Abstract
Background:
Bolus thermodilution is the standard bedside method of cardiac output measurement in the intensive care unit (ICU). The Baxter Vigilance monitor uses a modified thermodilution pulmonary artery catheter with a thermal filament to give a continuous read-out of cardiac output. This has been shown to correlate very well with both the 'gold standard' dye dilution method and the bolus thermodilution method. Bioimpedance cardiography using the Bomed NCCOM 3 offers a noninvasive means of continuous cardiac output measurement and has been shown to correlate with the bolus thermodilution method. We investigated the agreement between the continuous bioimpedance and continuous thermodilution methods, enabling acquisition of a large number of simultaneous measurements.
Results:
A total of 2390 paired data points from seven patients were collected. There was no correlation (r2 = 0.01) between the methods. The precision (1.16 l/min/m2) of agreement between the Vigilance and the Bomed, assessed by the Bland-Altam method, was very poor although the bias (-0.16 l/min/m2) appeared fair.
Conclusions:
The Bomed NCCOM 3 bioimpedance monitor shows poor agreement with the Baxter Vigilance continuous thermodilution monitor in a group of general ICU patients and cannot be recommended for cardiac output monitoring in this situation.
Keywords: measurement techniques, impedance cardiography, thermodilution, monitoring, cardiac output
Introduction
The fluid bolus thermodilution method of cardiac output measurement, using a pulmonary artery catheter (PAC), has gained wide acceptance over the past 25 years. The advantages and disadvantages of the use of this method of monitoring critically ill patients are well established [1].
A recent development has been the introduction of 'continuous' cardiac output monitoring using a modified PAC (Continuous Cardiac Output/SvO2 Catheter model 746H8F, Baxter Healthcare Corporation, Round Lake, Illinois, USA). This catheter has a thermal filament that produces pulses of heat at the level of the right ventricle, and a thermistor at the tip in the pulmonary artery senses temperature change. A dedicated computer (Vigilance, Baxter Healthcare Corporation) is required, which updates calculated cardiac output every 30–60 s. This system has been previously investigated [2] and has shown a very strong correlation with both the 'gold standard' dye dilution technique (r2 = 0.91) and fluid bolus thermodilution (r2 = 0.97). It has also been evaluated specifically for use in critically ill patients [3] and in a bench model of pulmonary artery blood flow [4].
Bioimpedance cardiography has been developed over the past 30 years as a noninvasive technique to measure cardiac output. Monitors such as the Bomed Noninvasive Computerized Cardiac Output Monitor (NCCOM 3, Bomed Medical Manufacturing Ltd, Cheshire, UK) are commercially available to measure cardiac output. However, in the United Kingdom and elsewhere they have not achieved widespread usage. The NCCOM 3 uses eight spot electrodes, placed at the root of the neck and chest wall. A constant sinusoidal alternating current (2.5 mA rms, 70 kHz) is passed through the subject's chest and the impedance measured. By measuring the maximum rate of change of thoracic impedance during systole, timed from the electrocardiogram (ECG), the stroke volume is calculated. The Sramek-Bernstein formula is used, which calculates stroke volume as the volume of electrically participating intrathoracic tissue × ventricular ejection time × index of contractility, which is the ratio of the peak rate of change in thoracic bioimpedance and the thoracic fluid index (or total thoracic impedance). Cardiac output is then calculated from the product of heart rate and stroke volume, averaged over 16 cardiac cycles.
We have investigated the correlation between these two methods of continuous cardiac output measurement to determine their suitability for use in critically ill patients in the intensive care unit (ICU).
Methods
We compared the Bomed NCCOM 3 with the Baxter Vigilance in a mixed group of seven ICU patients. All patients required pulmonary artery catheterization on clinical grounds. In two patients, an existing PAC was exchanged for a continuous cardiac output PAC, using the same introducer sheath. An explanation of the use of noninvasive bioimpedence monitoring as part of a research study was given to the patients' relatives and assent was obtained. The primary pathologies of the patients were acute pancreatitis (one), emergency repair of an abdominal aortic aneurysm (two), appendix abscess (one), probable pulmonary embolism (one), cholangitis following cholecystectomy (one) and respiratory failure (one). The study was purely observational, and required no alterations in therapy.
