Abstract
The purpose of this study was to examine the repairing ability of nano-hydroxyapatite (nano-HA) artificial bone with different pore sizes. Animal models of bone defects were created in both radii of 60 New Zealand white rabbits. The bone defects in A, B, and C groups were repaired with nano-HA artificial bone with three different pore sizes while those in group D were left unrepaired. The repairing ability of material was evaluated by gross observation, histopathological study, X-ray examination, scanning electron microscope (SEM), and biomechanical analysis in the fourth, eighth, and 12th weeks. Group B stimulated more bone formation than the other groups. Nano-HA artificial bone is capable of good bone formation and biocompatibility. The ability of bone formation of nano-HA artificial bone may be affected significantly by the pore size. The material with pore size in the range of 100 –250 μm has a greater ability to form bone.
Résumé
L’objectif de cette étude est d’étudier les possibilités de réparation osseuse avec la nano-hydroxyapatite (nano-HA) sur des os artificiels avec différentes tailles de pores osseux. Méthode : le modèle animal choisi l’a été avec constitution d’un défect osseux au niveau du radius bilatéral de 60 lapins blancs de Nouvelle Zélande. Les défects osseux ont été réalisés sur trois groupes A, B et C avec réparation par nano-hydroxyapatite et trois calibrages différents et un dernier groupe D sans mise en place de nano-hydroxyapatite. Les possibilités de réparation ont été évaluées par l’observation macroscopique des études histopathologiques, des études radiologiques, le microscope électronique, l’étude biomécanique. Toutes ces études ont été réalisées à 4, 8 et 12 semaines. Résultats, le groupe B permet une cicatrisation osseuse plus importante que dans les autres groupes. En conclusion : l’os artificiel constitué par la nano-hydroxyapatite permet une bonne régénération osseuse et cette régénération est influencée par la taille des pores osseux. Le matériel avec des pores compris entre 100 et 250 Im est la dimension optimum pour permettre cette régénération.
Introduction
In the application of bone defect repairing material, it is desired that the structure be simulated, imitated, or replicate the porous structure of biological bone, so that it can adapt to the stress changes in a certain range, circulate blood well, ensure normal growth and metabolism of bone tissue, and accelerate bone reconstruction [13]. Hydroxyapatite (HA) is a kind of implanted material widely used clinically to repair bone defects. With increased pore size of HA, bone material conductibility gradually strengthens, but the increase of pore size and gap rate will decrease the biological intensity at the same time. So an optimal combination of pore size, gap rate, and mechanical intensity of HA material needs to be found to conform to the needs of biological activity and mechanical intensity based on different functions [4]. In this study, nano-HA material was manufactured into artificial bone with different pore sizes through various techniques. Thus, the purpose of this study was to evaluate the bone formation ability of nano-HA material with different pore sizes to repair bone defects through animal experiments.
Materials and methods
Materials
Nano-HA artificial bone was manufactured with three pore sizes (50–150 μm, 100–250 μm, and 300–500 μm) by Shenzhen Second People’s Hospital associated with the Study Centre of Powder Metallurgy Engineering of Zhongnan University [8].
Animal grouping
Sixty New Zealand white rabbits provided by the Animal Centre of Southern Medical University (all male, weight 1.5–2.0 kg) were randomly divided into four groups:
Nano-HA artificial bone with pore size of 50–150 μm was implanted in the bone defect.
Nano-HA artificial bone with pore size of 100 –250 μm was implanted in the bone defect.
Nano-HA artificial bone with pore size of 300–500 μm was implanted in the bone defect.
Nothing was implanted in the bone defect (control group).
