Abstract
We analysed the results of 24 cases of aggressive benign and malignant tumours of the distal radius treated by resection and prosthetic replacement between 1995 and 2006. Patient ages ranged from 18 to 74 years, averaging 33 years; 18 were males. Recurrent giant cell tumour was the most common tumour. The prosthesis used was a bipolar hinge custom mega prosthesis manufactured locally. Average follow-up was 78 months. The average Musculoskeletal Tumor Society (MSTS) functional score achieved was 75%. The ten-year prosthesis survival was rate 87.5%. Infection was the most common complication.
Resume
Prothèses sur mesure dans les tumeurs du radius distal. Indications de remplacement. Nous avons analysé les résultats de 24 cas de tumeurs malignes et bégnines agressives de l’extrémité distale du radius traitées par résection et prothèses entre 1995 et 2006. Les patients étaient âgées de 18 à 74 ans avec un âge moyen de 33 ans dont- 18 étaient des hommes. La cause la plus fréquente a été la récidive de tumeur à cellules géantes. La prothèse utilisée était une prothèse bipolaire à charnière sur mesure fabriquée localement. Le suivi moyen a été de 78 mois. Le score moyen MSTS après traitement était de 75%, la courbe de survie à 10 ans de 87,5%. La complication la plus fréquente a été l’infection.
Introduction
The distal radius is a relatively common skeletal site for primary bone tumours and is the third most common location for giant cell tumour [7]. En bloc resection of the distal radius is indicated for malignant lesions and aggressive benign lesions [3]. This poses a dual problem of skeletal reconstruction and functional restoration because of the high functional demands of the hand, the youth and long life expectancy of the patients especially in benign tumours, the limited surrounding soft tissue and the proximity of the adjacent nerves and tendons. The various procedures described include arthrodesis using bulk autograft [14], ulnar translocation [20], reconstruction with non-vascularised or vascularised fibular grafts [13, 19], osteoarticular allograft [11], and prosthetic replacement [6]. Despite extensive experience with prosthetic replacement of juxta-articular tumours of the lower limb, there are few reports of prosthetic replacement of the distal radius along with any long-term outcome [9]. We present here our experience over a decade with endoprosthethic replacement for distal radial tumours.
Materials and methods
Twenty-four patients with distal radial tumours who underwent wide resection and replacement by custom mega prosthetic arthroplasty during the period 1995 to 2006 were analysed in this study. There were 18 males and six females with a mean age of 33 years (range 18–74 years). The indication for wide resection and reconstruction was a benign tumour that extended through the cortex or articular surface of the distal radius, a recurrent benign lesion that was invasive or a malignant lesion. Nine patients had primary giant cell tumours, seven had giant cell tumours that were recurrent after a primary curettage and eight had osteosarcoma. All patients who had a primary lesion had a needle or incisional biopsy for diagnosis before the reconstruction. Staging evaluation consisted of plain radiographs, CT scan, MRI and bone scans when appropriate. According to the MSTS system [4] of staging, eleven patients had stage 3 GCT and five were in stage 2. Among patients with osteosarcoma, five patients were in stage IIA and three in stage IIB and all of them received pre-op chemotherapy.
The prosthesis
A bipolar hinge custom prosthesis of the distal radius with wrist joint manufactured in Chennai, India, was used in all cases. The design has been modified and upgraded over the years. The basic components include a carpal stabiliser, a bipolar hinge component and a distal radial shaft component (Fig. 1). The double hinge mechanism enables movements at the anteroposterior and mediolateral planes. The proximal end is fixed with screws or polymethylmethacrylate (PMMA) cement and the distal end is secured to carpal bones by screws. The components are made of surgical grade stainless steel (316 L) with ultra high molecular weight polyethylene inserts.
Fig. 1.
