Abstract
Objective
Peripheral nerve injury often leads to neuropathic pain, which is characterized by burning pain, allodynia, and hyperalgesia. The role of the sympathetic nervous system in neuropathic pain is a complex and controversial issue. It is generally accepted that the alpha adrenoreceptor (AR) in sympathetic nerve system plays a significant role in the maintenance of pain. Among alpha adrenoreceptor, alpha-1 receptors play a major role in the sympathetic mediated pain. The primary goal of this study is to test the hypothesis that sympathetically maintained pain involves peripheral alpha-2 receptors in human.
Methods
The study was a randomized, prospective, double-blinded, crossover study involving twenty patients. The treatments were : Yohimbine (30 mg mixed in 500 mL normal saline), and Phentolamine (1 mg/kg in 500 mL normal saline) in 500 mL normal saline at 70 mL/hr initially then titrated. The patients underwent infusions on three different appointments, at least one month apart. Thus, all patients received all 2 treatments. Pain measurement was by visual analogue scale, neuropathic pain questionnaire, and McGill pain questionnaire.
Results
There were significant decreases in the visual analogue scale, neuropathic score, McGill pain score of yohimnine, and phentolamine.
Conclusion
We conclude that alpha-2 adrenoreceptor, along with alpha-2 adrenoreceptor, may be play role in sympathetically maintained pain in human.
Keywords: Reflex sympathetic dystrophy, Yohimbine, Alpha-2 antagonist
INTRODUCTION
Sympathetically maintained pain (SMP) refers to pain which is dependent upon sympathetic efferent activity or humoral sympathetic mediators reaching the affected region. Standard diagnosis and treatment protocol for SMP involve blockade of sympathetic activity to the painful region. Previous studies suggest that alpha-adrenergic receptors (AR) play a significant role in the maintenance of pain16). The intravenous administration of the nonspecific alpha-blocker phentolamine can result in similar pain relief as that of blockade of the sympathetic ganglion (e.g., stellate ganglion or lumbar sympathetic ganglia)9,16,21). Hence, it was presumed that alpha-1 receptors play a major role in the SMP. However, in an animal model18), following nerve injury, there appears to be an increase in alpha-2 like AR sites at peripheral sensory neurons. We hypothesize that SMP in human results in increased numbers of alpha-2 like adrenergic receptors; therefore a specific alpha-2 blocker such as yohimbine may be useful as diagnostic or therapeutic agent.
The aim of the present study is to determine whether SMP in humans is based on proliferation or upregulation of alpha-2 receptors on nerve terminals.
MATERIALS AND METHODS
We performed a randomized, prospective, double-blinded, crossover study involving twenty patients and two treatments). The study was conducted with the full approval of the Institutional Review Board. Inclusion criteria were : 1) all patients had clinical evidence of SMP. In practical terms, this would mean pain symptoms in an appropriate distribution characterized by burning, shooting, or stabbing pain after injury, associated with allodynia, hyperpathia, edema, trophic changes, or alterations in temperature or color; 2) > 50% pain relief from sympathetic blockade (stellate ganglion block for upper extremity, lumbar sympathetic block for lower extremity); and 3) ages greater than 18. The exclusion criteria were patients with history of gastric or duodenal ulcer, vascular disease, arrhythmia, ischemic heart disease, or renal insufficiency. No pregnant or breast feeding patients enrolled in this study. For women of childbearing age, serum pregnancy test was performed at beginning of the study.
The patient received an intravenous infusion of either : Yohimbine (Y, 30 mg mixed in 500 mL normal saline), or Phentolamine (P, 1 mg/kg) in 500 mL normal saline in 500 mL normal saline at 70 mL/hr initially then titrate to keep heart rate < 130 rate/min or 160 rate/min > systolic blood pressure > 85 mmHg.
The patient underwent infusions on three different days, at least one month apart. Each day, the subject received one of the two infusions in randomized order. Both the patient and person doing the pain evaluations were blinded to the drug infused. Pain measurement was based on a visual analogue scale (VAS) score, neuropathic pain questionnaire, short-form McGill pain questionnaire. The baseline pain score was recorded for spontaneous pain as well as pain elicited by moving or touching the affected extremity. The subjects were asked to answer pain questionnaire before and after the infusion and to provide a log of their pain symptoms for 24 hours following each infusion. Blood pressure was recorded every 5 minutes. Electrocardiography (ECG) was monitored continuously. Sympathetic block was assessed through cutaneous vasodilatation and nasal stiffness. Following the completion of the study, all patients received a one month follow-up.
The effect for each infusion was estimated as the change in pain score from before to after the infusion (change after Y, and P). Then the differences between effects of treatments were calculated as the differences between pairs of these pre-to-post change scores (change after Y minus change after P, etc.) The significance of both sets of differences was tested with Wilcoxon signed rank tests. The effect of patient gender on the differences between treatment effects was tested with Wilcoxon rank sum tests, and the effects of patient age and pre-treatment duration of pain on the differences between treatment effects was tested with Spearman correlation tests. Non-parametric statistical tests were used due to the expected non-normal distribution of change scores.
