Abstract
Although hyperbaric oxygen therapy has been used for diabetic foot ulcer since the 1980s, there is little information on its efficacy. The aim of this study is to evaluate whether hyperbaric oxygen can decrease major amputation rates and to determine the predictive factors. A total of 184 consecutive patients were treated with hyperbaric oxygen therapy as an adjunct to standard treatment modalities for their diabetic foot ulcer. Of these patients, 115 were completely healed, 31 showed no improvement and 38 underwent amputation. Of the amputations, nine (4.9%) were major amputations (below knee) and 29 were minor. Major amputations were associated with the Wagner grade (p < 0.0001), with the age of the patients (p = 0.028) and with the age of the wounds (p = 0.018). Hyperbaric oxygen therapy can help to reduce the major amputation rates in diabetic foot ulcer. However, further large, multicentre, randomised controlled studies are needed to make more accurate conclusions.
Résumé
Dès 1980, le traitement par oxygène hyperbare a été utilisé pour les lésions des pieds diabétiques avec ulcérations. Les données de la litterature sur l’efficacité de ce traitement semble limitées. Le but de cette étude est d’évaluer si l’utilisation de l’oxygène hyperbare peut diminuer le taux d’amputation et permettre de faire une prédiction concernant l’évolution. Matériel et méthode: 184 patients consécutifs ont été traités par oxygène hyperbare pour leurs lésions sur pieds diabétiques. 115 cas ont complètement guéris, 31 cas n’ont pas du tout été améliorés, 38 ont été amputés. Sur ces amputations 9 (4,9%) ont été des amputations majeures (sous le genou) et 29 mineures. Les amputations majeures sont associées au grade de Wagner (p < 0,0001) à l’âge des patients (p = 0,028) et la durée d’évolution des lésions. Conclusions: l’oxygène hyperbar peut permettre de réduire le taux d’amputations majeures dans les pieds diabétiques avec ulcères. Cependant une étude plus large, multicentrique, randomisée est nécessaire pour conclure de façon plus stricte.
Introduction
Diabetic foot ulcers occur in 1.9% of adults with diabetes annually, resulting in amputation in 15–20% of patients within five years. Loss of nociceptive and autonomic nerves results in a dry, hyperkeratotic surface that is subject to mechanical cracking, infection and tissue destruction; local ischaemia and tissue injury result in chronic, nonhealing wounds that remain a portal of entry for deep tissue infection [3].
Because the infection and tissue hypoxia are the major factors for the nonhealing diabetic foot ulcers, hyperbaric oxygen therapy (HBOT) is thought to carry potential benefits for treating infected diabetic wounds [17, 19]. Increased tissue oxygen levels instigate wound healing in hypoxic tissues by a mechanism of angiogenesis, fibroblast replication, collagen synthesis, revascularisation and epithelialisation and increased leukocyte bactericidal activity [9, 14, 16].
HBOT has been in use for the treatment of diabetic foot ulcers as an adjunct to standard multidisciplinary therapies to improve limb salvage since the 1980s [19]. However, despite vigorous studies, limited information exists on the role and efficacy of HBOT.
The aim of this study is to evaluate whether HBOT can decrease major amputation rates in diabetic foot ulcers and to determine the predictive factors for major amputation.
Materials and methods
From 2005 to 2007, 209 diabetic patients were treated with HBOT for their diabetic foot ulcers as an adjunct to standard treatment modalities. Of these patients, three died and 22 withdrew from treatment for various reasons. The remaining 184 patients were evaluated for this study.
On admission, wounds were classified according to Wagner [18]. Diabetes mellitus type, duration of diabetes, type of diabetes treatment, age of diabetic wound, previous diabetic ulcer history, diabetic foot deformity and smoking habits were recorded. Glycosylated haemoglobin levels were measured. Consultations were obtained so as to determine neuropathy, nephropathy, retinopathy and arterial disease requiring surgical intervention. The latter was considered an exclusion criterion for the study. Specimens from foot lesions were collected for culture and for antimicrobial susceptibility tests.