Process of operation
The animals were under general anaesthesia with an injection of ketamine hydrochloride (20 mg/kg). After depilation and sterilisation, the specimens were covered with a sterile twel towel on both forearms and the middle sections of both radial diaphyses were exposed through a 2.0 cm longitudinal incision. The radius and periosteum was sawed through using a wire saw at a point 2.5 cm from the distal radius. The same process was performed at a site 1.5 cm distal to the first osteotomy and the bone segment between the two cuts was resected with the periosteum. After irrigation with saline, three types of nano-HA artificial bone with different pore sizes were respectively implanted in the bone defects of groups A, B, and C, while nothing was implanted in group D. The same material was implanted in the contralateral forearm. After operation, the rabbits were left to move about without internal or external fixation. Penicillin was used daily to avoid infection.
Measurement
General observation
The diet, activity, and wounds of the rabbits were observed after operation. The superficial state, osteogenesis, inflammatory reaction of implanted material, bone repairing state, and growth of callus were studied after taking out the sample.
X-ray examination
X-ray photographys were taken at four, eight, and 12 weeks postoperatively. Bone formation and moulding were evaluated according to Lane-Sandhu X-ray score standard.
Biomechanical analysis
Four samples were extracted at four, eight, and 12 weeks postoperatively. Then a three point anti-bend test was performed with 858 mini Bionix (provided by Southern Medical University). In the test, acceleration was 1 mm/min, spacing between two points was 30 mm. The maximum bend point of radius was in the middle of it. The sample was forced downward and the peak was recorded when it broke. Bend intensity was calculated according to White's formula, 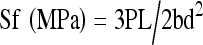 , in order to study the biomechanical property and the state of bone defect repair of every group.
, in order to study the biomechanical property and the state of bone defect repair of every group.
Scanning electron microscope (SEM) observation
Two samples were taken at every period for each group. The site of the bone defect and about 5 mm normal radius of both sides were all resected. Fixed with 3% glutaraldehyde, the samples were cut across by quick cutter knife. After desiccation, the samples were coated with gold, and then the state of compatibility and bone defect repair under scanning electron microscope (HITACH S-450) were observed.
Statistical analysis
The data were collected and statistically analysed by SPSS 11.0.
Results
General observation
All rabbits had normal diet and movement after operation, and there were no deaths or wound infections. The wound achieved primary healing in the first postoperative week. The rabbits moved freely without limp.
Radiographic examination
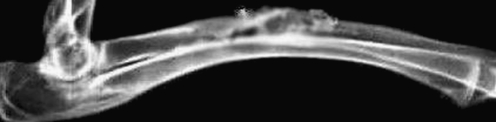
Group A At four weeks, the implanted material began to degrade and a little callus formed. At eight weeks together with the degradation of implanted material, its margins and the bone tissue had become cloudy. At 12 weeks, the implanted material was incompletely broken down. The medullary cavity achieved partial recanalisation and bone defects were partly repaired (Figs. 1 and 2).
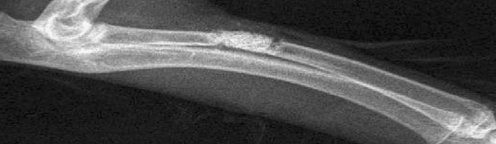
Fig. 1.
X-ray showing the bone defect repaired with artificial bone (1 day postoperative)
Fig. 2.
X-ray of group A. The material was degraded incompletely. The bone defect was partly repaired (12 weeks postoperative)
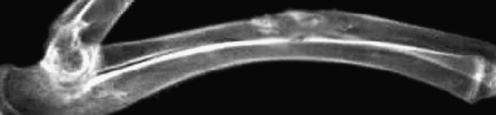
Group B At four weeks, the implanted material was partly degradated. There was confluence between the implanted material and bone tissue, and a little callus formed. At eight weeks, mass callus had formed and the boundary of implanted material and bone tissue had become cloudy. At 12 weeks, the implanted material was completely degraded. The medullary cavity achieved full recanalisation, and bone defects were repaired completely (Fig. 3).
Fig. 3.