For bioimpedance cardiography, eight standard ECG gel electrodes and, if necessary, two additional electrodes for ECG monitoring were applied, according to directions printed on the NCCOM 3 monitor. Timed data points were saved on a personal laptop computer connected to the NCCOM 3 using the CDDP version SI 4.05 software (CDIc, Irvine, California, USA).
For thermodilution measurements, a continuous cardiac output PAC connected to the Vigilance monitor was inserted via the internal jugular or subclavian vein. Timed data was stored in the patient monitoring system (Hewlett Packard Ltd, Boise, Idaho, USA), using the 'Vue-link' software to connect the two devices. A print-out of cardiac output data at 1-min intervals was obtained at the end of the study period.
Any discrepancy between the clocks on the two monitors was accounted for by noting the times displayed at the start of measurement and allowing for this when pairing the data. In this way we ensured that the paired data points were accurately synchronized.
Body surface area was calculated by each device in order to obtain 'indexed' measurements, by entering the patients' height and weight, estimated if necessary. We verified that both devices produced the same body surface area, so eliminating this source of bias error.
Each patient was monitored for a period of approximately 6 h, acquiring simultaneous paired cardiac index data points at 1-min intervals. The data points were analysed using SPSS for Windows release 6.1 (SPSS Inc, Chicago, Illinois, USA) and r2 was calculated using regression analysis. A plot of the difference between measurements against the mean of the measurements was then constructed according to the technique for assessing agreement between two methods of clinical measurement described by Bland and Altman [5].
Results
Seven patients were studied; the mean (± SD) age was 63 ± 16 years, mean weight 86 ± 31 kg and mean body surface area 2.0 ± 0.34 m2. A total of 2390 simultaneous paired cardiac index data points were analysed, with approximately equal numbers of data points from each patient. The patients were all mechanically ventilated; other ongoing supportive care included vasopressor and inotropic support and renal replacement therapy (continuous haemofiltration or intermittent haemodialysis) as required by the individual patient. Positive end-expiratory pressure (PEEP) was used as clinically indicated, up to 10 cm H2O. During the study none of the patients suffered new dysrhythmias requiring treatment or interfering with cardiac output measurement.
The range of cardiac index measurements was 1.40–7.20 l/min/m2 (mean 3.50 ± 0.95 l/min/m2), by the Bomed, and 1.60–5.60 l/min/m2 (mean 3.65 ± 0.77 l/min/m2) by the Vigilance monitor. There was essentially no relationship between the two methods (r2 = 0.01; Fig 1). The correlation coefficients for individual patients were -0.25, -0.21, 0.41, 0.25, -0.39, -0.16 and 0.06, respectively.
Figure 1.
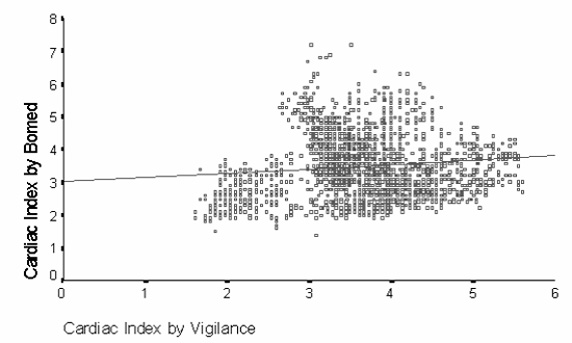
Scatter plot of Bomed bioimpedance vs Vigilance thermodilution continuous measurement of cardiac index (r2 = 0.01).
