Abstract
Between May 2002 and October 2006, 19 patients (17 men and 2 women; average age 57.2; range 47–71 years) received anterior corpectomy and fusion for severe ossification of the posterior longitudinal ligament (OPLL) in our department. Preoperative radiological evaluation showed the narrowing by the OPLL exceeded 50% in all cases, and OPLL extended from one to three vertebrae. We followed-up all patients for 12–36 months (mean 18 months). The Japanese Orthopaedic Association (JOA) score before surgery was 9.3 ± 1.8 (range 5–12) which significantly increased to 14.2 ± 1.3 (range 11–16) points at the last follow-up (P < 0.01). The improvement rate (IR) of neurological function ranged from 22.2–87.5%, with a mean of 63.2% ± 15.2%. The operation also provided a significant increase in the cervical lordosis and the cord flatting rate (P < 0.01). No severe neurological complication developed. We therefore concluded that anterior decompression and fusion was effective and safe in the treatment of the selected patients, although OPLL exceeded 50% diameter of the spinal canal.
Résumé
Entre mai 2002 et octobre 2006, 19 patients (17 hommes et 2 femmes) âgés en moyenne de 57,2 ans (47 à 71 ans) ont bénéficié d’une corporectomie antérieure avec arthrodèse pour une ossification sévère postérieure du ligament (OPLL). L’évaluation radiologique préopératoire a montré que l’importance de l’ossification était supérieure à 50% dans tous les cas et cette ossification (OPLL) s’étendait sur 1 à 3 niveaux vertébraux. Tous les patients ont été suivis en moyenne de 12 à 36 mois (18 mois en moyenne). Le score de la JOA a été de 1,8 en préopératoire (5 à 12) et a progressé jusqu’à 9,3 à 14,2 (11 à 16) au dernier suivi (P < 0,01). Le pourcentage d’amélioration neurologique a été de 22,2% à 87,5% avec une moyenne de 63,2%. L’intervention entraîne également une amélioration significative de la lordose cervicale et du taux d’applatissement de la moelle (P < 0,01). Aucune complication neurologique sévère n’a été observée. Nous pouvons conclure que la décompression antérieure associée à une greffe est une technique efficace et sûre du traitement des OPLL chez les patients sélectionnés dont le diamètre du canal rachidien doit être supérieur à 50%.
Introduction
Ossification of the posterior longitudinal ligament (OPLL) has recently been recognised as one of the most common causes of severe cervical meylopathy in China. Since OPLL is a multifactorial disease and no conservative treatment has been found to be effective, surgical treatment is necessary in most cases. Surgical strategies for cervical myelopathy due to OPLL can be divided into two approaches: anterior and posterior. Anterior corpectomy and resection of OPLL seems to be a radical surgical option, owing to direct decompression and satisfactory results [4, 13]. However, when it comes to severe OPLL with narrowing exceeding 50%, anterior decompression becomes more technically demanding. In particular, if OPLL is associated with dural ossification (DO), which is reported to be more common in the cases of severe OPLL, the risk of unexpected complications including cerebrospinal fluid leakage and iatrogenic neurological deterioration significantly increases [1, 5, 6, 8]. Although the superiority of the anterior approach has been reported in several studies, the surgical strategy for severe OPLL still remains controversial [10, 14, 19]. Starting in 2002, anterior corpectomy and fusion was performed for select patients with severe OPLL in our department. The purpose of this study was to discuss the indications for anterior decompression for severe OPLL and evaluate its effectiveness and safety.
Materials and methods
Patient population
Between May 2002 and October 2006, a total of 19 consecutive patients (17 men and 2 women) were selected to receive anterior corpectomy and fusion for severe OPLL (ossification occupying more than 50% diameter of the spinal canal on CT images) in our department of Changzheng Hospital, Second Military Medical University, Shanghai, China. The mean age at operation was 57.2 years, ranging from 43 to 71 years. The patients presented moderate to severe spastic limb paresis, and the duration of symptoms was at least 12 months. Most patients chose to have surgical decompression because of recent neurological aggravation, and one patient needed revision surgery after an unsuccessful posterior laminoplasty. The preoperative Japanese Orthopaedic Association (JOA) score ranged from 5 to 12 points, averaging 9.3 ± 1.8 points.