X-ray of group B. The material was broken down. The medullary cavity recanalised completely, and the bone defect had been repaired (12 weeks postoperative)
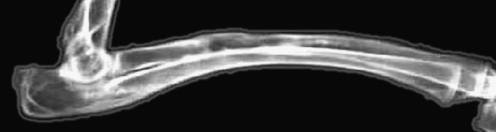
Group C At four weeks, the implanted material was partly degraded. There were interspaces between implanted material and bone tissue in which callus formed. At eight weeks, mass callus had formed and implanted material was partly degraded. At 12 weeks, implanted material was degraded completely. There was clouding at the boundary and interspaces between new formed and normal bone (Fig. 4).
Fig. 4.
X-ray of group C. The material was completely degraded. The medullary cavity partly recanalised (12 weeks postoperative)
Group D At 12 weeks, there was no osteoid connection in the defects. The extremities of the bone defects were sclerotic, and the medullary cavities were blocked. Bone defects had not been repaired.
Radiographical analysis of bone formation and moulding
The bone formation scores were evaluated according to Lane-Sandhu radiographical score standard and the average scores were used (Table 1). It was shown that at four, eight, and 12 weeks postoperatively the scores of groups A, B, and C were higher than those of group D and the differences were statistically significant between groups A and D, groups B and D, and groups C and D (P < 0.05). The scores of group B were higher than those of groups A and C in every period and the differences were statistically significant between groups A and B, and groups B and C (P < 0.05). The differences between the scores of groups A and C were not statistically significant (P > 0.05). Thus, it might be considered that the ability of bone formation of nano-HA artificial bone with pore size of 100–250 μm was better than that of 50–150 μm and 300–500 μm.
Table 1.
X-ray scores of bone formation and moulding in different groups ( )
)
| Time (weeks) | Group A | Group B | Group C | Group D | ||||
|---|---|---|---|---|---|---|---|---|
| N | Score | N | Score | N | Score | N | Score | |
| 4 | 15 | 2.8 ± 0.30*△ | 15 | 4.5 ± 0.45* | 15 | 2.6 ± 0.34*△ | 15 | 1.0 ± 0.30 |
| 8 | 10 | 5.8 ± 0.74*△ | 10 | 8.3 ± 0.67* | 10 | 5.2 ± 0.42*△ | 10 | 1.5 ± 0.29 |
| 12 | 5 | 8.8 ± 1.05*△ | 5 | 10.6 ± 0.73* | 5 | 8.9 ± 0.78*△ | 5 | 2.2 ± 0.57 |
* Compared with group D P < 0.05
△ Compared with group B P < 0.05
Biomechanical test
Samples of the experimental groups were tested in the three point anti-bend test at four, eight, and 12 weeks postoperatively. The biomechanical strength measurements were statisticaly analysed by paired t test (Table 2). The differences were statistically significant (P < 0.05). At four, eight, and 12 weeks postoperatively, the biomechanical strengths of group B were stronger than those of groups A and C and the differences were statistically significant (P < 0.05). The differences between groups A and C were not statistically significant (P > 0.05).
Table 2.
Biomechanical strength of different groups ( , n = 4)
, n = 4)
| Time (weeks) | Biomechanical strength (MPa) | ||
|---|---|---|---|
| Group A | Group B | Group C | |
| 4 | 15.36 ± 2.19* | 21.77 ± 4.03 | 15.23 ± 2.25* |
| 8 | 30.64 ± 2.42* | 45.43 ± 6.38 | 30.77 ± 2.53* |
| 12 | 52.24 ± 5.00* | 71.55 ± 5.79 | 52.26 ± 5.15* |
*Compared with group B P < 0.05
Scanning electron microscope test
Group A At four weeks, the implanted material began degradation. Callus occurred and grew towards the centre of the material. At eight weeks, the interspaces between implanted material and normal bone tissue were decreased and a significant amount of new bone tissue was formed. At 12 weeks, the implanted material was incompletely degraded. The bone defect was filled by new lamellar bone. The medullary cavity achieved partial recanalisation and the bone defect was partly repaired.