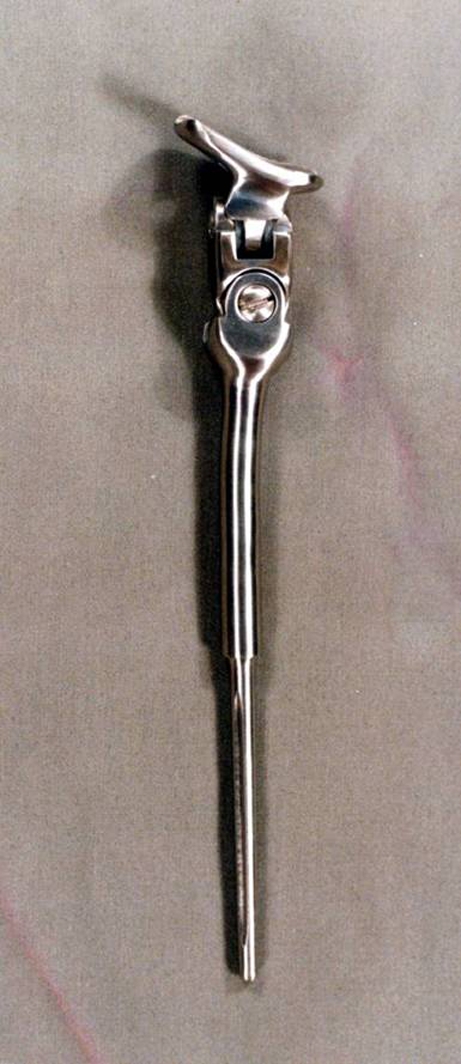
Distal radius custom prosthesis with bipolar hinge joint
Surgical technique
Under general anaesthesia, through a standard dorsal longitudinal incision overlying the radius and including the previous biopsy site, en bloc resection of the tumour was performed. A wide margin of resection was achieved in 16 patients, while a marginal margin was accepted in eight patients. Tumours which extended into radioulnar or radiocarpal joints required resection of the distal ulna or the proximal carpal row. A custom made distal radius with wrist joint prosthesis with appropriate dimensions obtained from the preoperative radiographs was used for reconstruction in all patients. The proximal stem was fixed with PMMA cement to the radial shaft and distally screws were used to fix the prosthesis to the carpal bones. The limb was immobilised in an above-the-elbow cast for four weeks following which mobilisation was started.
Results
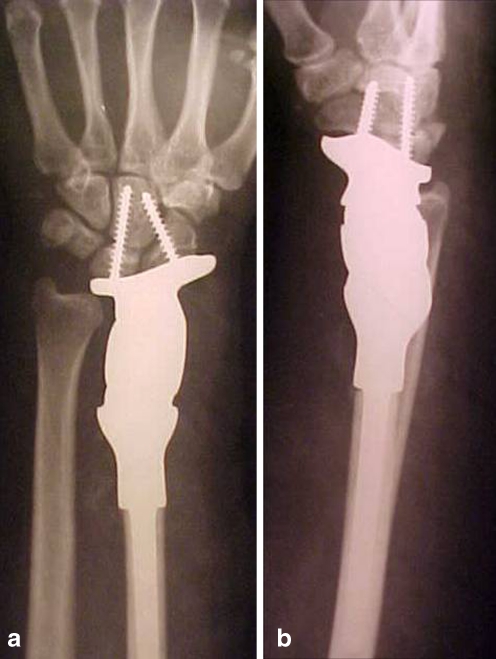
Patients were evaluated every three months during the first year and every six months thereafter with a physical examination and plain radiography. The minimum follow-up was 24 months and maximum follow-up was 156 months, averaging 78 months. On analysing the oncological outcome, all patients were alive with no evidence of recurrence of the disease at the latest follow-up. The Kaplan Meier estimator [10] was used to calculate the ten-year prosthesis survival rate which was 87.5%. The preoperative and six-year follow-up radiographs of a patient are given in Figs. 2 and 3a,b.
Fig. 2.
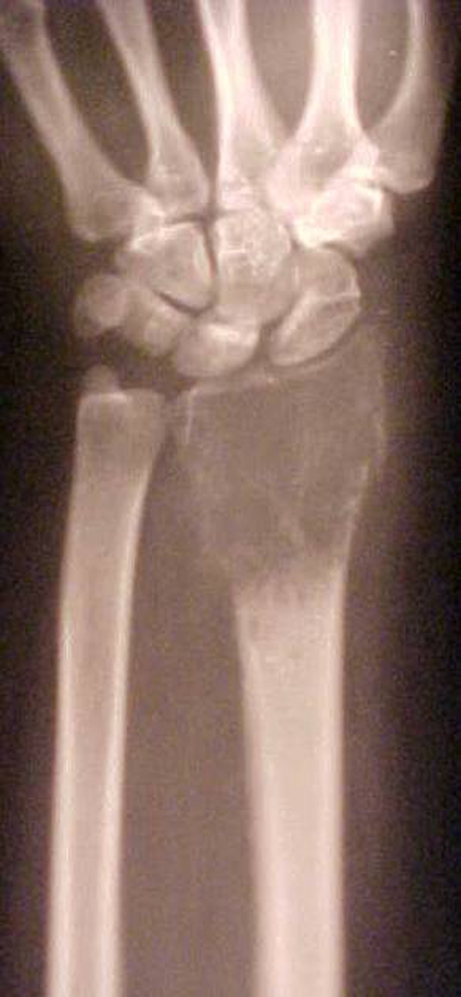
Preoperative X-ray (anteroposterior) of a patient (no. 8)
Fig. 3.