Radiological evaluation
All patients had preoperative plain radiographs, computed tomography (CT) scans, and magnetic resonance images (MRIs). The parameters described as follows were investigated before operation.
Plain radiographs The diameter of the spinal canal was measured from the middle of the posterior surface of the vertebral body to the nearest point on the corresponding spinolaminar line. The mean value of the measures at C3 to C6 represented the spinal canal diameter for the patient. The cervical lordosis was measured as the angle between a line parallel to the posterior aspect of the C2 vertebral body and that of the C6 body.
Computed tomography The narrowing rate was defined as the thickness of OPLL divided by the anteroposterior diameter of the bony spinal canal on the axial image. The double-layer sign indicating the association with dural ossification (DO) was recorded. On the sagittal image, OPLL was classified into four types: local, segmental, continuous, or mixed. The extent of OPLL was also investigated.
Magnetic resonance imaging The cord flattening rate was defined as the minor axis length of the spinal cord divided by its major axis length at the level of maximal cord compression on axial T1-weighted MR images. This parameter has been used to evaluate compression of the spinal cord without individual differences. When it is less than 0.4 after operation, surgical decompression is considered to be inadequate [19].
Surgical technique
Under general anaesthesia, the patients were placed in the supine position with neck slightly extended. The cervical spine was exposed through a standard right-side anterior approach. The appropriate surgical level was confirmed by intraoperative radiography. After necessary discectomies, the one or two vertebral bodies were partially removed using an appropriate rongeur until the posterior cortex of the vertebrae was exposed and thinned as much as possible. The OPLL was then separated from dura using a specialised micro dissector. The head of this dissector was a hook with a narrow slot. It was inserted under the OPLL from the nonossified ligament, rotated 90 degrees, and slightly lifted. The ligament was cut off by scalpel along the slot. Next, the OPLL was meticulously separated using the micro dissector and removed by 1–2 mm Kerrison rongeur or micro curettes. If the OPLL was associated with dural ossification (DO), this portion of dura was carefully preserved using the anterior floating method to avoid dural tears. Titanium mesh cages were then used and filled with autologous bone fragments from the excised vertebrae to restore the bone defect. Finally, anterior cervical plates were applied across the segments to be fused. The procedure involved one-level corpectomy in two cases, and two-level in 17.
Clinical assessment
The Japanese Orthopaedic Association (JOA) scoring system was used to evaluate the neurological status before and after the operation. An improvement rate (IR) was calculated as IR=(postoperative JOA score – preoperative JOA score/17 – preoperative JOA score)×100%. Surgical outcome was defined by the IR as follows: excellent (IR ≥ 75%), good (75% > IR ≥ 50%), fair (50% > IR ≥ 25%), and poor (IR < 25%).
Statistical analysis
The student t test was used for comparison of paired data. The results were considered significant when a P value was less than 0.05. Results were presented as mean ± standard deviation.
Results
Radiological findings
On preoperative plain radiographs, the mean diameter of the spinal canal was 14.2 ± 1.1 mm (range, 12–15 mm), and the mean cervical lordosis was 9.2° ± 2.6° (range, −4° – 18°). Preoperative CT scans showed the mean narrowing rate reached 65.4% ± 8.2% (50–78%). The double-layer sign was observed in five cases (26.3%). On the sagittal images, OPLL extended from one to three vertebrae, with an average of 2.3 ± 0.2 vertebrae. The type of OPLL was distributed as follows: 11 local, 6 segmental, 2 continuous, and 0 mixed. On preoperative MR images, the mean cord flattening rate was 0.23 ± 0.06 (0.10–0.34).
At 1 week postoperative, all patients underwent plain radiographs, CT, and MRI scans. Postoperative cervical lordosis and cord flattening rate were studied and are presented in Table 1. The results showed that postoperative cervical lordosis averaged 13.2° ± 1.4° (8°–23°) and was significantly greater than preoperative cervical lordosis (P < 0.01). The operation also provided a significant increase in the cord diameter (P < 0.01), and postoperative cord flattening rate averaged 0.52 ± 0.12 (0.37–0.63).