Initially, aggressive débridement was performed and the wound was dressed. Dressings were changed at required intervals. After the collection of swabs from the wound, patients were given empirical antibiotic treatment. This was modified if necessary according to the sensitivity tests. Blood glucose levels were optimised with insulin. The feet were protected from uncontrolled mechanical stresses. After that, the patients underwent HBOT.
HBOT was applied in a multiplace chamber (Hiperbot Model 101, 2005, Turkey) which makes it possible to treat ten patients simultaneously. First, the chamber was pressurised with compressed air for 15 min. When the pressure in the chamber reached a level equivalent to 42 feet (12.80 m) depth, the patients breathed 100% oxygen by using a mask. Thus, they were exposed to 2.4 absolute atmosphere (ATA) pressure when breathing 100% oxygen. Each therapy session consisted of three oxygenation periods. These periods lasted 25 min each and were separated by 5-min air breaks. After that, the chamber was decompressed for 15 min and the therapy was finished. Therefore, one treatment session took approximately 120 min.
A member of the medical staff accompanied the patients in the chamber during the whole session. The HBOT sessions were repeated once or twice daily and six days in a week. The patients were observed throughout their HBOT treatment and control examinations were done at the end of the third, sixth and 12th month.
The results were evaluated as healed, not improved and amputated.
Descriptive statistics, chi-square test, Fischer’s exact test and multiple comparison tests for univariate analysis were performed where appropriate. The SPSS version 13 was used to evaluate statistical analysis.
Results
There were 184 patients in the study group consisting of 132 men and 52 women. The clinical characteristics of the study group are shown in Table 1.
Table 1.
Clinical characteristics of the patients
| Minimum | Maximum | Mean ± SD | |
|---|---|---|---|
| Age (years) | 27 | 88 | 60.19 ± 10.78 |
| Duration of DM (years) | 0a | 66 | 14.70 ± 9.39 |
| Duration of the wound (weeks) | 1 | 260 | 25.27 ± 44.72 |
| Glucose level on admission (mg/100 ml) | 51 | 643 | 208.17 ± 95.72 |
| HbA1C (mg/100 ml) | 4.80 | 14.70 | 8.52 ± 2.07 |
| HBOT session | 8 | 90 | 39.01 |
| N | % | ||
| Sex | |||
| Male | 132 | 71.7 | |
| Female | 52 | 28.3 | |
| Type of DM | |||
| Type 1 DM | 14 | 7.6 | |
| Type 2 DM | 170 | 92.4 | |
| Type of DM treatment | |||
| Insulin | 172 | 93.5 | |
| OAD | 12 | 6.5 | |
| Neuropathy | |||
| Yes | 171 | 92.9 | |
| No | 13 | 7.1 | |
| Nephropathy | |||
| Yes | 24 | 13.0 | |
| No | 160 | 87.0 | |
| Retinopathy | |||
| Yes | 90 | 48.9 | |
| No | 94 | 51.1 | |
| Foot deformity | |||
| Yes | 103 | 55.9 | |
| No | 81 | 44.0 | |
| Previous wound history | |||
| Yes | 99 | 53.8 | |
| No | 85 | 46.2 | |
| Smoking | |||
| Yes | 95 | 51.6 | |
| No | 89 | 48.4 | |
| Peripheral arterial disease | |||
| Yes | 72 | 39.1 | |
| No | 112 | 60.9 | |
| Wagner classification of the wounds on admission | |||
| Grade 2 | 57 | 31.0 | |
| Grade 3 | 76 | 41.3 | |
| Grade 4 | 41 | 22.3 | |
| Grade 5 | 10 | 5.4 |
DM diabetes mellitus, HbA1C glycosylated haemoglobin, OAD oral antidiabetic drug
a0 means undiagnosed diabetes mellitus before foot ulcer
The bacteriological culture of the wound swab was positive in 91.85% of the patients. In 121 cases single species (65.76%) and in 48 cases (26.08%) multiple species were observed in culture tests. The most common infectious agents were Pseudomonas aeruginosa (40.5%) and Staphylococcus aureus (24.6%). The others were as follows: Enterobacter (13.0%), Klebsiella (8.6%), Proteus (5.7%), Acinetobacter (2.8%), Citrobacter (1.4%), Escherichia coli (1.4%), Morganella (1.4%) and others (0.6%).