a Postoperative X-ray after six years (anteroposterior view). b Postoperative after six years (lateral view)
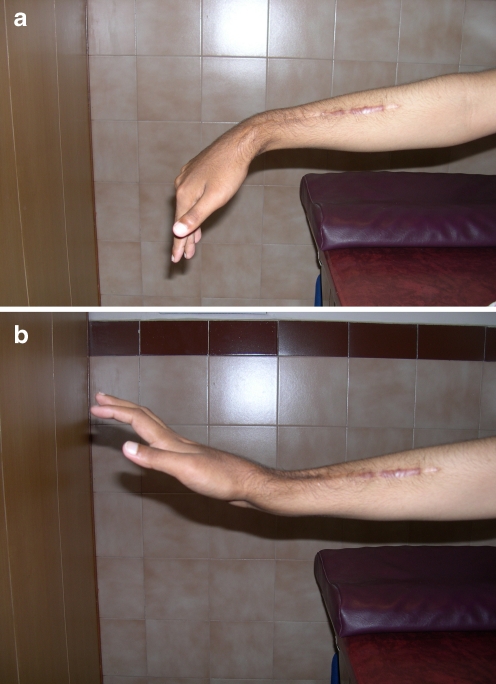
Functional results reveal that the average range of movement at the operated wrist were: active extension 20° (range 10–50°), flexion 25° (range 5–40°), radial deviation 10° (range 5–15°), ulnar deviation 15° (range 5–20°), pronation 60° (range 25–85°), and supination 40° (range 5–80°). Movement and sensation of the fingers and thumb were satisfactory in all patients. The modified rating system of the Musculoskeletal Tumour Society [5] was used to determine the functional outcome which was 74%. The functional photographs of a patient at six-year follow-up are shown in Fig. 4a,b.
Fig. 4.
a Functional photograph of wrist after six years (volar flexion). b Functional photograph of wrist after six years (dorsi flexion)
Complications, both major and minor in nature, occurred in four patients. Among early complications, skin flap necrosis occurred in two patients. An additional plastic surgical procedure failed in both of them requiring removal of the prosthesis and wrist arthrodesis with bone grafts. Wound infection occurred in two patients. One of them responded to wound lavage and antibiotics while the other required removal of the prosthesis and wrist arthrodesis. Aseptic loosening was observed as a late complication in two patients. Both of these patients were asymptomatic and were satisfied with their function despite radiological evidence of loosening.
Discussion
Reconstruction of defects that remain after excision of the neoplastic lesion of the distal radius remains a challenge in orthopaedic oncology. Achieving adequate clearance of the tumour without the risk of local recurrence while preserving good hand function proves to be a daunting task, due to the unique anatomical constraints such as proximity to complex joints, limited soft tissue cover and the close relationship to important vessels, nerves and tendons. When the architecture of the distal radius is preserved, extended curettage is the procedure of choice for primary benign lesions [7, 15]. However, when the lesion is malignant or locally aggressive with extra capsular or extra compartmental soft tissue extension, excision permits a lower rate of recurrence [8, 12, 15, 18], especially in the distal radius [1]. Local recurrence needs to be dealt more radically [22].
Radiocarpal arthrodesis using bone grafts gives good stability at the cost of mobility but was associated with fairly high rates of fracture of the graft and donor site morbidity [14, 21]. Translocation of ulna with carpoulnar arthrodesis gives restricted mobility with poor hand function [2].
Osteoarticular allograft reconstruction has the advantages of no donor site morbidity, shorter operating time and greater congruency of the radiocarpal joint. Complications include nonunion, fractures, slow incorporation of the allograft and possibility of transmitting infectious diseases [11, 17]. Furthermore, allografts are not easily procurable in most countries.
Replacement using non vascularised and free vascularised fibular grafts with and without arthrodesis has been the method of choice for reconstruction of distal radius. It gives limited range of motion of the wrist, due to improper articulation, which leads to progressive and accelerated degenerative changes of the carpo-fibular joint [13]. Complications include nonunion, delayed union, fracture of the graft, subluxation of the wrist joint and donor site morbidity [16].
Initial attempts to replace distal radius by prosthesis have failed, necessitating arthrodesis or amputation. This was largely because these prostheses were primitive, did not include the built-in joint for wrist mobility, and did not attempt to stabilise or reconstruct the radiocarpal articulation [6, 9]. By using a distal radius prosthesis with bipolar wrist joint we were able to achieve a good functional outcome, comparable to those of fibular grafting [13, 16] without its inherent complications. Long-term follow-up of our patients shows satisfactory function with no pain or clinical signs of loosening, which suggests that this method may be an acceptable alternative for functional joint reconstruction in adults.