Table 1.
Radiological and neurological improvement after operation
| Cervical lordosis (°) | Flattening rate (%) | JOA score (points) | |
|---|---|---|---|
| Before operation | 9.2° ± 2.6° (−4°–18°) | 0.23 ± 0.06 (0.10–0.34) | 9.3 ± 1.8 (5–12) |
| After operation | 13.2° ± 1.4° (8°–23°)* | 0.52 ± 0.12 (0.37–63)* | 14.2 ± 1.3 (11–16)* |
JOA Japanese Orthopaedic Association
*P < 0.01, compared with the data before operation using the student t test
Surgical outcome
In this series, all cases were followed-up for a mean period of 18 months (range, 12–36). Generally, the neurological status was significantly improved (Table 1). The mean JOA score increased to 14.2 ± 1.3 (11–16) points at the last follow-up, significantly greater than that prior to surgery (P < 0.01). The improvement rate (IR) of neurological function ranged from 22.2% to 87.5%, with a mean of 63.2% ± 15.2%. The surgical outcome was excellent in seven (36.8%) patients, good in nine (47.4%) patients, fair in two (10.5%) patients, and poor in one (5.3%) patient.
Complications
In this series, the complications after anterior corpectomy and fusion included cerebrospinal fluid (CSF) leakage in four cases, bilateral C5 palsy in one case, and haematoma in one case. CSF leakage occurred after a dural tear during the operation due to tight adhesion with the dura or dural ossification. Luckily, there was no significant dural defect in these cases, because dural ossification was preserved by careful dissection. If CSF leakage occurred after operation, the drainage tube was pulled out 12 hours after operation and continuous pressure to the wound was applied. In our experience, CSF leakage usually stopped after 3- to 5-day conservative treatment of local pressure, although one patient experienced a cerebrospinal fluid pseudocyst. Dural tear healed and the pesudocyst gradually disappeared after 2 months of repeated aspiration. C5 palsy developed at 8 hours postoperatively, and the strength of related deltoid and biceps muscles decreased to grade 1 in a manual muscle test (MMT). This patient was treated conservatively including neurotrophy drugs, high-pressure oxygen therapy, and functional exercises. Strength of the paralysed muscles recovered to grade 4 in MMT after 3 months. The patient who experienced haematoma recovered in neurological function after an emergency operation.
Case reports
Case 1
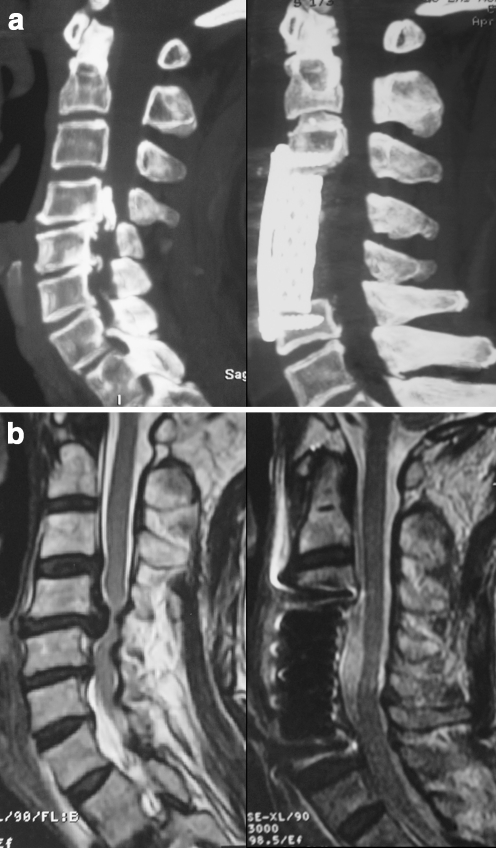
A 67-year-old man underwent anterior corpectomy for severe OPLL with a narrowing rate of 80% (Fig. 1). His preoperative JOA score was 6, postoperative JOA score was 15, and IR was 81.8%. Preoperative CT scan showed a massive local type OPLL at C4-5. Preoperative MR image showed severe compression of the spinal cord anteriorly. Postoperative CT scan demonstrated complete resection of OPLL, and postoperative MR image demonstrated satisfying decompression of the spinal cord.