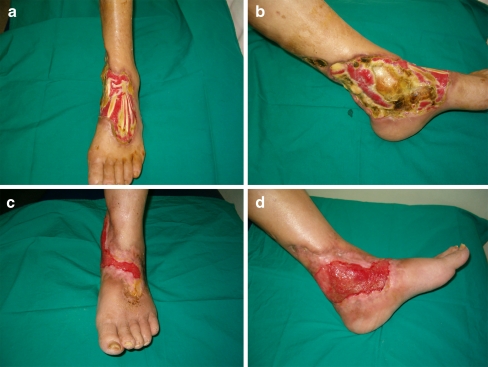
At the end of HBOT, 115 patients were completely healed (Figs. 1, 2 and 3), 31 showed no improvements and 38 underwent amputation, 29 of which were minor amputations and nine major amputations. Distribution of the major amputation cases according to Wagner grade is shown in Table 2. The Wagner grades were closely associated with the major amputation rates (p < 0.0001).
Fig. 1.
a–e An 85-year-old woman with Wagner grade 5 diabetic foot ulcer. She had type 2 DM for 25 years; 75 sessions of HBOT were applied over 3 months. Serial clinical photos throughout HBOT and the final result
Fig. 2.
a–f A 50-year-old man with Wagner grade 5 diabetic foot ulcer; 60 sessions of HBOT were applied
Fig. 3.
a–d A 63-year-old woman with Wagner grade 3 diabetic foot ulcer and 25 years of type 2 DM; 60 sessions of HBOT were applied
Table 2.
Distribution of the major amputations according to Wagner classification
| Wagner grade | Major amputations | Percentage in group |
|---|---|---|
| Grade 2 | 0 | 0* |
| Grade 3 | 0 | 0* |
| Grade 4 | 2 | 4.9* |
| Grade 5 | 7 | 70.0* |
| Total | 9 | 4.9 |
*p < 0.0001
The mean age of the amputated group was older than the healed group: 59.86 versus 69.44 years and the difference was statistically significant (p = 0.028). When the groups were compared, the difference between the age of the wounds was also statistically significant (p = 0.018). The other clinical parameters did not show a significant statistical difference between the amputated and healed groups (Table 3).
Table 3.
Comparison of the clinical parameters between the amputated and healed group
| Clinical parameter | p value |
|---|---|
| Wagner grade | <0.0001 |
| Duration of wound | 0.018 |
| Age | 0.028 |
| Peripheral arterial disease | 0.073 |
| HbA1C | 0.295 |
| Foot deformity | 0.363 |
| Retinopathy | 0.402 |
| Neuropathy | 0.459 |
| DM type | 0.653 |
| Sex | 0.776 |
| DM duration | 0.803 |
| DM treatment | 0.840 |
| Glucose level at admission | 0.849 |
| Smoking | 0.906 |
| Nephropathy | 0.978 |
| Previous wound history | 0.980 |
DM diabetes mellitus, HbA1C glycosylated haemoglobin
Discussion
Diabetic foot ulcers are the most common chronic wounds in Western industrialised countries. They take a long time to heal, fail to heal or recur and cause significant pain and discomfort. In these patients the amputation rates is 15–70 times higher than in the general population [15]. The three most common causes of amputations are ischaemia, infection and the retarded wound healing.
Wound hypoxia is well documented in nonhealing wounds, representing the strongest risk factor in those patients [1]. Wound healing is oxygen dependent and is limited by its availability at the cellular level [2, 11]. Elevated tension of oxygen in plasma causes upregulation of growth factors, downregulation of inflammatory cytokines, increased fibroblast activation, angiogenesis, antibacterial effects and enhanced antibiotic action [5, 15, 16]. Consequently, it has been used in the treatment of these wounds since the 1980s.