Group B At four weeks, the implanted material was partly degraded. There were tiny interspaces and contact connections between implanted material and normal bone tissue. Among the interspaces, no abnormal tissue was found. At eight weeks, there were regional connections and the interspaces were not clear between implanted material and normal bone tissue. Bone tissues grew into implanted material to form confluence of directions. At 12 weeks, the implanted material was mostly degraded. The rest of the material connected tightly with bone tissues with no interspaces existing between them; their interfaces appeared confluent. The medullary cavity recanalised and the bone defect had been repaired (Fig. 5).
Fig. 5.
Scanning electron microscope photo of group A. The material was completely degraded. The bone defect area had been filled with lamellar bone tissue and the bone defect had been repaired (×1000)
Group C At four weeks, the implanted material was quickly degraded. At eight weeks, there was new bone tissue formed on the edges of implanted material, which was further degraded. At 12 weeks, the implanted material was completely degraded. The bone defect was filled by lamellar bone. The medullary cavity achieved recanalisation and bone defect was partly repaired.
Discussion
Polyporous HA is a satisfactory biological material for filling bone defects; it has good biological activity, biocompatibility, and conjunctive ability with bone tissues. For many years, HA has been widely studied and products have been manufactured of different forms, gap rates, and degradation rates. Satisfactory results have been achieved since its use was approved ten years ago. But the polyporous HA ceramic material has the obvious shortcoming of lower mechanical strengh which greatly limits the application at the weight-bearing sites in bone transplantation. To strengthen the HA, researchers have carried out many investigations in all directions and the focus of contension in the development of HA is whether the biological properties can satisfactorily match the mechanical properties [5].
Currently, consideration is being given to the mechanism of implanted HA to promote bone reconstruction and providing support for the formation of microvessels and the attachment of host osteocytes in the early stage of bone defect repair. The stimulation function of HA crystal makes osteocytes active and forms zones of ossification around HA particles, namely, polycentric osteogenesis. HA can provide crystal nuclei to accelerate calcification osteogenesis in the course of bone mineral deposition, i.e. bone conduction [9]. The combination of implanted and host bone depends on the invasive achievement of blood vessels. Even though implanted bone shows negative activity, it’s existence and structure can stimulate the growth of surrounding tissues, and its three-dimensional structure is the major factor for determining the speed and completeness combination which can be confirmed by the different phenomena observed in the implantation of cortical and spongy bone. The differences indicate the need of interspace in the course of bone conduction, and the matrix materials with larger gap rate and superficial acreage can promote faster and more complete bone conduction. Thus, the bone conduction of transplanted and implanted bone is obviously dependent on their structural configuration [2, 11].
Currently, the focus of study in this area is development of several synthetic transplanted matrices of artificial bone. Investigation and analysis of these materials makes people appreciate the significance of the pore size of HA. For polyporous HA artificial bone, there is not yet agreement on suitable pore size. Researchers have suggested that material with pore size larger than 150 μm could provide ideal room for bone tissues growth [10]. Ei et al. [3] proposed that the minimum pore size for bone ingrowth is 100 μm, and that 150 μm is the ideal pore size. Chang et al. [1] thought that 500 μm was a more suitable pore size. But experiments in vitro showed that the increase of pore size and gap rate could decrease the biomechanical strength of artificial bone [6, 7].
It was seen in recent years that HA in human normal bone was mainly needle and single crystal structure, with a particle diameter at nanometer level and distributed among the net of collagen in specific directions [12]. Thus, the material structure of HA at nanometer grade is similar to that of the human body and has better biological properties [1].
In this study, nano-HA material was manufactured by the method of Collosol-Flocculation to improve the mechanical property and to overcome the shortcoming of higher fragility, lower intensity to resist extrusion and breakage, and poor weight bearing. At the same time, polyporous nano-HA artificial bone with a gap rate above 90% and pore sizes of 50 –150 μm, 100– 250 μm and 300 –500 μm were produced by different techniques in order to perform the animal experiments of bone defect repair. The purpose of this experiment was to screen out appropriate pore sizes to achieve good unification of bone conduction and biomechanical property.