Fig. 1.
Case 1. a CT reconstruction images. Preoperative CT image (left) shows a massive local type OPLL at C4-5, and postoperative CT image (right) shows complete resection of OPLL. b MR images. Preoperative MR image (left) shows severe compression of the spinal cord, and postoperative MR image (right) shows satisfying decompression of the spinal cord
Case 2
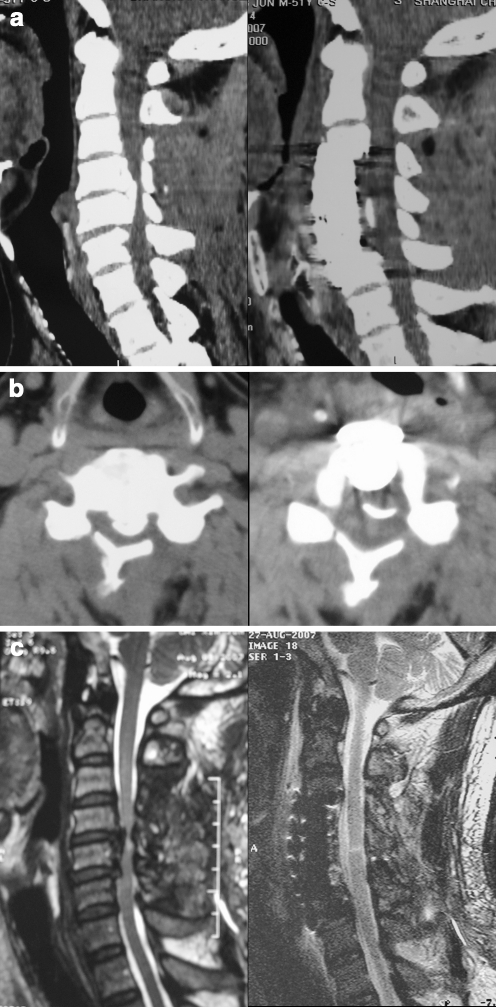
Anterior decompression and the floating method were used for the treatment of a 51-year-old man with severe OPLL, who had just undergone an unsuccessful laminoplasty 2 months earlier (Fig. 2). His preoperative JOA score was 7, postoperative JOA score was 14, and IR reached 70%. Preoperative reconstructed CT scan showed a segmental type OPLL at C4-5, and an axial image showed OPLL occupying 75% diameter of the spinal canal with a typical double-layer sign. Preoperative MR image showed no decompression of the spinal cord after laminoplasty. Postoperative CT scan demonstrated residual dural ossification (DO) after a C4-5 anterior corpectomy and fusion. Postoperative MR image demonstrated sufficient decompression of the spinal cord.
Fig. 2.
Case 2. a CT reconstruction images. Preoperative CT image (left) shows a massive segmental type OPLL at C4-5, and postoperative CT image (right) shows resection of OPLL. b Axial CT scans. Preoperative CT scan (left) shows a typical double-layer sign, and postoperative CT scan (right) shows residual of dural ossification (DO) and anterior shifting. c MR images. Preoperative MR image (left) shows insufficient decompression of the spinal cord after unsuccessful laminoplasty, and postoperative MR image (right) shows sufficient decompression of the spinal cord
Discussion
Some controversy still exists over the surgical options for cervical myelopathy due to OPLL. In general, anterior decompression by cervical corpectomy and resection of OPLL seems to be an ideal surgical option, and posterior decompression (laminoplasty or laminectomy) is an alternative choice for severe OPLL when the anterior approach threatens iatrogenic deterioration of the neurological status. Indeed, severe OPLL presents a significant challenge for spinal surgeon when attempting to remove the OPLL through the anterior approach, and the early reports indicated discouraging results. In most cases, the anterior floating method without resection of OPLL was usually used to avoid dural tears and injury to the spinal cord. The results of anterior procedures varied due to insufficient decompression resulting from DO or massive bleeding from the epidural space [5, 11, 15, 17, 20]. Cervical laminoplasty was one of the most widely used surgical options and good clinical results have been published. However, laminoplasty in patients with massive OPLL may result in insufficient decompression because OPLL remains ventral to the spinal cord. Limitation of laminoplasty may become more significant in patients with cervical kyphotic deformity. In addition, progressive kyphosis and progression of OPLL after laminoplasty may cause neurological deterioration in the long-term follow-up [9]. Therefore, this study focussed on the questions of when to choose anterior decompression for severe OPLL and how to remove the ossified lesions.