Type I error rate < 0.05 was considered significant. No correction was made for multiple comparisons. In a follow up analysis, the method of generalized estimating equations with unstructured correlation structure was used to test effects of carry-over and treatment period (1st, 2nd, or 3rd)22). The sample size of 20 was specified in advance to provide 90% power to detect a difference in the amount of change in pain score between treatments of at least 2.8.
RESULTS
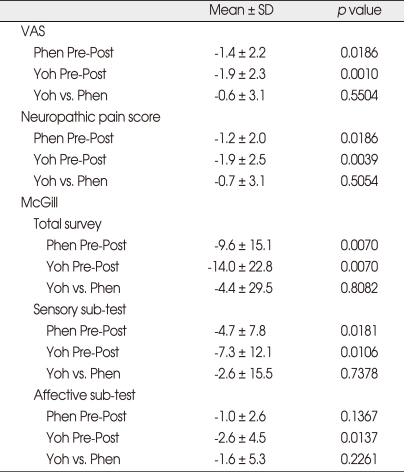
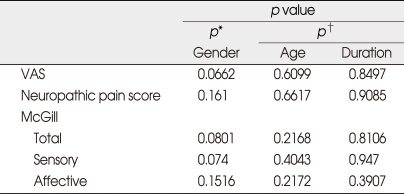
Twenty patients completed the study. Thirteen (65%) were female. The painful location was in the upper extremities in 12 patients, in the lower extremities in 8. Descriptive statistics on the amount of change in pain scores from before to after infusion, and on differences between treatments in amount of change, are shown in Table 1. For two treatments, changes from before to after infusions were significant in almost all measures and sub-tests of pain (p < 0.05) except affective scores of phentolamine. However, the amount of change was not significantly different between two active treatments themselves, for any of the pain responses (Table 1). No significant effect of gender, age, or pre-infusion duration of pain on difference between treatments was seen (Table 2). No effect of carry-over or treatment period on response was found to be significant.
Table 1.
Descriptive statistics on amount of change pre-post treatment and differences between treatments in amount of change
Phen: phentolamine, Post: posttreatment, Pre: pretreatment, VAS: visual analogue scale, Yoh: yohimbine
Table 2.
Tests for effect of Covariables on pain measure differences : testing effect of single covariable on difference between yohimbine and phentalamine in response to treatment (pre-post change)
*p value from Wilcoxon rank-sum test of gender groups. †p value from Spearman correlation test. Phen: phentolamine, Post: posttreatment, Pre: pretreatment, VAS: visual analogue scale, Yoh: yohimbine
DISCUSSION
In this study, we found the amount of change was not statistically significant different between yohimbine and phentolamine. The main results indicate that alpha-1 and/or alpha-2 AR involved SMP, and unknown mechanism might exist.
Peripheral nerve injuries sometimes create ectopic alpha AR that render them sensitive to sympathetic activity2,12) and lead to neuropathic pain, including such symptoms as spontaneous burning pain, hyperalgesia and allodynia1). This is often referred to as SMP. It is generally accepted that the alpha AR plays a significant role in the maintenance of pain. The intravenous administration of the nonspecific alpha-blocker phentolamine can result in similar pain relief as that of blockade of the sympathetic ganglion16). Hence, it was presumed that alpha-1 AR play a major role in the SMP13,17). However, in an animal model19) following nerve injury, there appears to be an increase in alpha-2 like AR sites at peripheral sensory neurons and cutaneous nociceptor in rabbit become sensitive to adrenergic agonist. And also chronic inflammation initiates circumstances in which alpha-2 AR-mediated sympathetic activity excites some nociceptor20). Some studies demonstrated that alpha-2 AR is more important in neuropathic pain3,11,14,18,26,29,31).
We have shown that yohimbine relieves pain. After a chronic nerve constriction, the dorsal root ganglion becomes a source of abnormal activity modulated by sympathetically released norepinephrine acting on alpha-2 AR in dorsal root ganglion somata. This neuropathic activity may contribute to cutaneous pain and hyperalgesia31). About 40% of the nociceptive C fibers developed an evoked response to close arterial injection of norepinephrine (NE) after a partial injury of the great auricular nerve in the rabbit. The adrenergic sensitivity of cutaneous nociceptors developed as early as 7 days after partial nerve injury18). Thus, the increased sensitivity of cutaneous nociceptors to NE may contribute to the hyperalgesia in the early stages of the nerve injury26). Later, more alpha-2 AR will be expressed in the dorsal root ganglion cells that will induce NE sensitivity and possibly contribute to hyperalgesia and ongoing pain. This effect was blocked by yohimbine and we obtained the same result.