With the samples studied by X-ray examination, histological observation, biomechanical test, and scanning electron microscope detection, it was shown that there was degradation of nano-HA artificial bone and bone formation to different extents at the site of bone defect, but there was also repair to different extents. At 12 weeks, the cortical bone had basically completed moulding and the medullary cavity of bone had recanalised of nano-HA artificial bone with a pore size of 100–250 μm. The results indicated that nano-HA artificial bone had the capability to repair bone defects and excellent biomechanical properties, which might become promising bone transplantation material. But the repair characteristics of nano-HA artificial bone with different pore sizes also displayed differences. The ability of bone formation of nano-HA artificial bone with pore size of 100–250 μm was obviously higher than those of 50–150 μm and 300–500 μm.
Through the evaluation of bone formation of nano-HA artificial bone with different pore sizes, it is suggested from these results that the suitable pore size of polyporous nano-HA artificial bone is 100–250 μm. That will provide a good foundation for further improving the material structure and creating an ideal replacement material for bone transplantation.
References
- 1.Chang BS, Lee CK, Hong KS, et al. Osteoconduction at porous HA with various pore configurations. Biomaterials. 2000;20:1291–1295. doi: 10.1016/S0142-9612(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 2.Dorner-Reisel A, Klenn V, Irmer G, et al. Nano and microstructure of short fibre reinforced and unreinforced hydroxyaptite. Biomed Tech. 2002;47(suppl 1Pt1):397–400. doi: 10.1515/bmte.2002.47.s1a.397. [DOI] [PubMed] [Google Scholar]
- 3.Ei GH, Annam A, Ducheyne P, et al. Reconstruction of alveolar bone defect by calcium phosphate compounds. Biomed Mater Res. 1995;29(3):359–370. doi: 10.1002/jbm.820290311. [DOI] [Google Scholar]
- 4.Jiang HP, Wang DP, Ruan JM, Zhu WM. Biocompatibility study of nano-hydroxyapatite artificial bone. China J Modern Medicine. 2005;6(10):24–29. [Google Scholar]
- 5.Jouve JL, Mottet V, Cottalorda J, et al. Reimplantation of growth plate chondrocyte cultures in central growth plate defects: part 1. Characterization of cultures. J Pediatr Orthop B. 1998;7:167–173. doi: 10.1097/01202412-199804000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Liu DM. Preparation and characterization of porous hydroxya-patite bioceramicvia as lip-casting route. Ceram Int. 1998;24:441–445. doi: 10.1016/S0272-8842(97)00033-3. [DOI] [Google Scholar]
- 7.Milosevski M, Bossert J, Milosevski D, et al. Preparation and properties of dense and porous calcium phosphate. Ceram Int. 1999;25:693–695. doi: 10.1016/S0272-8842(99)00003-6. [DOI] [Google Scholar]
- 8.Ruan JM (2000) Chinese patent. Produce method of polyporous biomaterial. ZL 91106753.1
- 9.Wang D, Zheng CQ, Hu YY. The new progress of biological material study. Biomed Engin For Med Sci. 1999;22(6):364–367. [Google Scholar]
- 10.Yang ZJ, Yuan HP, Zhang XD, et al. Effect of sarsasapogenin and its derivatives on the stimulus coupled responses of human neutrophils. Biomaterials. 1996;17:2131–2137. doi: 10.1016/0142-9612(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhu WM, Wang DP, Meng ZB, et al. Experimental study of the nano-hydroxyapatite artificial bone in repairing the bone defect. Chinese J Clin Anat. 2006;6(10):13–18. [Google Scholar]
- 12.Zhu WM, Wang DP, Xiong JY, et al. Revascularization study of the nano-hydroxyapatite artificial bone in repairing the bone defect. J Chinese Microcirculation. 2006;5(4):37–42. [Google Scholar]
- 13.Zhu WM, Wang DP, Xiong JY. Biological characteristics and clinical application of scaffold materials for bone tissue engineering. J Clin Rehab Tissue Eng Res. 2007;48:23–26. [Google Scholar]