The results of this study demonstrated the advantages of anterior decompression and fusion for severe OPLL including sufficient decompression and restoration of cervical lordosis, but we still suggest caution for anterior decompression when OPLL occupies over 50% diameter of the spinal canal. In fact, the patients who underwent anterior corpectomy and fusion were strictly selected in this series. Indications for anterior decompression were concluded as follows: (1) a local or segmental type OPLL extending less than three vertebrae, (2) absence of congenital canal stenosis, and (3) the lesion below C2 and above T1. The difficulties for an anterior approach lie not only in resection of OPLL but also in restoration of bone defect after corpectomy. Iliac crest struts could be grafted for two-level corpectomy and a technique of fibula grafting was used for three- or more level corpectomy [4, 10, 19]. However, the traditional autologous bone graft was reported to have a high incidence of donor site complications, including subcutaneous haematoma, wound infection, and chronic wound pain. The patients also required prolonged postoperative immobilisation of the neck with a cervical orthosis. As a solution to these problems, titanium mesh cages have been developed and used together with anterior cervical plates, which provide the advantages of immediate stability, comparable fusion rate, and no donor site complications. However, when it comes to three- or more level corpectomy, restoration with titanium mesh cages has a clear risk of subsidence or dislocation [3, 7, 12, 16]. Hence, only patients with a local or segmental type OPLL extending less than three vertebrae were chosen for anterior corpectomy and fusion.
Anterior decompression was thought to be more risky with increasing OPLL narrowing rate. Intraoperative mechanical injury to the spinal cord resulted in immediate deterioration of the neurological status, and haematoma could induce delayed paralysis. The safety of the anterior procedure depends on less traumatic manipulation to the spinal cord and protection of the epidural vascular plexus. A specialised micro dissector was designed and used to separate OPLL from the dura, and resection of OPLL should begin from a nonossified ligament. The floating method seems to be helpful when OPLL is associated with DO, which is more frequent with severe OPLL. If a double-layer sign is presented on preoperative CT images, which is the specific indicator for DO described by Hida, separation and resection of the OPLL should be done with greater care [6, 8]. Because there is a thin layer of nonossified ligament between the OPLL and DO in cases of the double-layer pattern, meticulous dissection of the OPLL from DO can be performed without removing DO. The residual DO preserved to avoid dural tears, gradually floated anteriorly, and eventually did not compress the spinal cord.
CSF leakage was the most common complication after anterior surgery for OPLL and its incidence rate varied from 4.5% to 32%. Accumulation of CSF in the wound may cause delay of wound healing, infection, or airway obstruction [2, 18, 21]. Avoiding this complication depends on preoperative identification of DO and meticulous dissection during the operation. In this series, CSF leakage resulting from dural tears occurred in four patients due to tight adherence of OPLL with the dura or associated DO, but the area of dural defect was limited and suture or fascial graft reported in the literature seemed to be unnecessary because CSF leakage could be cured simply by conservative treatment.
Based on the results of this study, anterior decompression and fusion was effective and safe in the treatment of the selected patients, although OPLL exceeded 50% diameter of the spinal canal. Preoperative detailed radiological evaluation was critical to anterior decompression of OPLL, and severe neurological complications could be avoided by meticulous dissection.