Generally accepted care for diabetic foot ulcers includes optimised nutritional support and glycaemic control, off-loading, débridement of nonviable tissue, appropriate dressing, management of infection and other adjunctive therapies including HBOT [4, 16, 17].
HBOT is administration of oxygen pressures greater than 1 ATA. More oxygen is dissolved in the blood with HBOT.
Although HBOT has been used for diabetic foot ulcers and a success rate of 70–95% reported, there are few clinical details regarding effectiveness [15].
There are limited randomised controlled trials (RCT) about HBOT efficacy in the treatment of diabetic foot ulcer [1, 6–8, 12, 13]. The numbers of cases in these studies were equal to or lower than 30 except Faglia et al.’s study [7]. In Lin et al.’s study [13] the wound grades were 0–2, in Kessler et al.’s study [12] 1–3 and in Abidia et al.’s study [1] 1–2. Our study consisted of 41 grade 4 and 10 grade 5 cases in addition to 76 grade 3 and 57 grade 2.
According to the randomisation and study design, only one of the above-mentioned studies received the full Jadad score of 5 [1] and this study comprised 18 cases including only Wagner grades 1 and 2. This is a potential problem for comparing results.
Still, in these studies lower major amputation rates were reported in HBOT groups.
Our results are parallel with the above-mentioned comparative studies showing positive effects of adjunctive HBOT on reduction of amputation rates. No major amputation was applied to grades 2 and 3. The amputation rates in grades 4 and 5 were 4.9 and 70.0%, respectively. The differences between grades are highly significant (p < 0.0001). We did not apply HBOT to grades 0 and 1 patients and applied to grade 2 patients only if they had not showed improvement after at least four weeks conservative standard treatment modalities.
In our study the amputated group was older than the healed group and the difference was statistically significant (p = 0.028). The younger patients may have had a better wound healing potential.
The age of the wound was shorter in the amputated group when compared with the healed group and the difference was statistically significant (p = 0.018). This can be explained by the quick progression of the wound in the higher graded and amputated patients.
The mean number of HBOT sessions in our study was 39.01 and in accordance with the literature. Transcutaneous oxymetry could not be performed because we had no proper equipment.
HBOT may cause some complications such as oxygen toxicity in the brain or lung and barotraumas in the middle ear [10]. In our study we did not observe any permanent complications or patient compliance problems. The patients experienced some mild side effects.
This study has some weak points. The most important is the absence of a control group. Thus, randomisation could not be realised. Moreover, strict conclusions on direct HBOT efficacy could not be reached. However, it is difficult to apply sham therapy; in our clinical conditions that would pose a problem with receiving payment from the social security company. Ethical issues were another problem because our study group had higher graded patients. Because our institute has no HBOT treatment unit, we had a private HBOT centre undertake treatment of our patients. But on the other hand, the HBOT centre and the clinicians were blinded to the other part of the treatment and to evaluation. The number of cases is relatively high and the follow-up period is sufficient. The study group consisted of consecutive patients admitted to our clinic and no selection of cases was applied. Approximately two thirds of the patients were Wagner grades 3 and 4 and the study group involved all types of Wagner grade (except grades 0 and 1).
In this study, the major amputation rates were decreased with adjunctive HBOT therapy in addition to standard treatments and the major amputation rates are compatible with the literature. It appears that HBOT is effective in the treatment of diabetic foot ulcers; nevertheless, this treatment cannot be considered a substitute for standard treatments and is to be accepted as adjunctive therapy.
The Wagner score, the age of the patients and the age of the wounds were observed to be prognostic factors for the major amputation rates. When an attempt was made to evaluate the effectiveness of HBOT, not enough information was available on a suitable control group to conduct a more traditional comparison of cohort groups in the literature, but HBOT must be considered as an alternative treatment option before amputation for some patients.
