Abstract
We report a case of an acute spontaneous epidural hematoma (EDH) due to skull base metastasis in a 46-year-old male patient with hepatocellular carcinoma (HCC). The patient presented with the acute onset of severe headache followed by unconsciousness, and computed tomography showed a large EDH in the right temporal and parietal lobes with midline shift. Emergency evacuation of the EDH was performed, and the hemorrhage was determined to be secondary to skull base metastasis of HCC.
Keywords: Epidural hematoma, Skull base metastasis, Hepatocellular carcinoma
INTRODUCTION
Skull and intracranial metastases from hepatocellular carcinoma (HCC) have been rarely reported2,5,9,14). Epidural hematoma (EDH), subdural hematomas (SDH), and intracerebral hemorrhage from skull metastasis of HCC are also extremely rare5). We report a case of an acute spontaneous EDH associated with skull base metastasis in a 46-year-old male patient with HCC, with a review of the pertinent literature.
CASE REPORT
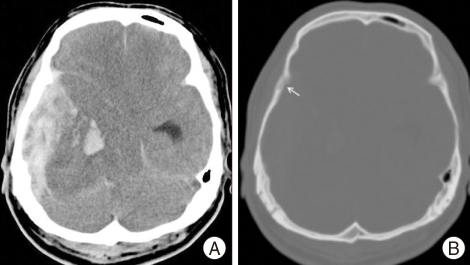
A 46-year-old man was brought to our hospital in a state of unconsciousness. His wife noted that he had complained of intermittent headaches for one day prior to admission. A few hours before admission, he had complained of a sudden onset of severe headache. He lost consciousness during transfer to the hospital. He had no history of recent head trauma, and no scalp or facial swelling or injury was identified. He had a history of transarterial hepatic embolization due to HCC that was performed three months previously. Initially, hepatic encephalopathy was suspected, but he showed decerebrate posture and shallow, irregular respirations. The right pupil was dilated and unresponsive to light. Computed tomography showed a large EDH in the right temporal and parietal lobes with midline shift (Fig. 1A). Decreased bone density in the greater wing of the right sphenoid bone compared with the contralateral part was suspected in the bone setting image (Fig. 1B). Laboratory examination demonstrated liver dysfunction and borderline coagulopathy. There was no thrombocytopenia.
Fig. 1.
Non-contrast brain tomography and bone setting image. A : Brain computerized tomography shows epidural hematoma in the right temporal base with midline shift, obliteration of basal cistern, and enlarged left temporal horn of lateral ventricle. B : Decreased bone density in the greater wing of the right sphenoid bone (arrow) compared with the contralateral part is suspected in the bone setting image.
Emergent craniotomy and evacuation of the EDH were performed, with simultaneous transfusion of fresh frozen plasma and injection of vitamin K. During the operation, no scalp mass or skull fracture was found. A right frontotemporoparietal craniotomy demonstrated that the inner table and diploic space of the temporal bone were destroyed, suggesting the presence of a bone tumor. Following evacuation of the EDH, a soft, yellow-brown, hypervascular mass was found in the right temporal skull base, which extended to near the cavernous sinus. The mass was resected, leaving a small portion near the cavernous sinus. No gross invasion or thickening of the dura was observed. However, thinning and a small defect in the dura were observed in the anteromedial temporal skull base. A small amount of acute SDH had formed as a result of blood draining through the defect in the dura, but the cerebral cortex was normal. There was considerable oozing of blood from the scalp, temporalis muscle, and skull during surgery. However, no active bleeding was seen from the dura or vessels. It was assumed that the bleeding focus was the destroyed temporal bone and the tumor itself.
