as to cross-react with cell-surface Fas from either species [13, 20, 22]. In addition, no species specificity is observed among human and rat FasL. Therefore, both human or rat FasL can induce apoptosis in the cells expressing either human Fas or rat Fas. The results of our study were in line with those of previous studies that demonstrated no species specificity of Fas and FasL interaction [13, 20, 22].
It might be possible that another biochemical compound released by nucleus pulposus cells into the supernatant increases the apoptosis of cancer cells. Therefore, we cannot definitely say that it was just the FasL in the supernatant of nucleus pulposus cells that increased the apoptosis of cancer cells. However, while there may be some unknown substance, it is well known that FasL is sufficient to induce the apoptosis of Fas-positive cancer cells. Further study is needed to investigate this issue.
In conclusion, our results demonstrate that Fas-bearing cancer cells undergo apoptotic cell death by FasL produced by nucleus pulposus cells by the activation of caspase pathways, which might be a possible biochemical explanation of the disc’s resistance to metastatic cancer.
Acknowledgement
This study was supported by the 2005 Catholic Cancer Center Research Funding Program.
References
- 1.Asdourian PL, Weidenbaum M, DeWald RL, et al. The pattern of vertebral involvement in metastatic vertebral breast cancer. Clin Orthop Relat Res. 1990;250:164–170. [PubMed] [Google Scholar]
- 2.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112:138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrettoni BA, Carter JR. Current concepts review: Mechanisms of cancer metastasis to bone. J Bone Joint Surg Am. 1986;68:308–312. [PubMed] [Google Scholar]
- 4.Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop. 1982;169:95–102. [PubMed] [Google Scholar]
- 5.Fujita T, Ueda Y, Kawahara N, et al. Local spread of metastatic vertebral tumors. A histologic study. Spine. 1997;22:1905–1912. doi: 10.1097/00007632-199708150-00020. [DOI] [PubMed] [Google Scholar]
- 6.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 7.Griffith TS, Ferguson TA. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18:240–244. doi: 10.1016/S0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Ashraf N, Kilburn J, et al. Vertebral endplate architecture and vascularization: application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine. 2005;30:2593–2600. doi: 10.1097/01.brs.0000187877.30149.83. [DOI] [PubMed] [Google Scholar]
- 9.Harrington KD. Metastatic disease of the spine. J Bone Joint Surg Am. 1986;68:1110–1115. [PubMed] [Google Scholar]
- 10.Kauppila LI. Ingrowth of blood vessels in disc degeneration. Angiographic and histological studies of cadaveric spines. J Bone Joint Surg Am. 1995;77:26–31. doi: 10.2106/00004623-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Langer R, Brem H, Falterman K, et al. Isolation of a cartilage factor that inhibits tumor neovascularization. Science. 1976;193:70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- 12.Müllauer L, Mosberger I, Grusch M, et al. Fas ligand is expressed in normal breast epithelial cells and is frequently up-regulated in breast cancer. J Pathol. 2000;190:20–30. doi: 10.1002/(SICI)1096-9896(200001)190:1<20::AID-PATH497>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Muneta Y, Shimoji Y, Inumaru S, et al. Molecular cloning, characterization, and expression of porcine Fas ligand (CD95 Ligand) J Interferon Cytokine Res. 2001;21:305–312. doi: 10.1089/107999001300177493. [DOI] [PubMed] [Google Scholar]
- 14.Park JB, Chang H, Kim KW. Expression of Fas ligand and apoptosis of disc cells in herniated lumbar disc tissue. Spine. 2001;26:618–621. doi: 10.1097/00007632-200103150-00011. [DOI] [PubMed] [Google Scholar]
- 15.Reichmann E. The biological role of the Fas/FasL system during tumor formation and progression. Semin Cancer Biol. 2002;12:309–315. doi: 10.1016/S1044-579X(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 16.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88:S10–S14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 17.Sprague JE, LI QP, Liang K, et al. In vitro and in vivo investigation of matrix metalloproteinase expression in metastatic tumor models. Nucl Med Biol. 2006;33:227–237. doi: 10.1016/j.nucmedbio.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada T, Nishida K, Doita M, et al. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine. 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Tanaka M, Inazawa J, et al. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Itai T, Adachi M, et al. Downregulation of Fas ligand by shedding. Nature Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 22.Tsuyuki S, Kono M, Bloom ET. Cloning and potential utility of porcine Fas ligand: overexpression in porcine endothelial cells protects them from attack by human cytolytic cells. Xenotransplantation. 2002;9:410–421. doi: 10.1034/j.1399-3089.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 23.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 24.Yasuma T, Yamauchi Y, Arai K, et al. Histopathologic study on tumor infiltration into the intervertebral disc. Spine. 1989;14:1245–1248. doi: 10.1097/00007632-198911000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Yuh WTC, Quets JP, Lee HJ, et al. Anatomic distribution of metastases in the vertebral body and modes of hematogenous spread. Spine. 1996;21:2243–2250. doi: 10.1097/00007632-199610010-00012. [DOI] [PubMed] [Google Scholar]