References
- 1.Choi S, Lee SH, Lee JY, et al. Factors affecting prognosis of patients who underwent corpectomy and fusion for treatment of cervical ossification of the posterior longitudinal ligament: analysis of 47 patients. J Spinal Disord Tech. 2005;18:309–314. doi: 10.1097/01.bsd.0000161236.94894.fc. [DOI] [PubMed] [Google Scholar]
- 2.Chiang HS, Kondo S, Mizuno J, et al. Airway obstruction caused by cerebrospinal fluid leakage after anterior cervical spine surgery. A report of two cases. J Bone Joint Surg (Am) 2004;86:370–372. doi: 10.2106/00004623-200402000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Dorai Z, Morgan H, Coimbra C. Titanium cage reconstruction after cervical corpectomy. J Neurosurg. 2003;24:3–7. doi: 10.3171/spi.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 4.Epstein N. Anterior approaches to cervical spondylosis and ossification of the posterior longitudinal ligament: review of operative technique and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313–324. doi: 10.1016/S0090-3019(01)00464-5. [DOI] [PubMed] [Google Scholar]
- 5.Epstein N. The surgical management of ossification of the posterior longitudinal ligament in 51 patients. J Spinal Disord. 1993;6:432–454. doi: 10.1097/00002517-199306050-00011. [DOI] [PubMed] [Google Scholar]
- 6.Epstein N. Identification of ossification of the posterior longitudinal ligament extending through the dura on preoperative computed tomographic examinations of the cervical spine. Spine. 2001;26:182–186. doi: 10.1097/00007632-200101150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Hee HT, Majd ME, Holt RT, et al. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1–9. doi: 10.1097/00024720-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Hida K, Iwasaki Y, Koyanagi I, et al. Bone window computed tomography for detection of dural defect associated with cervical ossification posterior longitudinal ligament. Neurol Med Chir (Tokyo) 1997;37:173–175. doi: 10.2176/nmc.37.173. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: clinical results and limitations of laminoplasty. Spine. 2007;32:647–653. doi: 10.1097/01.brs.0000257560.91147.86. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32:654–660. doi: 10.1097/01.brs.0000257566.91177.cb. [DOI] [PubMed] [Google Scholar]
- 11.Kojima T, Waga S, Kudo Y, et al. Anterior cervical vertebrectomy and interbody fusion for multilevel spondylosis and ossification of the posterior longitudinal ligament. Neurosurgery. 1989;24:864–871. doi: 10.1097/00006123-198906000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama M, Hashimoto T, Shigenobu K, et al. Pitfalls of anterior cervical fusion using titanium mesh and local autograft. J Spinal Disord Tech. 2003;16:513–518. doi: 10.1097/00024720-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno J, Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6:282–288. doi: 10.1016/j.spinee.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Masaki Y, Yamazaki M, Okawa A, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka T, Yamaura I, Kurosa Y, et al. Long-term results of the anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Spine. 2001;26:241–248. doi: 10.1097/00007632-200102010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Narotam PK, Pauley SM, McGinn GJ. Titanium mesh cages for cervical spine stabilization after corpectomy: a clinical and radiological study. J Neurosurg. 2003;24:172–180. doi: 10.3171/spi.2003.99.2.0172. [DOI] [PubMed] [Google Scholar]
- 17.Onari K, Akiyama N, Kondo S, et al. Long-term follow-up results of anterior interbody fusion applied for cervical myelopathy due to ossification of the posterior longitudinal ligament. Spine. 2001;26:488–493. doi: 10.1097/00007632-200103010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Smith MD, Bolesta MJ, Leventhal M, et al. Postoperative cerebrospinal fluid fistula associated with erosion of the dura. Findings after anterior resection of ossification of the posterior longitudinal ligament in the cervical spine. J Bone Joint Surg. 1992;74:270–277. [PubMed] [Google Scholar]
- 19.Tani T, Ushida T, Ishida K, et al. Relative safety of anterior microsurgical decompression versus laminoplasty for cervical myelopathy with a massive ossified posterior longitudinal ligament. Spine. 2002;27:2491–2498. doi: 10.1097/00007632-200211150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Yamaura I, Kurosa Y, Matuoka T, et al. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res. 1999;359:27–34. doi: 10.1097/00003086-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Yonenobu K, Hosono N, Iwasaki M, et al. Neurologic complications of surgery for cervical compression myelopathy. Spine. 1991;16:1277–1282. doi: 10.1097/00007632-199111000-00006. [DOI] [PubMed] [Google Scholar]