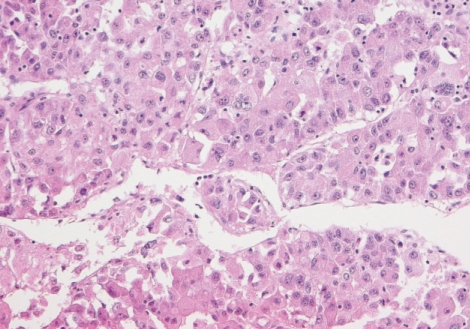
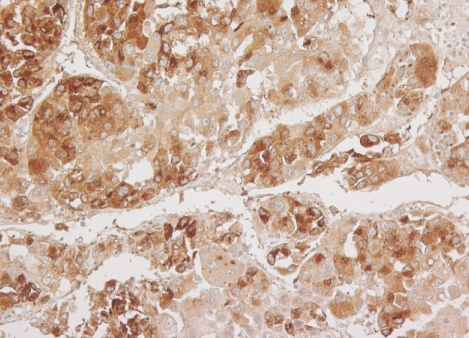
Microscopically, the tumor revealed a characteristic trabecular pattern with thickened cords of cells separated by vascular sinusoids, mimicking the cell plates and sinusoids of normal liver. The tumor cells had a polygonal shape with prominent nuclei and nucleoli, granular eosinophilic cytoplasm, and intercellular canaliculi resembling liver cells (Fig. 2). The immunohistochemical staining for α-fetoprotein demonstrated strong immunoreactivity on cytoplasm of tumor cells (Fig. 3). These findings were compatible with the diagnosis of metastatic HCC.
Fig. 2.
Histologic features of tumor. The tumor shows thick trabecular growth pattern with intercellular canaliculi resembling liver cell plates and sinusoids. The tumor cells maintain a polygonal shape and have abundant granular eosinophilic cytoplasm, round vesicular nuclei and prominent nucleoli (H&E, ×200).
Fig. 3.
The immunohistochemical finding. The tumor cells show strong cytoplasmic immunoreactivity for α-fetoprotein.
Post-operatively, the patient did not regain consciousness. Laboratory examination demonstrated liver dysfunction, anemia, thrombocytopenia, and coagulopathy, suggesting disseminated intravascular coagulopathy, which might have been caused by blood loss during surgery. Five days after the operation, the patient died of multi-organ failure, including liver failure.
DISCUSSION
HCC commonly metastasizes to regional lymph nodes and the lungs, but rarely to the skeletal system. The most common osseous metastatic sites are the vertebrae, pelvis, and ribs. The incidence of skull metastasis in the setting of HCC has been reported to be 0.4-1.6%5). In a review of 60 articles published between January 1966 and November 2005, Hsieh et al.5) found the total number of reported cases of HCC with skull metastasis to be 68. The most common presentation is a subcutaneous mass with an occasional painful sensation, which has been reported in 59% of the patients. Cranial nerve deficits, such as visual disturbance, dysphagia, deafness, and facial numbness were present in cases that had skull base involvement1,5-7). In our case, the presenting symptom was a headache followed by unconsciousness without definite cranial nerve palsy. Because metastatic HCC was located in the skull base, a palpable subcutaneous mass was not formed.
Several authors have reported chronic or acute EDH associated with skull metastasis from HCC4,9-13). We found four cases of acute EDH reported in the literature4,9-11). Except for one case, which occurred after trivial trauma10), all of the cases occurred spontaneously. Among these reports, however, there were no acute EDHs from skull base metastasis of HCC. Our report is unique in this regard. In our case, bleeding derived from the tumor mass and the diploic space of the involved skull. We did not obtain a biopsy of the dura, so microscopic dural invasion could not be excluded. However, dural metastasis of HCC is extremely rare, and we found no thickening or nodular patterning of the dura, which are typical findings of dural metastasis8). Dural metastasis may produce a SDH, rather than EDH, although one case of combined acute EDH and chronic SDH associated with dural metastasis from HCC has also been reported3,8). Thinning and a small defect in the dura might have been caused by compression of the dura by the growing tumor mass, and the small SDH might have derived from EDH spilling into the subdural space through the defect in the dura.
EDH usually occurs after head trauma or bleeding from a middle meningeal artery, emissary vein, or venous sinus. Because no history of recent head trauma and no radiologic or operative evidence of scalp injury or swelling or skull fracture were detected in this patient, EDH presumably occurred spontaneously.
Tumor bleeding may occur from thin, fragile tumor vessels. The present tumor had many sinusoid-like blood vessels, which probably caused hemorrhage and formation of the EDH. In the operative specimen, some intratumoral hemorrhage was observed, but no active bleeding from these vessels was observed intra-operatively. The EDH was thought to be from tumor bleeding, which might have been brought on by liver dysfunction and coagulopathy.