Conclusions
HBOT may be regarded as a promising adjunctive treatment option to standard treatment modalities and multidisciplinary interventions; nevertheless multicentre, large, prospective, randomised, controlled, double-blinded studies are needed to make more accurate conclusions.
Acknowledgements
All authors of this study confirm that they have no financial and personal relationships with other people or organisations that could inappropriately influence their work. We thank Dr. Timur Kose for statistical analysis.
References
- 1.Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513–518. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 2.Al-Waili NS, Butler GJ, Beale J, Abdullah MS, Finkelstein M, Merrow M, et al. Influences of hyperbaric oxygen on blood pressure, heart rate and blood glucose levels in patients with diabetes mellitus and hypertension. Arch Med Res. 2006;37:991–997. doi: 10.1016/j.arcmed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Barnes RC. Point: hyperbaric oxygen is beneficial for diabetic foot wounds. Clin Infect Dis. 2006;43:188–192. doi: 10.1086/505207. [DOI] [PubMed] [Google Scholar]
- 4.Berendt AR. Counterpoint: hyperbaric oxygen for diabetic foot wounds is not effective. Clin Infect Dis. 2006;43:193–198. doi: 10.1086/505223. [DOI] [PubMed] [Google Scholar]
- 5.Chen SJ, Yu CT, Cheng YL, Yu SY, Lo HC. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin Biochem. 2007;40:30–36. doi: 10.1016/j.clinbiochem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Doctor N, Pandya S, Supe A. Hyperbaric oxygen therapy in diabetic foot. J Postgrad Med. 1992;38:112–114. [PubMed] [Google Scholar]
- 7.Faglia E, Fevales F, Aldeghi A, Calia P, Quarantiello A, Barbano P, et al. Change in major amputation rate in a center dedicated to diabetic foot care during the 1980s: prognostic determinants for major amputation. J Diabetes Complications. 1998;12:96–102. doi: 10.1016/S1056-8727(97)98004-1. [DOI] [PubMed] [Google Scholar]
- 8.Faglia E, Fevales F, Aldeghi A, Calia P, Quarantiello A, Oriani G. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338–1343. doi: 10.2337/diacare.19.12.1338. [DOI] [PubMed] [Google Scholar]
- 9.Fife CE, Buyukcakir C, Otto G, Sheffield P, Love T, Warriner R., III Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15:322–331. doi: 10.1111/j.1524-475X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. Q J Med. 2004;97:385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 11.Kalani M, Jörneskog G, Naderi N, Lind F, Brismar K. Hyperbaric oxygen (HBO) therapy in treatment of diabetic foot ulcers. Long term follow-up. J Diabetes Complications. 2002;16:153–158. doi: 10.1016/S1056-8727(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 12.Kessler L, Bilbault P, Ortega F, Grasco C, Passemard R, Stephan D, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26:2378–2382. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- 13.Lin TF, Chen SF, Niu KC.The vascular effects of hyperbaric oxygen therapy in treatment of early diabetic foot Undersea Hyperb Med 2001286311908697 [Google Scholar]
- 14.Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- 15.Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg. 2005;92:24–32. doi: 10.1002/bjs.4863. [DOI] [PubMed] [Google Scholar]
- 16.Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14:680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 17.Putten M. Consensus conference on hyperbaric oxygen in the treatment of foot lesions in diabetic patients. Foot. 1999;9:53–55. doi: 10.1054/foot.1999.0513. [DOI] [Google Scholar]
- 18.Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2:64–122. doi: 10.1177/107110078100200202. [DOI] [PubMed] [Google Scholar]
- 19.Zgonis T, Garbalosa JC, Burns P, Vidt L, Lowery C. A retrospective study of patients with diabetes mellitus after partial foot amputation and hyperbaric oxygen treatment. J Foot Ankle Surg. 2005;44(4):276–280. doi: 10.1053/j.jfas.2005.04.007. [DOI] [PubMed] [Google Scholar]