Yohimbine produces antinociception4,15,20). Shannon and Lutz20) demonstrated that yohimbine produces dose-related antinociception in formalin test in rats which is mediated in part by agonistic action at 5-HT1A receptor. The affinity for serotonin receptor is only approximately twofold lower than its affinity alpha-2 receptor30). In contrast, yohimbine does not produce antinociception23,25) or systemic administration of alpha-1 antagonist attenuated cold allodynia, where alpha-2 antagonist exacerbates it10).
Yohimbine administed intravenously can induce transient moderate increases in blood pressure and heart rate6). Systemic administration of yohimbine augments sympathoadrenal outflow and blocks presynaptic alpha-2 AR, releasing neurotransmitter norepinephrine into blood stream5,24). Dose dependent adverse reactions from yohimbine reported previously by Guthrie et al.7) include tachycardia, elevation of blood pressure, nausea, vomiting, and anxiety. To minimize the cardiovascular effects of yohimbine, we used 30 mg yohimbine mixed 500 mL saline by basal infusion 70 mL/hr. We experienced one patient who developed severe tachycardia after 50 mg yohimbine infusion with 500 mL saline. After that, we reduced the yohimbine dose to 30 mg. Also, patients were kept supine.
These results were similar to the extent that phentolamine reduced pain. Phentolamine is mixed alpha-1/alpha-2 AR antagonist. Raja et al.16) demonstrated that intravenous phentolamine achieved pain relief in 20 patients with pain and hyperalgesia to mechanical and cooling stimuli in extremity and was a sensitive alternative test to identify sympathetic ganglion block. Tracey et al.26) found that subcutaneous injection of phentolamineinto affected hid paw eliminated mechanical hyperalgesia. But, Verdugo and Ochoa28) reported that rate of pain reduction was 9.2% (7 in 77) during phentolamine infusion. The pharmacological manipulation of the alpha-1 AR by either agonist or antagonist drugs does not influence neuropathic pain28). In this study, phentolamine was infused 1 mg/kg. By preparing these drugs in 500 mL volume, patient safety is enhanced by infusing a more diluted solution. The risk of volume overload is minimal, since yohimbine, phentolamine, and nitroglycerin cause direct peripheral vasodilation. The vital signs (blood pressure, heart rate, and oxygen saturation) and visual analog pain score were documented every 5 minutes during the infusion and up to 60 min after completion of the infusion. Due to the short half life of intravenous phentolamine, yohimbine, and nitroglycerin, 60 minutes of post-infusion monitoring period is considered adequate.
The side effects often associated with the phentolamine administration are tachycardia, nausea, vomiting, headache, dizziness, nasal stuffiness, and hypotension. Rare side effects of phentolamine include cardiac arrhythmias, angina, abdominal pain, diarrhea, and exacerbation of peptic ulcer disease.
Hord et al.8) reported that both alpha-1 and alpha-2 AR are involved in thermal hyperalgegia caused by chronic constriction injury. And, α1 and mixed alpha-1/alpha-2 receptor antagonists have been used in human to treat RSD, drugs that are highly specific postjunctional alpha-2 antagonists may also be useful in the treatment of sympathetically mediated pain. Our result did not show differenence between yohimbine and phentolamine. A alpha-2 receptor is located on the primary afferent terminal and an several brainstem27). After systemic injection of drug, all these sites were involved. The type of pain, and animal strain may have played for the different results.
CONCLUSION
In present study, the patients' pain was relieved by alpha-1 and alpha-2 AR antagonist. Therefore, results of the present study indicate that SMP is thought to be mediated by alpha-1 or alpha-2 AR, or both in humans. However, the reason for similar efficacy of both antagonists and the reason for efficacy of placebo are unknown.