CONCLUSION
We emphasize that acute intracranial hemorrhage associated with brain or skull metastasis from HCC should always be considered in the differential diagnosis of patients with known HCC who present with headache or unconsciousness. Only early diagnosis and emergent treatment can prevent morality and morbidity in these patients.
References
- 1.Aung TH, Po YC, Wong WK. Hepatocellular carcinoma with metastasis to the skull base, pituitary gland, sphenoid sinus, and cavernous sinus. Hong Kong Med J. 2002;8:48–51. [PubMed] [Google Scholar]
- 2.Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma : review of 45 cases. Surg Neurol. 2004;62:172–177. doi: 10.1016/j.surneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Endo M, Hamano M, Watanabe K, Wakai S. [Combined chronic subdural and acute epidural hematoma secondary to metastatic hepatocellular cancer : case report.] No Shinkei Geka. 1999;27:331–334. [PubMed] [Google Scholar]
- 4.Hayashi K, Matsuo T, Kurihara M, Daikoku M, Kitange G, Shibata S. Skull metastasis of hepatocellular carcinoma associated with acute epidural hematoma : a case report. Surg Neurol. 2000;53:379–382. doi: 10.1016/s0090-3019(00)00208-1. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh CT, Sun JM, Tsai WC, Tsai TH, Chiang YH, Liu MY. Skull metastasis from hepatocellular carcinoma. Acta Neurochir (Wien) 2007;149:185–190. doi: 10.1007/s00701-006-1071-3. [DOI] [PubMed] [Google Scholar]
- 6.Isaka T, Nakagawa H, Suzuki T, Yamada J, Wada K, Kadota T. Successful removal of a giant skull base metastasis from hepatocellular carcinoma after direct ethanol injection : case report. Skull Base Surg. 2000;10:81–86. doi: 10.1055/s-2000-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SR, Kanda F, Kobessho H, Sugimoto K, Matsuoka T, Kudo M, et al. Hepatocellular carcinoma metastasizing to the skull base involving multiple cranial nerves. World J Gastroenterol. 2006;12:6727–6729. doi: 10.3748/wjg.v12.i41.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005;75:57–61. doi: 10.1007/s11060-004-8098-1. [DOI] [PubMed] [Google Scholar]
- 9.Lee KW, Chung DS, Huh PW, Hong YK, Rha HK, Kang JK. Intracranial metastasis of hepatocellular carcinoma associated with epidural hematoma : a case report. J Korean Neurosurg Soc. 1996;25:1738–1742. [Google Scholar]
- 10.McIver JI, Scheithauer BW, Rydberg CH, Atkinson JL. Metastatic hepatocellular carcinoma presenting as epidural hematoma : case report. Neurosurgery. 2001;49:447–449. doi: 10.1097/00006123-200108000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa Y, Yoshino E, Suzuki K, Tatebe A, Andachi H. Spontaneous epidural hematoma from a hepatocellular carcinoma metastasis to the skull--case report. Neurol Med Chir (Tokyo) 1992;32:300–302. doi: 10.2176/nmc.32.300. [DOI] [PubMed] [Google Scholar]
- 12.Nakao N, Kubo K, Moriwaki H. Cranial metastasis of hepatocellular carcinoma associated with chronic epidural hematoma--case report. Neurol Med Chir (Tokyo) 1992;32:100–103. doi: 10.2176/nmc.32.100. [DOI] [PubMed] [Google Scholar]
- 13.Nakao S, Sato S, Fukumitsu T, Ogata M, Shirane H. [Cranial metastasis of hepatocellular carcinoma. Report of three cases.] Neurol Med Chir (Tokyo) 1985;25:229–234. doi: 10.2176/nmc.25.229. [DOI] [PubMed] [Google Scholar]
- 14.Tunc B, Filik L, Tezer-Filik I, Sahin B. Brain metastasis of hepatocellular carcinoma : A case report and review of the literature. World J Gastroenterol. 2004;10:1688–1689. doi: 10.3748/wjg.v10.i11.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]