A Bland–Altman plot (Fig 2) showed a poor degree of agreement between the methods. Although the degree of bias was acceptable, the precision was very poor. The mean of the differences (bias) was -0.16 l/min/m2, but with a standard deviation (precision) of ± 1.16 l/min/m2. The lower and upper limits of agreement were -2.48 and 2.16 l/min/m2 respectively. Bland–Altman analysis of individual patients showed bias measurements of -1.31, -0.95, 0.28, 0.59, 0.88, 0.76 and -1.32 l/min/m2, with precision of 0.59, 0.63, 0.59, 0.61, 1.10, 0.60 and 0.74 l/min/m2, respectively. Three of the patients showed a poor precision throughout the measured range of cardiac output. The other four patients showed a fair degree of precision, but with a changing bias, from negative to positive, with increasing cardiac output. There were no clear factors which could be used to predict which group individual patients would fall into.
Figure 2.
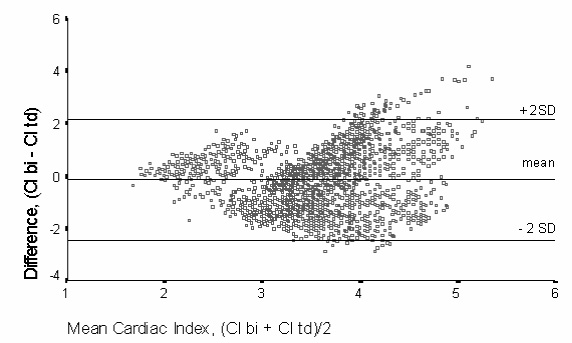
Bland–Altman plot of difference in cardiac index measured by bioimpedance cardiography (bi) and continuous thermodilution (td) against mean measured cardiac index (Cl; l/min/m2). The degree of bias (measured by the mean) and precision (± 2SD) are shown.
Discussion
The morbidity and mortality associated with the use of PACs is well recognized and must be weighed against the potential benefits for the individual patient of gaining valuable information regarding the cardiovascular system and oxygen delivery [6]. Bioimpedance cardiography offers an apparently attractive noninvasive way of estimating cardiac output and obtaining derived haemodynamic parameters. However, this is of no benefit if the information acquired is unreliable, leading to inappropriate management. We investigated the use of bioimpedance in the critically ill to assess whether it can be a reliable method of cardiac output measurement for this group of patients.
The thermodilution method is an indirect measure of cardiac output, but has been shown to correlate well with the gold standard dye dilution method. Moreover, it is the method most commonly used to measure cardiac output in patients in the ICU and upon which much of our understanding of the cardiovascular changes in critical illnesses is based.
Bioimpedance cardiography has been validated in some patient groups [7], and newer systems with improved software for advanced signal processing may be valid in critically ill patients [8]. However, there have been concerns raised as to its accuracy and reliability in ICU patients [9,10]. Correct placement of the eight electrodes is important in obtaining accurate information; in ICU patients this may be hampered by dressing covering internal jugular line sites, thoracotomy wounds and chest drain sites. In this study, the directions for electrode placement detailed on the NCCOM 3 monitor were followed as a closely as practically possible, aiming to reproduce the conditions that would pertain to routine clinical use of the monitor.
The use of positive pressure ventilation with PEEP, and the presence of endotracheal tubes, chest drains and sternal wires may affect bioimpedance measurements by affecting the rate of change of thoracic impedance [11]. However, cardiological studies in patients with pacemakers have shown bioimpedance to be a useful technique [12]. The presence of the thermal filament in the modified PAC used by the Vigilance monitor may also affect bioimpedance measurements; standard PACs may also affect measurements by the presence of the thermistor wire. In any case, if the bioimpedance data are affected by foreign material in the thorax, this makes the use of the Bomed in ICU patients very problematic.
The design of this study is novel in two ways, giving major advantages over previous studies. Firstly, by comparing two 'continuous' methods of cardiac output measurement, the inevitable errors of synchronization using intermittent methods are virtually eliminated. (Previous studies comparing bioimpedance with thermodilution have needed to average several bolus measurements before or after the acquisition of bioimpedance data.) Secondly, we were able to collect a very large number of simultaneous paired data points from the two methods, averaged over the whole respiratory cycle, enabling a more accurate comparison.