Conclusion
Custom prosthetic replacement for malignant and aggressive benign tumours of the distal radius has been proven to produce a good functional outcome with an acceptable rate of complications. However, careful patient selection, a precise preoperative work-up, and a meticulous surgical technique are essential to achieve the desired outcome.
References
- 1.Briggs TWR, Cobb J, McAuliffe T, Pringle J, Kemp H. Giant cell tumours of bone. J Bone Joint Surg. 1990;72B:937. [Google Scholar]
- 2.Campanacci M, Laus M, Boriani S. Resection of the distal end of the radius. Ital J Orthop Traumatol. 1979;5(2):145–152. [PubMed] [Google Scholar]
- 3.Eckardt JJ, Grogan TJ (1986) Giant cell tumor of bone. Clin Orthop Relat Res (204):45–58 [PubMed]
- 4.Enneking WF, Spanier SS, Goodman MA (2003) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res (415):4–18 [DOI] [PubMed]
- 5.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ (1993) A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res (286):241–246 [PubMed]
- 6.Gold AM. Use of a prosthesis for the distal portion of the radius following resection of a recurrent giant-cell tumor. J Bone Joint Surg Am. 1957;39(6):1374–1380. [PubMed] [Google Scholar]
- 7.Goldenberg RR, Campbell CJ, Bonfiglo M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg. 1970;52:619–663. [PubMed] [Google Scholar]
- 8.Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg [Am] 2004;29(2):188–193. doi: 10.1016/j.jhsa.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Hatano H, Morita T, Kobayashi H, Otsuka H. A ceramic prosthesis for the treatment of tumours of the distal radius. J Bone Joint Surg Br. 2006;88(12):1656–1658. doi: 10.1302/0301-620X.88B12.17989. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 11.Kocher MS, Gebhardt MC, Mankin HJ. Reconstruction of the distal aspect of the radius with use of an osteoarticular allograft after excision of a skeletal tumor. J Bone Joint Surg Am. 1998;80(3):407–419. doi: 10.2106/00004623-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Labs K, Perka C, Schmidt RG. Treatment of stages 2 and 3 giant-cell tumor. Arch Orthop Trauma Surg. 2001;121(1–2):83–86. doi: 10.1007/s004020000158. [DOI] [PubMed] [Google Scholar]
- 13.Lackman RD, McDonald DJ, Beckenbaugh RD, Sim FH (1987) Fibular reconstruction for giant cell tumor of the distal radius. Clin Orthop Relat Res (218):232–238 [PubMed]
- 14.Leung PC, Chan KT (1986) Giant cell tumor of the distal end of the radius treated by the resection and free vascularized iliac crest graft. Clin Orthop Relat Res (202):232–236 [PubMed]
- 15.Malek F, Krueger P, Hatmi ZN, Malayeri AA, Faezipour H, O’Donnell RJ. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int Orthop. 2006;30:495–498. doi: 10.1007/s00264-006-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruthainar N, Zambakidis C, Harper G, Calder D, Cannon SR, Briggs TW. Functional outcome following excision of tumours of the distal radius and reconstruction by autologous non-vascularized osteoarticular fibula grafting. J Hand Surg [Br] 2002;27(2):171–174. doi: 10.1054/jhsb.2001.0707. [DOI] [PubMed] [Google Scholar]
- 17.Matejovsky Z, Jr, Matejovsky Z, Kofranek I. Massive allografts in tumour surgery. Int Orthop. 2006;30:478–483. doi: 10.1007/s00264-006-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76(12):1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Pho RW. Malignant giant-cell tumor of the distal end of the radius treated by a free vascularized fibular transplant. J Bone Joint Surg Am. 1981;63(6):877–884. [PubMed] [Google Scholar]
- 20.Seradge H. Distal ulnar translocation in the treatment of giant-cell tumors of the distal end of the radius. J Bone Joint Surg Am. 1982;64(1):67–73. [PubMed] [Google Scholar]
- 21.Sheth DS, Healey JH, Sobel M, Lane JM, Marcove RC. Giant cell tumor of the distal radius. J Hand Surg [Am] 1995;20(3):432–440. doi: 10.1016/S0363-5023(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 22.Szendröi M. Giant-cell tumour of bone. J Bone Joint Surg Br. 2004;86(1):5–12. [PubMed] [Google Scholar]