References
- 1.Bonica JJ. Causalgia and other reflex sympathetic dystrophies. In: Bonica JJ, editor. The Management of Pain. Vol. 2. Philadelphia: Lea and Febiger; 1990. pp. 220–243. [Google Scholar]
- 2.Bridge D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth. 2001;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Michaelis M, Janig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721–3730. doi: 10.1152/jn.1996.76.6.3721. [DOI] [PubMed] [Google Scholar]
- 4.Dennis SG, Melzack R, Gutman S, Boucher F. Pain modulation by adrenergic agents and morphine as measured by three pain tests. Life Sci. 1980;26:1247–1259. doi: 10.1016/0024-3205(80)90070-3. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DS, Grossman E, Listwak S, Folio CJ. Sympathetic reactivity during a yohimbine challenge test in essential hypertension. Hypertension. 1991;18:III40–III48. doi: 10.1161/01.hyp.18.5_suppl.iii40. [DOI] [PubMed] [Google Scholar]
- 6.Grossman E, Rea RF, Hoffman A, Goldstein DS. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol. 1991;260:R142–R147. doi: 10.1152/ajpregu.1991.260.1.R142. [DOI] [PubMed] [Google Scholar]
- 7.Guthrie SK, Hariharan M, Grunhaus LJ. Yohimbine bioavailability in humans. Eur J Clinic Pharmacol. 1990;39:409–411. doi: 10.1007/BF00315421. [DOI] [PubMed] [Google Scholar]
- 8.Hord AH, Denson DD, Stowe B, Haygood RM. alpha-1 and alpha-2 Adrenergic antagonists relieve thermal hyperalgesia in experimental mononeuropathy from chronic constriction injury. Anesth Analg. 2001;92:1558–1562. doi: 10.1097/00000539-200106000-00042. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134:131–134. doi: 10.1016/0304-3940(91)90524-w. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Min BI, Kim JH, Hwang BG, Yoo GY, Park DS, et al. Effects of alpha1- and alpha2-adrenoreceptor antagonists on cold allodynia in a rat tail model of neuropathic pain. Brain Res. 2005;1039:207–210. doi: 10.1016/j.brainres.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M. The alpha (2A) adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain. 2000;85:345–358. doi: 10.1016/S0304-3959(99)00286-9. [DOI] [PubMed] [Google Scholar]
- 12.Korenman EM, Devor M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp Neurol. 1981;72:63–81. doi: 10.1016/0014-4886(81)90127-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- 14.Leem JW, Gwak YS, Nam TS, Paik KS. Involvement of alpha2-adrenoceptors in mediating sympathetic excitation of injured dorsal root ganglion neurons in rats with spinal nerve ligation. Neurosci Lett. 1997;234:39–42. doi: 10.1016/s0304-3940(97)00658-7. [DOI] [PubMed] [Google Scholar]
- 15.Miranda HF, Sierralta F, Pinardi G. An isobolographic analysis of the adrenergic modulation of diclofenac antinociception. Anesth Analg. 2001;93:430–435. doi: 10.1097/00000539-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 16.Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with with phentolamine : a diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698. doi: 10.1097/00000542-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Adelta- and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J Neurophysiol. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- 18.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 19.Sato J, Suzuki S, Iseki T, Kumazawa T. Adrenergic excitation of cutaneous nociceptors in chronically inflamed rats. Neurosci Lett. 1993;164:225–228. doi: 10.1016/0304-3940(93)90897-t. [DOI] [PubMed] [Google Scholar]
- 20.Shannon HE, Lutz EA. Yohimbine produces antinociception in the formalin test in rats : involvement of serotonin (1A) receptors. Psychopharmacology (Berl) 2000;149:93–97. doi: 10.1007/s002139900343. [DOI] [PubMed] [Google Scholar]
- 21.Shir Y, Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991;45:309–320. doi: 10.1016/0304-3959(91)90056-4. [DOI] [PubMed] [Google Scholar]
- 22.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. 2nd ed. Cary, NC: SAS Institute Inc.; 2000. pp. 487–494. [Google Scholar]
- 23.Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J Pharmacol Exp Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- 24.Tam SW, Worcel M, Wyllie M. Yohimbine : a clinical review. Pharmacol Ther. 2001;91:215–243. doi: 10.1016/s0163-7258(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 25.Tasker RA, Melzack R. Difference alpha-receptor subtypes are involved in clonidine produced analgesia in different pain tests. Life Sci. 1989;44:9–17. doi: 10.1016/0024-3205(89)90212-9. [DOI] [PubMed] [Google Scholar]
- 26.Tracey DJ, Cunningham JE, Romm MA. Peripheral hyperalgesia in experimental neuropathy : mediation by alpha 2-adrenoreceptors on post-ganglionic sympathetic terminals. Pain. 1995;60:317–327. doi: 10.1016/0304-3959(94)00141-z. [DOI] [PubMed] [Google Scholar]
- 27.Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system : analysis of some functional anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agent. Brain Res. 1984;1:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- 28.Verdugo RJ, Ochoa JL. Sympathetically maintained pain, I Phentolamine block questions the concept. Neurology. 1994;44:1003–1010. doi: 10.1212/wnl.44.6.1003. [DOI] [PubMed] [Google Scholar]
- 29.Vucković SM, Tomić MA, Stepanović-Petrović RM, Ugresić N, Prostran MS, Bosković B. The effects of alpha2-adrenoceptor agents on anti-hyperalgesic effects of carbamazepine and oxcarbazepine in a rat model of inflammatory pain. Pain. 2006;125:10–19. doi: 10.1016/j.pain.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Winter JC, Rabin RA. Yohimbine as a serotonergic agent : evidence from receptor binding and drug discrimination. J Pharmacol Exp Ther. 1992;263:682–689. [PubMed] [Google Scholar]
- 31.Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]