This study shows there is essentially no relationship between cardiac index as measured by the Bomed NCCOM 3 and the coninuous thermodilution method (r2 = 0.01); this is surprising as the two methods claim to measure the same variable. There is extremely poor agreement between the methods according to the Bland–Altman method. The lack of precision is quite unacceptable. From our data, a cardiac index of 4.0 l/min/m2 would be subject to an error of up to +54% or -62% at the 95% limits of agreement. Importantly, we were able to assess the changes in measured cardiac output in individual patients and these also showed poor agreement with variable precision and bias. This would indicate that the use of the NCCOM 3 is unlikely to be of value even if a subgroup of patients, in whom the precision is acceptable, could be identified.
The two methods cannot therefore be used interchangeably to monitor cardiac output. Furthermore, therapeutic interventions to improve cardiovascular function that have been shown to be beneficial in patients monitored by the thermodilution technique cannot be similarly applied to patients monitored by bioimpedance cardiography. Bioimpedance cardiography cannot be recommended for use in critically in patients such as this group from a general ICU.
References
- Soni N. Swan song for the Swan-Ganz catheter? BMJ. 1996;313:763–764. doi: 10.1136/bmj.313.7060.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Zollner C, Briegel J, Forst H. Evaluation of a new continuous cardiac output monitor in critically ill patients: a prospective criterion standard study. Crit Care Med. 1995;25:860–866. doi: 10.1097/00003246-199505000-00014. [DOI] [PubMed] [Google Scholar]
- Boldt J, Menges T, Wollbruck M, Hammermann H, Hemplemann G. Is continuous cardiac output measurement using thermodilution reliable in the critically ill patient? Crit Care Med. 1994;22:1913–1918. [PubMed] [Google Scholar]
- Mihaljevic T, von Segesser L, Tonz M, Leskosek B, Seifert B, Jenni R, Turina M. Continuous versus bolus thermodilution cardiac output measurements — a comparative study. Crit Care Med. 1995;23:944–949. doi: 10.1097/00003246-199505000-00025. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;I:370–310. [PubMed] [Google Scholar]
- Ginosar Y, Thijs LG, Sprung CL. Raising the standard of hemodynamic monitoring: targeting the practice or the practitioner? . Crit Care Med. 1997;25:209–211. doi: 10.1097/00003246-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Ciampani N, Costantini C, Blandini A, Purcaro A. Comparison of impedance cardiography with thermodilution and direct Fick methods for noninvasive measurement of stroke volume and cardiac output during incremental exercise in patients with ischaemic cardiomyopathy. Am J Cardiol. 1996;77:1293–1301. doi: 10.1016/s0002-9149(97)89153-9. [DOI] [PubMed] [Google Scholar]
- Shoemaker WC, Wo CCJ, Bishop MH, Appel PL, van de Water JM, Harrington GR, Wang X, Patil RS. Multicenter trial of a new thoracic electrical bioimpedance device for cardiac output estimation. Crit Care med. 1994;22:1907–1912. [PubMed] [Google Scholar]
- Young JD, McQuillan P. Comparison of thoracic electrical bioimpedance and thermodilution for the measurement of cardiac index in patients with severe sepsis. Br J Anaesth. 1993;70:58–62. doi: 10.1093/bja/70.1.58. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Raffin TA. Thoracic electrical bioimpedance measurement of cardiac output — Not ready for prime time. Crit Care Med. 1993;21:1111–1112. doi: 10.1097/00003246-199308000-00004. [DOI] [PubMed] [Google Scholar]
- Sageman WS, Amundsen DE. Thoracic electrical bioimpedance measurements of cardiac output in postaortocoronary bypass patients. . Crit Care Med. 1993;21:1139–1142. doi: 10.1097/00003246-199308000-00011. [DOI] [PubMed] [Google Scholar]
- Ovsyscher I, Furman S. Impedance cardiography for cardiac output estimation in pacemaker patients: review of the literature. Pacing Clin Electrophysiol. 1993;16:1412–1422. doi: 10.1111/j.1540-8159.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]


